-
PDF
- Split View
-
Views
-
Cite
Cite
Óscar R Peña-Vizcarra, María Fernanda Zavala-Miranda, Bernardo Juárez-Cuevas, Sofía E Márquez-Macedo, Adriana Hernández-Andrade, Alberto Nordmann-Gomes, Abril A Pérez-Arias, Luis E Morales-Buenrostro, Juan M Mejía-Vilet, Effect of antimalarials on clinical outcomes in lupus nephritis, Rheumatology, Volume 63, Issue 8, August 2024, Pages 2230–2238, https://doi.org/10.1093/rheumatology/kead576
Close - Share Icon Share
Abstract
To evaluate the effect of antimalarial drugs in response to therapy, incidence of LN flares, and progression of kidney disease in a large LN cohort.
We retrospectively studied 424 biopsy-proven LN patients followed for >3 years. We obtained demographic, clinical, laboratory, histopathological and treatment variables. Antimalarial use was approached as (i) users vs no users, (ii) according to prevalent vs incident use regarding the LN flare and (iii) according to the type of antimalarial. All outcomes were evaluated by time-to-event analyses. Adjusted hazard ratios were obtained by Cox regression.
The cohort included 424 patients, median age of 29 years (IQR 23–37), 96% female, with a median eGFR of 81 ml/min/1.73 m2 (IQR 48–118) and proteinuria of 3.4 g/g (IQR 1.9–5.5). Antimalarial use was associated with higher complete response (aHR 1.57, 1.08–2.27), lower incidence of kidney flares (aHR 0.63, 0.43–0.92) and lower progression to kidney failure (aHR 0.37, 0.23–0.53). The effect of antimalarials on these outcomes was modified by the presentation eGFR, histological class and/or concomitant initial immunosuppressor. These protective effects were observed in patients with prevalent or incident use regarding the LN flare and patients using hydroxychloroquine. The incidence of toxic retinopathy was 1.7%, 5.7% and 8.8% by 3, 5 and 7 years of continued antimalarial use, respectively.
The use of antimalarial drugs is associated with increased response to therapy, lower incidence of kidney flares, and lower progression to kidney failure in LN patients. Conversely, this population is at high risk of toxic maculopathy, and yearly ophthalmologic examination is recommended.
Antimalarial drugs are associated with increased response to therapy, lower incidence of LN flares, and reduced progression to kidney failure.
The effects of antimalarial drugs vary between the type of antimalarial and are modified by other factors, such as the concomitant initial immunosuppression.
The incidence of toxic retinopathy from antimalarials is increased in LN patients, so yearly examination is recommended.
Introduction
Kidney involvement occurs in 40% to 60% of systemic lupus erythematosus (SLE) patients and is associated with increased morbidity and mortality [1]. Despite advances in understanding the disease and novel therapies, 10% to 20% of patients with lupus nephritis (LN) will still progress to kidney failure within 10–20 years of diagnosis [2].
Antimalarial drugs, especially chloroquine and hydroxychloroquine, have been used since the 1950s as a therapy for SLE [3]. These drugs interfere with nucleic acid recognition and processing by the toll-like receptors and lysosomes within antigen-presenting cells and B cells, thereby blocking immune activation and cytokine production involved in SLE and LN pathogenesis [4].
Evidence for antimalarial drug efficacy in SLE proceeds from a few randomized controlled trials as well as observational prospective and retrospective cohorts [3]. Antimalarials have been associated with decreases in SLE disease severity [5–7], prevention of systemic disease flares [8–10], improvement in cutaneous and articular manifestations [11, 12], protection against flares in pregnancy [13, 14], lower risk for thrombosis and infections [15], improvement in the lipidic profile [16], protection against damage accrual [17, 18] and increased patient survival [19, 20], among others [3].
Specifically in LN, only one randomized controlled trial has suggested that hydroxychloroquine use is associated with higher rates of response to therapy, and few observational cohorts suggest that antimalarial use is associated with better response to therapy [5, 21, 22]. Even with the widespread recommendation for the use of antimalarials in patients with SLE and LN by the most recent practice guidelines [23, 24], there is a need for evidence supporting the role of antimalarials in improving kidney outcomes. In this study, we aimed to evaluate the association between antimalarial use (including the specific type of antimalarial and temporality of use regarding the LN flare) and response to therapy, the incidence of LN flares, and progression to kidney failure in a large LN cohort.
Methods
This is a retrospective cohort study performed at the National Institute of Medical Sciences and Nutrition Institute Salvador Zubirán in Mexico City. The study was approved by the National Institute of Medical Sciences and Nutrition Salvador Zubirán Ethics and Research Boards (reference 4665). The cohort was composed of patients classified with SLE according to the revised criteria of the American College of Rheumatology [25] who were diagnosed with biopsy-proven LN between 2008 and 2020. All patients were followed for a minimum of 36 months or death at any point of the follow-up.
The database included data from the previous history of SLE and LN, demographic, clinical, laboratory variables at LN flare, and evaluations over follow-up. Histological data from the diagnostic kidney biopsy included all items evaluated by the modified National Institutes of Health (NIH) activity and chronicity indices, the ISN/RPS classification and the vascular findings [26, 27]. We registered the immunosuppressive scheme used for initial and maintenance therapy. For antimalarial use, we revised each record for the date of start of an antimalarial, date of suspension, dose at each visit and type of antimalarial. From these data, we estimated the cumulative dose during the follow-up and registered the timing of antimalarial use in accordance with the LN flare.
