-
PDF
- Split View
-
Views
-
Cite
Cite
Nadège Cordel, Benoît Dechelotte, Fabienne Jouen, Janine A Lamb, Hector Chinoy, Paul New, Jiri Vencovsky, Herman Mann, Angeles S Galindo-Feria, Lara Dani, Albert Selva-O’Callaghan, Victoria P Werth, Adarsh Ravishankar, Océane Landon-Cardinal, Benoit Tressières, Olivier Boyer, Anti-transcription intermediary factor 1-gamma IgG2 isotype is associated with cancer in adult dermatomyositis: an ENMC multinational study, Rheumatology, Volume 62, Issue 4, April 2023, Pages 1711–1715, https://doi.org/10.1093/rheumatology/keac577
Close - Share Icon Share
Abstract
To assess the role of the anti-TIF1γ auto-antibody (aAb) IgG2 isotype as a biomarker of cancer in anti-TIF1γ aAb-positive adult DM.
International multicentre retrospective study with the following inclusion criteria: (i) diagnosis of DM according to ENMC criteria; (ii) presence of anti-TIF1γ IgG aAb determined using an in-house addressable laser bead immunoassay (ALBIA) from cryopreserved serums sampled at time of DM diagnosis and (iii) available baseline characteristics and follow-up data until the occurrence of cancer and/or a minimum follow-up of 1 year for patients without known cancer at diagnosis. Detection and quantification of anti-TIF1γ IgG2 aAb was done using the in-house ALBIA. In addition, a recent ELISA commercial kit was used for anti-TIF1γ IgG aAb quantification.
A total of 132 patients (mean age 55±15 years) of whom 72 (54.5%) had an associated cancer were analysed. The association between the presence of cancer and the presence of anti-TIF1γ IgG2 aAb was statistically significant (P = 0.026), with an OR of 2.26 (95% CI: 1.10, 4.76). Patients with cancer displayed significantly higher anti-TIF1γ IgG2 aAb ALBIA values with a median value of 1.15 AU/ml (IQR: 0.14–9.76) compared with 0.50 AU/ml (IQR: 0.14–1.46) for patients without cancer (P = 0.042). In addition, patients with cancer displayed significantly higher anti-TIF1γ IgG aAb ELISA values with a median value of 127.5 AU/ml (IQR: 81.5–139.6) compared with 93.0 AU/ml (IQR: 54.0–132.9) for patients without cancer (P = 0.004).
These results suggest considering anti-TIF1γ IgG2 ALBIA and IgG ELISA values as biomarkers of cancer in anti-TIF1 γ aAb-positive adult DM.
Identifying adult dermatomyositis patients at high risk of cancer remains a challenge for clinicians.
Anti-TIF1γ IgG2 isotype was previously identified as a potential biomarker of cancer in adult dermatomyositis.
We demonstrate an association between anti-TIF1γ IgG2 and cancer in a cohort of 132 adult dermatomyositis patients.
Introduction
Although the frequency of the association varies greatly among the studies, the risk of cancer in adults with DM has been widely documented in the literature [1]. Identifying adult DM patients at high risk of cancer remains a challenge for clinicians because, to date, neither biological nor clinical markers have been clearly determined despite being identified [2, 3].
Anti-transcription intermediary factor 1-gamma (TIF1γ) autoantibody (aAb)-associated DM patients represent a subset of adult DM patients which is strongly associated with the occurrence of cancer as illustrated by a high prevalence rate (i.e.: 46% to 84% among the series) and a risk of malignancy estimated at 4.7–9.7-fold in two recent meta-analyses [3–6]. We recently observed that among anti-TIF1γ IgG isotypes, the IgG2 isotype might represent a biomarker to identify adult DM patients who develop cancer [7]. This observation was made in a series of 51 patients from a single European country [7]. To confirm the potential role of the anti-TIF1γ aAb IgG2 isotype as a biomarker of cancer in anti-TIF1γ aAb-positive adult DM patients, we conducted herein a confirmatory study in a larger panel of patients from different European countries, Canada and the USA under the auspices of the European NeuroMuscular Center (ENMC).
