-
PDF
- Split View
-
Views
-
Cite
Cite
Wolfgang A Schmidt, Monitoring giant cell arteritis with ultrasound, Rheumatology, Volume 62, Issue 9, September 2023, Pages 2948–2950, https://doi.org/10.1093/rheumatology/kead134
Close - Share Icon Share
This editorial refers to ‘Ultrasonography in the assessment of disease activity in cranial and large-vessel giant cell arteritis: a prospective follow-up study’, by Nielsen et al., 2023;62:3084–94.
Berit Nielsen and colleagues conducted a prospective cohort study in newly diagnosed patients with GCA [1]. They utilized ultrasound to assess the intima-media thickness (IMT) of temporal, axillary and carotid arteries at diagnosis, after 8 weeks, 24 weeks and 15 months. They then compared several potential scores for evaluating structural changes over time.
Most scores included only temporal and axillary arteries. They were either based on the presence or absence of the ‘halo sign’ or on IMT. Scores considered either the sum of IMT, the maximum or mean IMT of all segments, or were restricted only to segments with a halo sign. Most of these scores were quantitative.
All scores including the temporal arteries showed good response from 8 weeks onwards. The response was poor in the axillary and carotid arteries. The recently published OMERACT GCA Ultrasound Score (OGUS) showed a large magnitude of change and was deemed the score least susceptible to potential bias. The authors suggest using the OGUS for evaluating treatment response in clinical trials [1].
Studies that calculated normal and cut-off values for IMT formed the foundation for developing scores [2]. The OGUS calculates the sum of IMT in six temporal artery segments (common superficial temporal artery, frontal and parietal branch, bilaterally) and both axillary arteries, divided by rounded cut-off values in each segment (0.4 mm for the common superficial temporal arteries, 0.3 mm for the branches and 1.0 mm for the axillary arteries). The resulting value is then divided by the number of available segments. A calculator can be found at http://scoring.multimedium.at/OMERACT [3].
The OGUS has been designed for monitoring purposes in clinical trials. It was originally tested in a prospective cohort, where IMT of temporal arteries continuously and significantly decreased and axillary artery IMT insignificantly decreased at weeks 1, 3, 6, 12 and 24 from baseline. This reduction correlated with ESR, CRP, BVAS and the cumulative dose of glucocorticoids [4]. The present study conducted a post-hoc analysis in an already existing independent cohort [1].
Another study, GUSTO, used a similar score. It considers normal IMT instead of rounded cut-off values as the denominator. Newly diagnosed GCA patients received glucocorticoid pulse therapy for three days and started treatment with tocilizumab without further glucocorticoids on day 4. The score rapidly decreased within the first three days and increased until week 4, before decreasing again once tocilizumab had become fully effective [5].
The Southend score was proposed earlier and converts the IMT of six temporal artery segments and two axillary artery segments into grades [6]. So far, it has mostly been investigated for diagnostic purposes, with a higher score correlating with correct diagnosis and disease severity. The OGUS can be considered a further development of the Southend score by an international expert group that followed strict methodology. It includes the same arterial segments, making it easy to convert values between the two scores. The OGUS has superior sensitivity to change, reliability and convergent construct validity, as compared with the Southend score. The Southend score gives a three times higher weight to the axillary arteries. Smaller changes may result in the same score. It may be elevated in healthy persons, when the IMT is within the upper normal range, particularly in the axillary arteries. Further, it does not consider missing or biopsied segments.
Ultrasound is increasingly becoming the first diagnostic test for confirming a suspected diagnosis of GCA [7]. It is widely available in rheumatology practice and allows trained rheumatologists to confirm or exclude GCA immediately in the examination room with high diagnostic accuracy.
When introducing ultrasound for confirming or excluding suspected GCA, we were pleased to discover that it was far easier and faster than temporal artery biopsy. The thickening of the intima and media, due to inflammatory cell infiltration, is displayed on ultrasound as a distinctive, circumferential and dark (hypoechoic) structure, known as the ‘halo sign’. It is non-compressible in superficial arteries like the temporal arteries and becomes more hyperechoic in chronic vasculitis (Fig. 1).