We used three approaches to stratify antimalarial use: (i) allocation into antimalarial users and non-users, (ii) segregation into ‘prevalent users’ (antimalarial started before the LN flare and continued during follow-up), and ‘incident users’ (antimalarial started within 3 months of the diagnosis of the LN flare) and (iii) segregation into groups according to the type of antimalarial (hydroxychloroquine, chloroquine, or use of both at different times over follow-up). We excluded 22 patients who started the antimalarial beyond 3 months of the LN flare (Supplementary Fig. S1, available at Rheumatology online). Medication persistence was assessed at each follow-up visit (average 3–4 visits per year). Treatment gaps were recorded with dates of suspension and dates of treatment re-initiation. Medication compliance was assessed from medical records.
All the patients included in the study had a minimum of two ophthalmology evaluations during follow-up to assess for antimalarial ocular toxicity. Screening modalities included fundoscopy, automated visual field assessment (VFA), spectral-domain optical coherence tomography (SD-OCT) in all cases, complemented with multifocal electroretinography (mf-ERG) in some cases. Mild retinopathy was defined as a patchy damage within the parafoveal zone documented by visual field or SD-OCT examinations, moderate retinopathy was defined as 50–100% parafoveal ring of damage and marked thinning of the parafoveal retina on SD-OCT, and severe stage as bull’s eye damage with retinal pigment epithelium involvement on SD-OCT and/or visible retinopathy on fundoscopy [28].
The following outcomes were evaluated by survival analyses (time-to-event) in the three approaches to antimalarial stratification: time to complete response (CR) or partial response (PR) to treatment, time to the first LN relapse, time to kidney failure, time of patient survival, and time to development of macular toxicity. Complete and partial response were defined according to the KDIGO guidelines[23]: complete response was defined as stable kidney function (eGFR within 15% of baseline) plus 24-h urine protein to creatinine ratio (24 h-uPCR) below 0.5 g/g. Partial response was defined as a 50% reduction of the 24 h-uPCR below 3.0 g/g plus stable kidney function. LN relapse was defined as a persistent increase of 24 h-uPCR to >1.0 g/g in patients with previous CR or persistent doubling of the previous 24 h-uPCR in patients with a previous PR, with or without a decline in the eGFR, that required intensification of the immunosuppressive therapy as judged by the treating physician. The time at risk of an LN flare was estimated from the date of PR attainment and was exclusively evaluated in patients with partial or complete response. Kidney failure was defined as a persistent requirement (>3 months) for any type of kidney replacement therapy.
Statistical analysis
For quantitative variables, distribution was evaluated by the Kolmogorov–Smirnov test and is presented as median (interquartile range). Categorical variables are shown as frequencies (percentages). All the outcomes were evaluated by survival analyses (time-to-event). The figures in this manuscript show the Kaplan–Meier plots. Univariate Cox regression analysis was performed to identify all factors associated with each outcome. All variables with a P-value < 0.05 or deemed clinically important were included in the multivariable adjusted analysis. Death was considered a competing risk. Stratified subgroup analyses were performed to identify modification of the antimalarial therapy effect in specific subgroups of patients. P-values below 0.05 were considered statistically significant. Data were analysed by SPSS 24.0 (IBM Corp., Armonk, NY, USA), and graphs constructed with GraphPad Prism 9.0 (GraphPad Software, Boston, MA, USA).
Results
A total of 424 patients with biopsy-proven LN were included in this study (Supplementary Fig. S1, available at Rheumatology online). The median age was 29 years (IQR 23–37), 405 (96%) were female, 245 (58%) had incident LN and 179 (42%) had a repeated LN flare. The median eGFR and proteinuria at presentation were 81 mL/min/1.73 m2 (IQR 49–118) and 3.4 g/g of creatinine (1.9–5.5), respectively (Table 1). The kidney biopsy showed mixed class III or IV plus V LN in 302 (71%), class III or IV in 72 (17%) and pure class V LN in 52 (12%) (Supplementary Table S1, available at Rheumatology online). Initial immunosuppressive therapy consisted of mycophenolate mofetil (MMF) plus glucocorticoids (GC) in 230 (54%), intravenous cyclophosphamide plus GC (modified NIH protocol) in 144 (34%), azathioprine (AZA) plus GC in 42 (10%) and triple therapy with calcineurin inhibitor (CNI) plus MMF and GC in 8 (2%). Maintenance immunosuppressive therapy was MMF in 278 (66%), AZA in 124 (29%) and CNI plus MMF in 22 (5%). An antimalarial drug was used in 349 (82%) patients: in 195 (56%) the use was classified as prevalent (started before the LN flare) and in 154 (44%) was incident (started within 3 months of the LN flare). Forty-nine (14%) of antimalarial users were administered chloroquine, 265 (76%) hydroxychloroquine and 35 (10%) used both (not concurrently but at different times). The complete specifications of antimalarial treatment are shown in Supplementary Table S2, available at Rheumatology online. By the end of a median follow-up of 91 months (IQR 63–119), 22 (5.1%) patients died of which 18 (82%) were under renal replacement therapy at the time of death. The mortality rates were 1.9%, 2.9% and 6.7% by 3, 5 and 10 years of follow-up.