Patients and methods
Study design and participants
We conducted a retrospective study in six university hospitals, national institutes or national collaborative network of the ENMC study group from Sweden, the Czech Republic, Spain, the UK, Canada and the USA. This study was approved by the Institutional Review Board of Rouen University Hospital (No. E2021-33). Inclusion criteria were: (i) adult patients with a diagnosis of DM according to ENMC criteria [8]; (ii) presence of anti-TIF1γ IgG aAb determined using an in house-addressable laser bead immunoassay (ALBIA) from cryopreserved serums sampled at time of DM diagnosis and (iii) available baseline characteristics and follow-up data until the occurrence of cancer and/or a minimum follow-up of 1 year for patients without known cancer at DM diagnosis, because this interval corresponds to the peak of the occurrence of cancer in the literature [9, 10].
Immunoassays
The immunological assays were performed blinded to clinical data.
To detect and quantify anti-TIF1γ IgG and IgG2 aAb, an in-house ALBIA was used as previously described [7]. Briefly, recombinant human TIF1γ protein (OriGene, Rockville, MD, USA) was coupled to fluorescent beads (Bio-Plex COOH-microspheres, Bio-rad, Hercules, CA, USA) using the Bio-Plex amine coupling kit according to the manufacturer’s instructions (Bio-rad). Serums diluted 1:300 were incubated with the beads, then with a mouse anti-human IgG biotinylated secondary antibody (Southern Biotech, Birmingham, AL, USA) and finally with streptavidin-R-phycoerythrin (Qiagen, Courtaboeuf, France). Mean fluorescence intensity (MFI) reading was done using a Bio-Plex apparatus (Bio-Rad) with Manager software (version 4.0). For anti-TIF1γ IgG aAb, a threshold of >2 AU/ml was retained as positive, yielding 96% sensitivity and 99% specificity [7]. For anti-TIF1γ IgG2 aAb, serums were diluted 1:100 and a mouse anti-human IgG2 biotinylated secondary antibody was used (Southern Biotech). To determine a positivity threshold for anti-TIF1γ IgG2 aAb, serums from 128 healthy donors (French people aged from 18 to 60 years, sex ratio 1/1) were quantified. They displayed a mean anti-TIF1γ IgG2 value of 0.25 AU/ml (0.37) [mean (s.d.)]. The positivity threshold for anti-TIF1γ IgG2 was set at a mean of +3 s.d. and established at 1.35 AU/ml.
In addition to the in-house ALBIA, a recent commercial kit ELISA (MBL, Fukushima, Japan) was used for anti-TIF1γ IgG aAb quantification in order to assess its practical use in routine analysis, as ELISA is faster and more widely used in clinical practice [11]. Briefly, according to the manufacturer’s instructions, serums diluted 1:100 were incubated on TIF1γ coated plates, and successively incubated with secondary antibody and chromogenic substrate with washings between each step. Optical density was measured using a PhD-Lx apparatus (Bio-Rad). To determine a positivity threshold for anti-TIF1γ ELISA values, serums from 156 healthy donors (French people aged from 18 to 60 years, sex ratio 1/1) were quantified. They displayed a mean anti-TIF1γ value of 1.40 AU/ml (2.6) [mean (s.d.)]. The positivity threshold was established at a mean of +3 s.d., i.e. 9.2 AU/ml.
Indirect immunofluorescence (IIF) on human epithelial (HEp-2000) cells (immunoconcept) and line-blot detection using Euroline autoimmune inflammatory myopathies 16AG with EuroBlot Master device and Euroline Scan software (Euroimmun, Lübeck, Germany) were performed as routine procedures. For antinuclear antibody assay, the screening dilution was 1/160 and the result was considered as evocative of anti-TIF1γ if a speckled AC-4b pattern was observed (ICAP nomenclature) [12]. For the line-blot, the threshold was superior than one cross, as recommended by the manufacturer.
