-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Korsten, Samy Hakroush, Ralph Rödel, Björn Tampe, Tongue necrosis in anti-neutrophil myeloperoxidase antibody-associated vasculitis, Rheumatology, Volume 63, Issue 2, February 2024, Pages e61–e62, https://doi.org/10.1093/rheumatology/kead342
Close - Share Icon Share
An 89-year-old Caucasian female patient presented with tongue necrosis (Fig. 1A), which had developed over 4 weeks. She complained of bilateral shoulder pain for 4 weeks, headache and visual impairment for 3 days. CRP levels were 101 mg/l (reference range: ≤5 mg/l), and anti-neutrophil myeloperoxidase antibodies (MPO-ANCAs) were positive at 11 IU/ml (reference range: <3.5 IU/ml). Indirect ANCA immunofluorescence (IIF) was positive at 1:320 (reference range: <1:10), consistent with small vessel ANCA-associated vasculitis (AAV). Urinalysis revealed leukocyturia, haematuria (no dysmorphic erythrocytes), few hyaline casts and mild proteinuria. A kidney biopsy excluded renal involvement of AAV. The patient received pulse methylprednisone for 3 days and oral prednisone with tapering thereafter. In addition, rituximab (RTX) was initiated at 375 mg/m2 per week, and tongue necrosis improved 2 weeks thereafter (Fig. 1B).
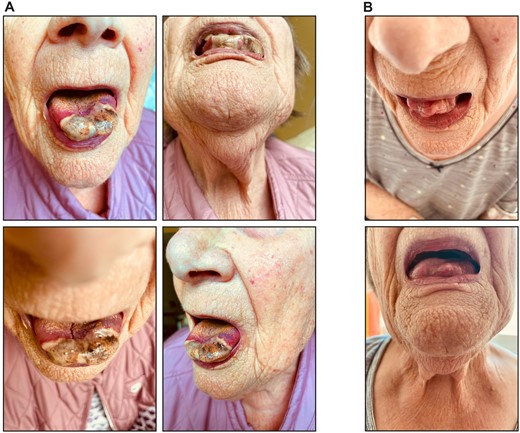
Necrotic lesions at the apex of the tongue. (A) Photographs of tongue necrosis at initial presentation. (B) Photographs of tongue necrosis 2 weeks after initial presentation
Tongue necrosis is a rare manifestation of systemic vasculitides and is more commonly seen in GCA. The most likely causes are the involvement of lingual arteries or thrombosis [1]. Previously only four cases of tongue necrosis have been reported with AAV positive for PR3-ANCA, and we present a case of tongue necrosis in vasculitis associated with MPO-ANCA, which to our knowledge appears to be the first [2].
Data availability
Data will be available from the corresponding author upon reasonable request.
Contribution statement
B.T. conceived the report, analysed data, and wrote the manuscript. P.K., R.R. and B.T. were directly involved in patient care, and S.H. performed histological analysis. All authors reviewed and approved the manuscript’s content before submission.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: P.K. has received honoraria or travel support from Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers-Squibb, Chugai, Galapagos, GlaxoSmithKline, Janssen-Cilag, Lilly, Novartis and Pfizer, all unrelated to this paper. P.K. has received research support from GlaxoSmithKline and Diamed Medizintechnik GmbH, all unrelated to this paper. B.T. has received research grants from Evotec SE and CSL Vifor, all unrelated to this paper. B.T. has received honoraria or travel support from CSL Vifor, all unrelated to this paper. The other authors have declared no conflicts of interest.
Ethics approval: The study was conducted according to the guidelines of the Declaration of Helsinki, and approval by the Institutional Review Board was not required for reporting individual case reports.
Consent: The case described does not contain any information on the identity of the patient. Written informed consent to publish this report was obtained directly from the patient.




Comments