| Parameter . | Full cohort . | Antimalarial use . | No antimalarial use . |
|---|---|---|---|
| n = 424 . | n = 349 . | n = 75 . | |
| Age, years | 29 (23–37) | 29 (23–37) | 30 (23–38) |
| Female, n (%) | 405 (96) | 330 (95) | 75 (100) |
| Mexican-mestizo race, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Hispanic ethnicity, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Socioeconomic status,a n (%) | |||
| Low | 385 (91) | 319 (91) | 66 (88) |
| Medium | 26 (6) | 19 (6) | 7 (9) |
| Social insurance | 13 (3) | 11 (3) | 2 (3) |
| Time from SLE diagnosis, months | 46 (5–109) | 40 (5–108) | 52 (6–118) |
| Previous history of LN, n (%) | |||
| First LN flare | 245 (58) | 212 (61) | 33 (44) |
| Repeated LN flare | 179 (42) | 137 (39) | 42 (56) |
| Year of lupus nephritis diagnosis | |||
| 2008–2013 | 209 (49) | 155 (44) | 56 (75) |
| 2014–2020 | 215 (51) | 194 (56) | 19 (25) |
| Body mass index, kg/m2 | 24.4 (21.9–27.5) | 24.6 (22.0–27.7) | 23.8 (21.1–26.9) |
| Mean arterial pressure, mmHg | 95 (87–103) | 94 (85–103) | 96 (87–103) |
| Haemoglobin, g/dL | 11.9 (10.5–13.4) | 12.0 (10.6–13.5) | 11.8 (10.2–13.0) |
| Platelets, × 103/mm3 | 238 (180–304) | 240 (180–305) | 235 (180–288) |
| Serum creatinine, mg/dL | 0.95 (0.67–1.43) | 0.92 (0.66–1.38) | 1.01 (0.69–1.78) |
| Serum creatinine, umol/L | 84.0 (59.2–16.9) | 81.3 (58.3–121.9) | 89.2 (61.0–157.4) |
| eGFR, ml/min/1.73 m2 | 81 (49–118) | 83 (51–119) | 74 (37–110) |
| Proteinuria (24 h-uPCR), g/g | 3.4 (1.9–5.5) | 3.4(1.9–5.4) | 3.7 (2.0–6.5) |
| Proteinuria (24 h-uPCR), mg/mmol | 304 (169–487) | 299 (169–479) | 326 (177–573) |
| Pyuria, n (%) | 331 (78) | 272 (78) | 59 (79) |
| Hematuria, n (%) | 310 (73) | 258 (74) | 52 (69) |
| Serum albumin, g/L | 2.8 (2.3–3.4) | 2.8 (2.3–3.4) | 2.6 (2.1–3.3) |
| Anti-dsDNA antibodies, UI/mL | 98.0 (22.7–423.8) | 97 (23–434) | 102 (20–271) |
| Positive anti-dsDNA antibodies, n (%) | 369 (87) | 304 (87) | 65 (87) |
| Complement C3, mg/dL | 65 (46–84) | 64 (46–84) | 71 (47–93) |
| Low C3, n (%) | 340 (80) | 279 (80) | 61 (81) |
| Complement C4, mg/dL | 10 (8–17) | 10 (8–16) | 12 (8–18) |
| Low C4, n (%) | 327 (77) | 267 (77) | 60 (80) |
| Parameter . | Full cohort . | Antimalarial use . | No antimalarial use . |
|---|---|---|---|
| n = 424 . | n = 349 . | n = 75 . | |
| Age, years | 29 (23–37) | 29 (23–37) | 30 (23–38) |
| Female, n (%) | 405 (96) | 330 (95) | 75 (100) |
| Mexican-mestizo race, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Hispanic ethnicity, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Socioeconomic status,a n (%) | |||
| Low | 385 (91) | 319 (91) | 66 (88) |
| Medium | 26 (6) | 19 (6) | 7 (9) |
| Social insurance | 13 (3) | 11 (3) | 2 (3) |
| Time from SLE diagnosis, months | 46 (5–109) | 40 (5–108) | 52 (6–118) |
| Previous history of LN, n (%) | |||
| First LN flare | 245 (58) | 212 (61) | 33 (44) |
| Repeated LN flare | 179 (42) | 137 (39) | 42 (56) |
| Year of lupus nephritis diagnosis | |||
| 2008–2013 | 209 (49) | 155 (44) | 56 (75) |
| 2014–2020 | 215 (51) | 194 (56) | 19 (25) |
| Body mass index, kg/m2 | 24.4 (21.9–27.5) | 24.6 (22.0–27.7) | 23.8 (21.1–26.9) |
| Mean arterial pressure, mmHg | 95 (87–103) | 94 (85–103) | 96 (87–103) |
| Haemoglobin, g/dL | 11.9 (10.5–13.4) | 12.0 (10.6–13.5) | 11.8 (10.2–13.0) |
| Platelets, × 103/mm3 | 238 (180–304) | 240 (180–305) | 235 (180–288) |
| Serum creatinine, mg/dL | 0.95 (0.67–1.43) | 0.92 (0.66–1.38) | 1.01 (0.69–1.78) |
| Serum creatinine, umol/L | 84.0 (59.2–16.9) | 81.3 (58.3–121.9) | 89.2 (61.0–157.4) |
| eGFR, ml/min/1.73 m2 | 81 (49–118) | 83 (51–119) | 74 (37–110) |
| Proteinuria (24 h-uPCR), g/g | 3.4 (1.9–5.5) | 3.4(1.9–5.4) | 3.7 (2.0–6.5) |
| Proteinuria (24 h-uPCR), mg/mmol | 304 (169–487) | 299 (169–479) | 326 (177–573) |
| Pyuria, n (%) | 331 (78) | 272 (78) | 59 (79) |
| Hematuria, n (%) | 310 (73) | 258 (74) | 52 (69) |
| Serum albumin, g/L | 2.8 (2.3–3.4) | 2.8 (2.3–3.4) | 2.6 (2.1–3.3) |
| Anti-dsDNA antibodies, UI/mL | 98.0 (22.7–423.8) | 97 (23–434) | 102 (20–271) |
| Positive anti-dsDNA antibodies, n (%) | 369 (87) | 304 (87) | 65 (87) |
| Complement C3, mg/dL | 65 (46–84) | 64 (46–84) | 71 (47–93) |
| Low C3, n (%) | 340 (80) | 279 (80) | 61 (81) |
| Complement C4, mg/dL | 10 (8–17) | 10 (8–16) | 12 (8–18) |
| Low C4, n (%) | 327 (77) | 267 (77) | 60 (80) |
The socioeconomic status variable was created by evaluating family income, household characteristics and family assets.