Statistical analysis
Quantitative data were summarized as median with interquartile range (IQR). Comparisons between patients with and without cancer were performed using the Mann–Whitney rank test. Assessment of the link between cancer and anti-TIF1γ IgG2 aAb positivity with ALBIA and between cancer and anti-TIF1γ IgG aAb positivity with ELISA was performed using a χ2 test. A logistic regression model was used to estimate crude odds ratios (OR) with corresponding 95% CI. Pearson's correlation coefficient was calculated to analyse the relationship between anti-TIF1γ IgG2 aAb values with ALBIA and anti-TIF1γ IgG aAb values with ELISA.
Statistical analysis was performed using R Statistical Software v4.1.1; R Core Team 2021. For all analyses, P-values of <0.05 were considered statistically significant. Anti-TIF1γ IgG2 aAb values with ALBIA and anti-TIF1γ IgG aAb values with ELISA were log-transformed to improve the readability of the graphs.
Results
Clinical characteristics of adult DM patients
A total of 132 DM patients met the diagnostic criteria and were enrolled in the study. The mean age of patients was 55 (±15) years. They were mostly women (112/132, 85%). The median follow-up period of the cohort was 5 years (IQR: 1–10; 95% CI: 3, 8). All the patients without known cancer at diagnosis of DM were followed for at least 3 years. Regarding systemic features reported to be associated with anti-TIF1γ IgG aAb in the literature, pharyngeal involvement with dysphagia was observed in 53/122 (43.4%) of patients and 14/127 (11%) patients had interstitial lung disease.
An associated cancer was diagnosed in 72 (54.5%) patients. The diagnosis of cancer was made before the diagnosis of DM in 20 cases (32.3%), concomitantly with that of DM in 12 cases (19.3%) and during the follow-up period [median 2 years (IQR: 1–10) in 30 cases (48.4%)]. The interval of cancer development was unknown for 10 patients. The types of cancer were various though the most represented were breast (28%), ovarian (22%) and lung (12%) tumours. Patients’ clinical characteristics are summarized in Table 1.
| . | n = 132 . | |
|---|---|---|
| Age at diagnosis of DM | Mean ± s.d. | 55 ± 15 |
| . | Median (Q1–Q3) | 56 (44–66) |
| Sex (F/M) | 112 (84.8%)/20 (15.2%) | |
| Duration of DM (years) | Median (Q1–Q3) | 5 (1–10) |
| Necrosis/ulcerations (unknown =30) | 13 (12.7%) | |
| Pharyngeal involvement (unknown =10) | 53 (43.4%) | |
| Interstitial lung disease (unknown n = 5) | 14 (11.0%) | |
| Presence of cancer | 72 (54.5%) | |
| Histology (unknown n = 4) | ||
| Breast | 19 (27.9%) | |
| Ovaries | 15 (22.1%) | |
| Lungs | 8 (11.8%) | |
| ENT | 5 (7.4%) | |
| Lymphoma | 4 (5.9%) | |
| Colon | 3 (4.4%) | |
| Cervical | 2 (2.9%) | |
| Liver | 1 (1.5%) | |
| Leukaemia | 1 (1.5%) | |
| Myeloma | 1 (1.5%) | |
| Esophageal | 1 (1.5%) | |
| Peritoneum | 1 (1.5%) | |
| Lungs/Kidney | 1 (1.5%) | |
| Prostate | 1 (1.5%) | |
| Thymoma | 1 (1.5%) | |
| Uterus | 1 (1.5%) | |
| Vagina | 1 (1.5%) | |
| Bladder | 1 (1.5%) | |