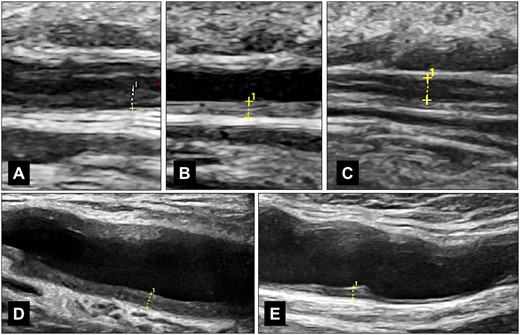
IMT measurements of temporal and axillary arteries in GCA. (A) shows the hypoechoic wall swelling of the frontal branch in a patient newly diagnosed with GCA, with an IMT of 0.60 mm. (B) displays the hyperechoic wall thickening of the parietal branch in a patient in remission, with an IMT of 0.49 mm. (C) illustrates the measurement of the frontal branch in acute GCA with compression, particularly in cases where a single wall measurement is difficult, with an IMT of both walls equal to 0.91 mm, or a single wall equal to 0.455 mm. (D) shows an axillary artery in a newly diagnosed patient, with an IMT of 1.21 mm. (E) depicts an axillary artery with chronic changes in remission, with an IMT of 0.88 mm. All images were captured using a GE Logiq E10 with a 6–24 MHz hockey stick probe (GE Healthcare, Chicago, IL, USA)
For many years, we have in no sense considered ultrasound for its potential use in monitoring GCA. However, advancements in technology have greatly improved the resolution of ultrasound, from 0.8 mm in the 1990s to below 0.1 mm today, allowing us to accurately depict and measure IMT.
Ultrasound transducers with >20 MHz are becoming more commonly used among rheumatologists and allow for reliable IMT measurement. The present study utilized a maximum frequency of 18 MHz, which was considered a satisfactory standard during the recruitment period between 2014 and 2018.
When measuring IMT, it is important to avoid areas with arteriosclerotic plaques or localized eccentric wall thickening, which may indicate arteriosclerosis. Arteriosclerosis is rare in temporal and axillary arteries but prevalent in carotid arteries, which are usually affected by GCA in conjunction with other arteries. Therefore, we recommend against using exclusive carotid wall swelling as a diagnostic indicator for GCA [8].
An IMT score in GCA should be comprehensive and sensitive to change, yet simple and feasible to use. It should focus only on frequently affected arteries, such as the temporal and axillary arteries.
All recent studies indicated that axillary artery IMT decreases more slowly than temporal artery IMT. However, another study found a relevant decrease in IMT of the axillary arteries over a period of up to 12 years [9].
We expect that future trials will include the OGUS. Sonographers can routinely measure IMT with minor additional effort. Most trials only include patients once they have reached remission on glucocorticoid therapy. After obtaining informed consent, IMT measurements and other important clinical data, such as CRP and ESR, should be retrospectively included in all GCA trials, in order to correctly characterize the cohorts. The greatest decrease in the score occurs during the first few weeks of treatment. It is important to confirm that a new drug has a positive impact on structural vascular damage and not just on inflammation.
The OGUS might be also considered for use in clinical practice, especially in unclear situations, for instance in symptomatic patients on tocilizumab with normal CRP.
In conclusion, the OGUS, a score based on IMT of temporal and axillary arteries, was tested in an independent cohort and shown to be a useful tool for evaluating structural changes during GCA. It should be employed in future trials in order to assess not only inflammation, but also structural changes during GCA.
Data availability
No new data were generated or analyzed in support of this article.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: W.A.S. has received consultant fees from Abbvie, Amgen, Bristol Myers Squibb, Chugai, GlaxoSmithKline, Novartis, Roche, Sanofi; speaker’s bureau fees from Abbvie, Amgen, Bristol Myers Squibb, Chugai, Lilly, Johnson & Johnson, Medac, Novartis, Pfizer, Roche, Sanofi, UCB. He is principal investigator in trials sponsored by Abbvie, GlaxoSmithKline, Novartis and Sanofi.




Comments