24 h-uPCR, urine protein to creatinine ratio estimated from a 24-h urine collection; anti-dsDNA, antibodies against double-strand DNA; eGFR, estimated glomerular filtration rate; LN: lupus nephritis; SLE: systemic lupus erythematosus.
| Parameter . | Full cohort . | Antimalarial use . | No antimalarial use . |
|---|---|---|---|
| n = 424 . | n = 349 . | n = 75 . | |
| Age, years | 29 (23–37) | 29 (23–37) | 30 (23–38) |
| Female, n (%) | 405 (96) | 330 (95) | 75 (100) |
| Mexican-mestizo race, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Hispanic ethnicity, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Socioeconomic status,a n (%) | |||
| Low | 385 (91) | 319 (91) | 66 (88) |
| Medium | 26 (6) | 19 (6) | 7 (9) |
| Social insurance | 13 (3) | 11 (3) | 2 (3) |
| Time from SLE diagnosis, months | 46 (5–109) | 40 (5–108) | 52 (6–118) |
| Previous history of LN, n (%) | |||
| First LN flare | 245 (58) | 212 (61) | 33 (44) |
| Repeated LN flare | 179 (42) | 137 (39) | 42 (56) |
| Year of lupus nephritis diagnosis | |||
| 2008–2013 | 209 (49) | 155 (44) | 56 (75) |
| 2014–2020 | 215 (51) | 194 (56) | 19 (25) |
| Body mass index, kg/m2 | 24.4 (21.9–27.5) | 24.6 (22.0–27.7) | 23.8 (21.1–26.9) |
| Mean arterial pressure, mmHg | 95 (87–103) | 94 (85–103) | 96 (87–103) |
| Haemoglobin, g/dL | 11.9 (10.5–13.4) | 12.0 (10.6–13.5) | 11.8 (10.2–13.0) |
| Platelets, × 103/mm3 | 238 (180–304) | 240 (180–305) | 235 (180–288) |
| Serum creatinine, mg/dL | 0.95 (0.67–1.43) | 0.92 (0.66–1.38) | 1.01 (0.69–1.78) |
| Serum creatinine, umol/L | 84.0 (59.2–16.9) | 81.3 (58.3–121.9) | 89.2 (61.0–157.4) |
| eGFR, ml/min/1.73 m2 | 81 (49–118) | 83 (51–119) | 74 (37–110) |
| Proteinuria (24 h-uPCR), g/g | 3.4 (1.9–5.5) | 3.4(1.9–5.4) | 3.7 (2.0–6.5) |
| Proteinuria (24 h-uPCR), mg/mmol | 304 (169–487) | 299 (169–479) | 326 (177–573) |
| Pyuria, n (%) | 331 (78) | 272 (78) | 59 (79) |
| Hematuria, n (%) | 310 (73) | 258 (74) | 52 (69) |
| Serum albumin, g/L | 2.8 (2.3–3.4) | 2.8 (2.3–3.4) | 2.6 (2.1–3.3) |
| Anti-dsDNA antibodies, UI/mL | 98.0 (22.7–423.8) | 97 (23–434) | 102 (20–271) |
| Positive anti-dsDNA antibodies, n (%) | 369 (87) | 304 (87) | 65 (87) |
| Complement C3, mg/dL | 65 (46–84) | 64 (46–84) | 71 (47–93) |
| Low C3, n (%) | 340 (80) | 279 (80) | 61 (81) |
| Complement C4, mg/dL | 10 (8–17) | 10 (8–16) | 12 (8–18) |
| Low C4, n (%) | 327 (77) | 267 (77) | 60 (80) |
| Parameter . | Full cohort . | Antimalarial use . | No antimalarial use . |
|---|---|---|---|
| n = 424 . | n = 349 . | n = 75 . | |
| Age, years | 29 (23–37) | 29 (23–37) | 30 (23–38) |
| Female, n (%) | 405 (96) | 330 (95) | 75 (100) |
| Mexican-mestizo race, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Hispanic ethnicity, n (%) | 424 (100) | 349 (100) | 75 (100) |
| Socioeconomic status,a n (%) | |||
| Low | 385 (91) | 319 (91) | 66 (88) |
| Medium | 26 (6) | 19 (6) | 7 (9) |
| Social insurance | 13 (3) | 11 (3) | 2 (3) |
| Time from SLE diagnosis, months | 46 (5–109) | 40 (5–108) | 52 (6–118) |
| Previous history of LN, n (%) | |||
| First LN flare | 245 (58) | 212 (61) | 33 (44) |
| Repeated LN flare | 179 (42) | 137 (39) | 42 (56) |
| Year of lupus nephritis diagnosis | |||
| 2008–2013 | 209 (49) | 155 (44) | 56 (75) |
| 2014–2020 | 215 (51) | 194 (56) | 19 (25) |
| Body mass index, kg/m2 | 24.4 (21.9–27.5) | 24.6 (22.0–27.7) | 23.8 (21.1–26.9) |
| Mean arterial pressure, mmHg | 95 (87–103) | 94 (85–103) | 96 (87–103) |
| Haemoglobin, g/dL | 11.9 (10.5–13.4) | 12.0 (10.6–13.5) | 11.8 (10.2–13.0) |
| Platelets, × 103/mm3 | 238 (180–304) | 240 (180–305) | 235 (180–288) |
| Serum creatinine, mg/dL | 0.95 (0.67–1.43) | 0.92 (0.66–1.38) | 1.01 (0.69–1.78) |
| Serum creatinine, umol/L | 84.0 (59.2–16.9) | 81.3 (58.3–121.9) | 89.2 (61.0–157.4) |
| eGFR, ml/min/1.73 m2 | 81 (49–118) | 83 (51–119) | 74 (37–110) |
| Proteinuria (24 h-uPCR), g/g | 3.4 (1.9–5.5) | 3.4(1.9–5.4) | 3.7 (2.0–6.5) |
| Proteinuria (24 h-uPCR), mg/mmol | 304 (169–487) | 299 (169–479) | 326 (177–573) |
| Pyuria, n (%) | 331 (78) | 272 (78) | 59 (79) |
| Hematuria, n (%) | 310 (73) | 258 (74) | 52 (69) |
| Serum albumin, g/L | 2.8 (2.3–3.4) | 2.8 (2.3–3.4) | 2.6 (2.1–3.3) |
| Anti-dsDNA antibodies, UI/mL | 98.0 (22.7–423.8) | 97 (23–434) | 102 (20–271) |
| Positive anti-dsDNA antibodies, n (%) | 369 (87) | 304 (87) | 65 (87) |
| Complement C3, mg/dL | 65 (46–84) | 64 (46–84) | 71 (47–93) |
| Low C3, n (%) | 340 (80) | 279 (80) | 61 (81) |
| Complement C4, mg/dL | 10 (8–17) | 10 (8–16) | 12 (8–18) |
| Low C4, n (%) | 327 (77) | 267 (77) | 60 (80) |
The socioeconomic status variable was created by evaluating family income, household characteristics and family assets.
24 h-uPCR, urine protein to creatinine ratio estimated from a 24-h urine collection; anti-dsDNA, antibodies against double-strand DNA; eGFR, estimated glomerular filtration rate; LN: lupus nephritis; SLE: systemic lupus erythematosus.
Outcomes in antimalarial users vs non-users
As a first approach, the study population was divided into antimalarial users (n = 349) and non-users (n = 75). The time to complete and partial response was shorter in antimalarial users than in non-users (log-rank P =0.011 and P = 0.010) (Fig. 1). The variables associated with complete and partial response in the univariate analysis are shown in Supplementary Tables S3 and S4 (available at Rheumatology online). In the adjusted multivariable Cox-regression analysis (Table 2), antimalarial use was associated with a higher probability of complete (aHR 1.57, 95%CI 1.08–2.27) and partial (aHR 1.44, 95%CI 1.06–1.96) response to the initial immunosuppressive therapy. The effect of the antimalarial use on response was modified by the initial immunosuppressive scheme (interaction P = 0.037), as the association with increased response was exclusively observed in combination with MMF and not with CYP or AZA. The effect of the antimalarial in complete and partial response was also modified by the LN history (first or recurrent LN flare) (Supplementary Figs S2 and S3, available at Rheumatology online).
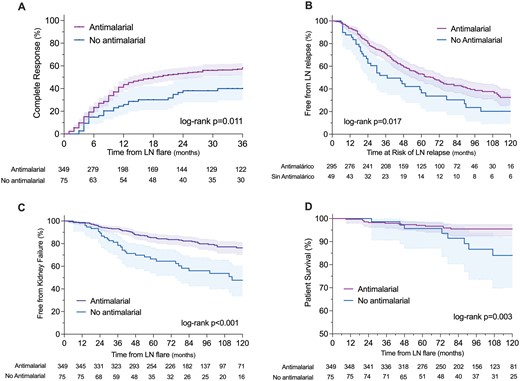
Study outcomes according to the use or non-use of antimalarial drugs
Adjusted hazard ratios derived from the multivariable Cox regression analysis for all outcomes
| . | Time to complete response . | Time to partial response . | Time to first LN flare . | Time to kidney failure . |
|---|---|---|---|---|
| Antimalarial use | 1.57 (1.08–2.27) | 1.44 (1.06–1.96) | 0.63 (0.43–0.92) | 0.37 (0.23–0.57) |
| Timing of antimalarial | ||||
| Prevalent | 1.51 (1.02–2.23) | 1.43 (1.03–1.97) | 0.67 (0.42–0.98) | 0.40 (0.25–0.63) |
| Incident | 1.65 (1.10–2.49) | 1.46 (1.04–2.06) | 0.54 (0.35–0.84) | 0.31 (0.17–0.56) |
| Type of antimalarial | ||||
| Chloroquine | 1.26 (0.74–2.15) | 1.48 (0.96–2.27) | 0.77 (0.46–1.28) | 0.72 (0.38–1.37) |
| Hydroxychloroquine | 1.66 (1.13–2.43) | 1.47 (1.07–2.01) | 0.59 (0.39–0.87) | 0.29 (0.18–0.48) |
| Both | 1.35 (0.76–2.38) | 1.23 (0.77–1.96) | 0.70 (0.39–1.24) | 0.44 (0.20–0.94) |
| . | Time to complete response . | Time to partial response . | Time to first LN flare . | Time to kidney failure . |
|---|---|---|---|---|
| Antimalarial use | 1.57 (1.08–2.27) | 1.44 (1.06–1.96) | 0.63 (0.43–0.92) | 0.37 (0.23–0.57) |
| Timing of antimalarial | ||||
| Prevalent | 1.51 (1.02–2.23) | 1.43 (1.03–1.97) | 0.67 (0.42–0.98) | 0.40 (0.25–0.63) |
| Incident | 1.65 (1.10–2.49) | 1.46 (1.04–2.06) | 0.54 (0.35–0.84) | 0.31 (0.17–0.56) |
| Type of antimalarial | ||||
| Chloroquine | 1.26 (0.74–2.15) | 1.48 (0.96–2.27) | 0.77 (0.46–1.28) | 0.72 (0.38–1.37) |
| Hydroxychloroquine | 1.66 (1.13–2.43) | 1.47 (1.07–2.01) | 0.59 (0.39–0.87) | 0.29 (0.18–0.48) |
| Both | 1.35 (0.76–2.38) | 1.23 (0.77–1.96) | 0.70 (0.39–1.24) | 0.44 (0.20–0.94) |
Adjusted for age, sex, previous lupus nephritis flare, presentation eGFR and proteinuria, ISN/RPS histological class, initial immunosuppressive treatment and maintenance immunosuppressive treatment (the latter only for time to first LN flare and time to kidney failure analyses).
Adjusted hazard ratios derived from the multivariable Cox regression analysis for all outcomes
| . | Time to complete response . | Time to partial response . | Time to first LN flare . | Time to kidney failure . |
|---|---|---|---|---|
| Antimalarial use | 1.57 (1.08–2.27) | 1.44 (1.06–1.96) | 0.63 (0.43–0.92) | 0.37 (0.23–0.57) |
| Timing of antimalarial | ||||
| Prevalent | 1.51 (1.02–2.23) | 1.43 (1.03–1.97) | 0.67 (0.42–0.98) | 0.40 (0.25–0.63) |
| Incident | 1.65 (1.10–2.49) | 1.46 (1.04–2.06) | 0.54 (0.35–0.84) | 0.31 (0.17–0.56) |
| Type of antimalarial | ||||
| Chloroquine | 1.26 (0.74–2.15) | 1.48 (0.96–2.27) | 0.77 (0.46–1.28) | 0.72 (0.38–1.37) |
| Hydroxychloroquine | 1.66 (1.13–2.43) | 1.47 (1.07–2.01) | 0.59 (0.39–0.87) | 0.29 (0.18–0.48) |
| Both | 1.35 (0.76–2.38) | 1.23 (0.77–1.96) | 0.70 (0.39–1.24) | 0.44 (0.20–0.94) |
| . | Time to complete response . | Time to partial response . | Time to first LN flare . | Time to kidney failure . |
|---|---|---|---|---|
| Antimalarial use | 1.57 (1.08–2.27) | 1.44 (1.06–1.96) | 0.63 (0.43–0.92) | 0.37 (0.23–0.57) |
| Timing of antimalarial | ||||
| Prevalent | 1.51 (1.02–2.23) | 1.43 (1.03–1.97) | 0.67 (0.42–0.98) | 0.40 (0.25–0.63) |
| Incident | 1.65 (1.10–2.49) | 1.46 (1.04–2.06) | 0.54 (0.35–0.84) | 0.31 (0.17–0.56) |
| Type of antimalarial | ||||
| Chloroquine | 1.26 (0.74–2.15) | 1.48 (0.96–2.27) | 0.77 (0.46–1.28) | 0.72 (0.38–1.37) |
| Hydroxychloroquine | 1.66 (1.13–2.43) | 1.47 (1.07–2.01) | 0.59 (0.39–0.87) | 0.29 (0.18–0.48) |
| Both | 1.35 (0.76–2.38) | 1.23 (0.77–1.96) | 0.70 (0.39–1.24) | 0.44 (0.20–0.94) |
Adjusted for age, sex, previous lupus nephritis flare, presentation eGFR and proteinuria, ISN/RPS histological class, initial immunosuppressive treatment and maintenance immunosuppressive treatment (the latter only for time to first LN flare and time to kidney failure analyses).
The time to the first LN flare was shorter in non-users of antimalarials than in users (log-rank P = 0.017) (Fig. 1). The factors associated with LN flares in the univariate analysis are shown in Supplementary Table S5, available at Rheumatology online. In the adjusted multivariable Cox regression analysis, antimalarial use was associated with a lower probability of LN flares (aHR 0.63, 95%CI 0.43–0.92) (Table 2). The effect of antimalarial use on flares was observed in all the analysed subgroups (Supplementary Fig. S4, available at Rheumatology online).
The time to progression to kidney failure was shorter in non-users of antimalarials than in antimalarial users (log-rank P < 0.001) (Fig. 1). The factors associated with progression to kidney failure in the univariate Cox regression analysis are shown in Supplementary Table S6, available at Rheumatology online. In the adjusted multivariable Cox regression analysis, antimalarial use was associated with a lower probability of progression to kidney failure (aHR 0.37, 95%CI 0.23–0.57) (Table 2). The effect of antimalarial use on kidney failure was modified by the concomitant initial immunosuppression, with a stronger effect on progression to kidney failure observed in patients who received initial immunosuppression with MMF (Supplementary Fig. S5, available at Rheumatology online). Patient survival was higher in antimalarial users than in non-users (log-rank P =0.003) (Fig. 1). The effect of antimalarial on patient survival was mediated by the prevention of progression to kidney failure (interaction P =0.003) as most deaths occurred in patients with kidney failure (Supplementary Tables S7 and S8, available at Rheumatology online).
Outcomes in prevalent and incident users of antimalarials vs non-users
A second approach to analysis was defined by the timing of the use of antimalarials regarding the LN flare. Prevalent antimalarial users had taken an antimalarial for a median 34 months (IQR 21–85) by the time of diagnosis of the LN flare, while incident users started them within 3 months of the LN flare. The time to complete and partial remission was shorter in prevalent and incident antimalarial users than in no antimalarial users (log-rank P =0.007 and P =0.016) (Fig. 2). In the adjusted multivariable Cox regression analysis prevalent (aHR 1.51, 95%CI 1.02–2.23 and aHR 1.43, 95%CI 1.03–1.97) and incident (aHR 1.65, 95%CI 1.10–2.49 and aHR 1.46, 95%CI 1.04–2.06) antimalarial use was associated with a higher probability of complete and partial response (Table 2). The effect on the response of antimalarial use was modified according to the LN history, with a higher effect in complete remission of both prevalent and incident antimalarial use in patients experiencing their first LN flare (Supplementary Fig. S6, available at Rheumatology online).
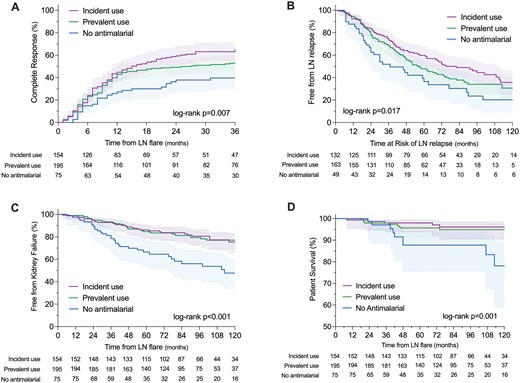
Study outcomes according to the timing of use of antimalarial drugs regarding the LN flare
The time to the first LN flare was shorter in patients without antimalarial than in both prevalent and incident antimalarial users (log-rank P =0.017) (Fig. 2). In the adjusted multivariable Cox regression analysis, both prevalent (HR 0.67, 95%CI 0.42–0.98) and incident (HR 0.54, 95%CI 0.35–0.84) antimalarial use was associated with a lower incidence of LN flares (Table 2). The effect of prevalent or incident antimalarial use on LN flares was not modified by other factors in the stratified analysis (Supplementary Fig. S7, available at Rheumatology online).
The time to kidney failure was shorter in patients without antimalarial use (log-rank P <0.001) (Fig. 2). In the adjusted multivariable Cox regression analysis, both prevalent (aHR 0.40, 95%CI 0.25–0.63) and incident (aHR 0.31, 95%CI 0.17–0.56) use of antimalarials was associated with lower progression to kidney failure (Table 2). The effect of prevalent or incident antimalarial use on kidney failure was modified by the estimated eGFR at presentation (Supplementary Fig. S8, available at Rheumatology online). Patient survival was higher in patients with prevalent and incident use of antimalarials (log-rank P =0.001) (Fig. 2). This effect on patient survival was mediated by the prevention of progression to kidney failure (interaction P =0.010).
Outcomes according to the type of antimalarial vs no antimalarial use
A third approach to analysis was defined by the type of antimalarial: chloroquine or hydroxychloroquine. The time to complete or partial response was shorter in hydroxychloroquine and chloroquine users than in no antimalarial use (log-rank P =0.004 and P =0.020) (Fig. 3). In the multivariable Cox regression analysis, only hydroxychloroquine use was associated with higher complete (aHR 1.66, 95%CI 1.13–2.43) and partial (aHR 1.47, 95%CI 1.07–2.01) response to therapy (Table 2). The effect of the type of antimalarial on response was modified by the LN history and the eGFR at presentation, with better complete response rates in patients using hydroxychloroquine in their first LN flare (Supplementary Fig. S9, available at Rheumatology online).
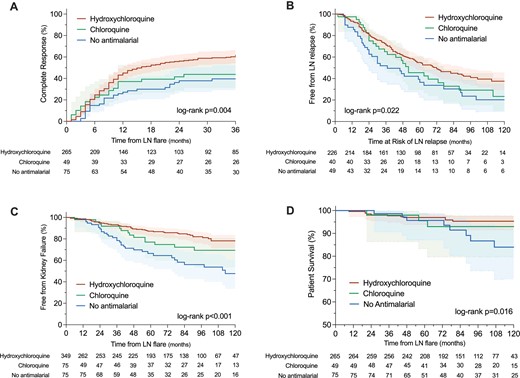
Study outcomes according to the type of antimalarial or no antimalarial use
The time to the first LN flare was longer in hydroxychloroquine users (log-rank P =0.022) (Fig. 3). In the multivariable analysis, only hydroxychloroquine use was associated with a lower incidence of LN flares (aHR 0.59, 95%CI 0.39–0.87) (Table 2). In the subgroup analysis, the effect of hydroxychloroquine in LN flare prevention was observed in all the subgroups of patients (Supplementary Fig. S10, available at Rheumatology online).
The time to kidney failure was longer in users of hydroxychloroquine and chloroquine than in patients without antimalarial (log-rank P <0.001) (Fig. 3). In the multivariable Cox regression analysis, only hydroxychloroquine was associated with a lower incidence of kidney failure (aHR 0.29, 95%CI 0.18–0.48) (Table 2). The protective effect of hydroxychloroquine was modified by the eGFR at presentation (Supplementary Fig. S11, available at Rheumatology online). Patient survival was higher with the use of hydroxychloroquine than with no antimalarial (log-rank P =0.009) but was not observed with chloroquine (Fig. 3). The effect of hydroxychloroquine on patient survival was also mediated by the prevention of progression to kidney failure (interaction P =0.003).
Adverse events from antimalarial use
Signs of macular toxicity were found in 46 patients in this cohort. Only 4 (7%) patients reported visual symptoms, and only 9 (20%) patients had an abnormal visual capacity at the time of the ophthalmologic assessment. All 46 patients were evaluated by fundoscopy, automated visual field assessment (VFA), and spectral-domain optical coherence tomography (SD-OCT). Additionally, 21 (46%) were performed a multifocal electroretinography (mfERG). The incidence of macular toxicity was 1.7%, 5.7% and 8.8% by 3, 5 and 7 years of continued antimalarial use (Fig. 4). The incidence was numerically but not statistically higher for chloroquine (4.2%, 8.4% and 8.4% by 3, 5 and 7 years, respectively) than for hydroxychloroquine (0%, 2.9% and 6.3% by 3, 5 and 7 years). The retinopathy was classified as mild, moderate or severe in 32 (70%), 13 (28%) and 1(2%) patients, respectively. The antimalarial therapy was suspended in all cases after diagnosis. We did not find any factor associated with macular toxicity in the univariate Cox regression analysis (Supplementary Table S9, available at Rheumatology online).
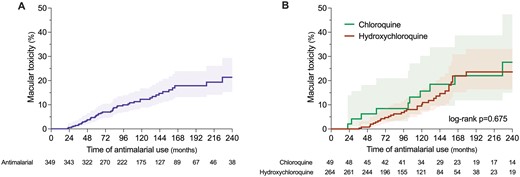
Time to documentation of toxic retinopathy in the whole cohort (A) and according to the type of antimalarial (B)
Discussion
The use of antimalarial drugs has been associated with beneficial effects in SLE patients with less evidence of its effects in LN patients [29]. In this study, we showed that antimalarial drug use is associated with an increased response to immunosuppressive therapy, lower incidence of LN flares and lower progression of kidney disease. These protective effects were observed in patients with prevalent and incident use of antimalarials, and more importantly in users of hydroxychloroquine than chloroquine. The effect on response to therapy was modified by the concomitant immunosuppressive drug, with a higher effect observed in combination with MMF. The incidence of subclinical macular toxicity was high and documented within the first two years of continued use of antimalarials by modern standard screening modalities.
Antimalarials are widely accepted drugs for the management of SLE and LN. Current clinical practice guidelines [23, 24] recommend their use in all patients with SLE unless contraindicated based on their multiple effects observed in a few randomized clinical trials and multiple observational cohorts [8, 9, 11, 13, 22, 30–32]. The specific effects of antimalarial drugs in LN have been less documented [29], with only one randomized clinical trial performed to date in children [22] and only a few observational cohorts showing a potential effect in response to therapy [21, 33–35], protection against kidney flares [36] and reduction in kidney damage [36, 37].
A recent small, randomized trial in children showed better 6- and 12-month responses with the addition of hydroxychloroquine to the initial immunosuppressive therapy [22]. Two small cohort studies of class V LN [35, 38] and an additional one in proliferative LN showed increased response to therapy with the addition of antimalarials [33]. Here, we observed an increase in response to therapy with the use of hydroxychloroquine, which was less clear for chloroquine, although the latter group was relatively small. We documented that patients with the prevalent use of these drugs and those starting them after an LN flare was diagnosed had an enhanced response to immunosuppression, especially when used in combination with an MMF-based immunosuppression scheme. The consistency of the effect when analysed by the timing of the start of antimalarial and the type of antimalarial strengthens this observation.
The protective effect of antimalarial drugs against systemic flares has been observed in multiple cohorts and a few randomized clinical trials [8–10, 13, 30, 32, 39, 40]. A link between the dose and the prevention of systemic flares was recently suggested [41] with a lower incidence of systemic flares with dosages above 5 mg/kg. The same dose-dependent effect has been suggested by studies measuring hydroxychloroquine blood levels where lower levels have been associated with disease and kidney flares [10, 32, 42, 43]. Interestingly, the average daily dose in this study was 3.3 mg/kg and 2.9 mg/kg for hydroxychloroquine and chloroquine, respectively. We did not document the specific dose used during the induction and maintenance phases of therapy, which may differentially affect the effects in response and LN flare prevention.
A definite effect of antimalarials on hard kidney outcomes has not been previously demonstrated. A recent retrospective study in 2050 SLE patients divided according to hydroxychloroquine prescription for less or >90 days showed no difference in the incidence of chronic kidney disease [44]. However, this study did not include specifically SLE patients with kidney involvement. In this biopsy-proven LN cohort, we show that antimalarial use is associated with an independent protective effect in the progression of kidney disease observed both in patients with previous use of antimalarials and those starting the drug after the LN flare and observed predominantly with hydroxychloroquine.
The prevalence of toxic retinopathy in long-term users of antimalarials varies according to the population evaluated and the methods used for screening but oscillates between 1.6% and 8.0% [28, 45, 46]. Due to a higher observed risk with doses above 5 mg/kg [45], recent guidelines recommend a dose not higher than 5 mg/kg [47, 48] and both baseline and follow-up ophthalmologic evaluations starting at the fith year of continuous use in patients with normal kidney function. Patients with impaired kidney function are at increased risk for toxic retinopathy and ophthalmologic examination is recommended annually [47]. We showed that toxic retinopathy may be documented as early as the second year of continuous use and a high incidence of maculopathy by modern standard screening techniques. A numerically higher percentage of maculopathy was observed with chloroquine than hydroxychloroquine, which may be explained by the lower volume of distribution of hydroxychloroquine compared with chloroquine [49]. This underlines the importance of yearly ophthalmologic examinations in LN patients, especially with the widespread and prolonged use of antimalarial drugs.
The present study has some limitations: (i) due to its retrospective nature we cannot exclude several confounding factors including confounding by antimalarial indication; (ii) there may be residual confounding from variables that were not registered in this study; (iii) we collected the total average dosage so we did not document if different antimalarial dosages were used at induction and maintenance phases; (iv) we evaluated adherence by medical records and hydroxychloroquine blood levels were not measured in any patient; (v) some sub analysis were underpowered to detect differences (i.e. chloroquine treated group); (vi) although all patients had at least two ophthalmologic evaluations, these evaluations were not strictly scheduled; and (vii) all findings correspond to a Hispanic and Mexican-Mestizo population, which may limit generalizability of the results.
In conclusion, we show that antimalarial use in LN patients is associated with an increased response to immunosuppressive therapy, a lower incidence of kidney relapses, and a lower risk of progressive kidney disease. Lupus nephritis patients are prone to toxic retinopathy and ophthalmologic evaluation is recommended yearly from the start of these drugs.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
All data relevant to this study are included in the article. The full database can be made available for future projects upon request to the authors.
Funding
No specific funding was received from any bodies in the public, commercial, or not-for-profit sectors to carry out the work described in this article. This work was performed with local funding from the Department of Nephrology and Mineral Metabolism of the National Medical Sciences and Nutrition Institute Salvador Zubirán.
Disclosure statement: The authors have declared no conflicts of interest.





Comments