| Bladder/Ovaries | 1 (1.5%) | |
| Timing DM/Cancer (uknown n = 10) | ||
| Cancer before DM | 20 (32.3%) | |
| Cancer concomitant to DM (+/- 30 days) | 12 (19.3%) | |
| Cancer after DM | 30 (48.4%) | |
| Metastatic cancer (unknown n = 39) | 21 (63.6%) | |
| Cancer in remission at the latest news (unknown n = 19) | 15 (28.3%) | |
| . | n = 132 . | |
|---|---|---|
| Age at diagnosis of DM | Mean ± s.d. | 55 ± 15 |
| . | Median (Q1–Q3) | 56 (44–66) |
| Sex (F/M) | 112 (84.8%)/20 (15.2%) | |
| Duration of DM (years) | Median (Q1–Q3) | 5 (1–10) |
| Necrosis/ulcerations (unknown =30) | 13 (12.7%) | |
| Pharyngeal involvement (unknown =10) | 53 (43.4%) | |
| Interstitial lung disease (unknown n = 5) | 14 (11.0%) | |
| Presence of cancer | 72 (54.5%) | |
| Histology (unknown n = 4) | ||
| Breast | 19 (27.9%) | |
| Ovaries | 15 (22.1%) | |
| Lungs | 8 (11.8%) | |
| ENT | 5 (7.4%) | |
| Lymphoma | 4 (5.9%) | |
| Colon | 3 (4.4%) | |
| Cervical | 2 (2.9%) | |
| Liver | 1 (1.5%) | |
| Leukaemia | 1 (1.5%) | |
| Myeloma | 1 (1.5%) | |
| Esophageal | 1 (1.5%) | |
| Peritoneum | 1 (1.5%) | |
| Lungs/Kidney | 1 (1.5%) | |
| Prostate | 1 (1.5%) | |
| Thymoma | 1 (1.5%) | |
| Uterus | 1 (1.5%) | |
| Vagina | 1 (1.5%) | |
| Bladder | 1 (1.5%) | |
| Bladder/Ovaries | 1 (1.5%) | |
| Timing DM/Cancer (uknown n = 10) | ||
| Cancer before DM | 20 (32.3%) | |
| Cancer concomitant to DM (+/- 30 days) | 12 (19.3%) | |
| Cancer after DM | 30 (48.4%) | |
| Metastatic cancer (unknown n = 39) | 21 (63.6%) | |
| Cancer in remission at the latest news (unknown n = 19) | 15 (28.3%) | |
| . | n = 132 . | |
|---|---|---|
| Age at diagnosis of DM | Mean ± s.d. | 55 ± 15 |
| . | Median (Q1–Q3) | 56 (44–66) |
| Sex (F/M) | 112 (84.8%)/20 (15.2%) | |
| Duration of DM (years) | Median (Q1–Q3) | 5 (1–10) |
| Necrosis/ulcerations (unknown =30) | 13 (12.7%) | |
| Pharyngeal involvement (unknown =10) | 53 (43.4%) | |
| Interstitial lung disease (unknown n = 5) | 14 (11.0%) | |
| Presence of cancer | 72 (54.5%) | |
| Histology (unknown n = 4) | ||
| Breast | 19 (27.9%) | |
| Ovaries | 15 (22.1%) | |
| Lungs | 8 (11.8%) | |
| ENT | 5 (7.4%) | |
| Lymphoma | 4 (5.9%) | |
| Colon | 3 (4.4%) | |
| Cervical | 2 (2.9%) | |
| Liver | 1 (1.5%) | |
| Leukaemia | 1 (1.5%) | |
| Myeloma | 1 (1.5%) | |
| Esophageal | 1 (1.5%) | |
| Peritoneum | 1 (1.5%) | |
| Lungs/Kidney | 1 (1.5%) | |
| Prostate | 1 (1.5%) | |
| Thymoma | 1 (1.5%) | |
| Uterus | 1 (1.5%) | |
| Vagina | 1 (1.5%) | |
| Bladder | 1 (1.5%) | |
| Bladder/Ovaries | 1 (1.5%) | |
| Timing DM/Cancer (uknown n = 10) | ||
| Cancer before DM | 20 (32.3%) | |
| Cancer concomitant to DM (+/- 30 days) | 12 (19.3%) | |
| Cancer after DM | 30 (48.4%) | |
| Metastatic cancer (unknown n = 39) | 21 (63.6%) | |
| Cancer in remission at the latest news (unknown n = 19) | 15 (28.3%) | |
| . | n = 132 . | |
|---|---|---|
| Age at diagnosis of DM | Mean ± s.d. | 55 ± 15 |
| . | Median (Q1–Q3) | 56 (44–66) |
| Sex (F/M) | 112 (84.8%)/20 (15.2%) | |
| Duration of DM (years) | Median (Q1–Q3) | 5 (1–10) |
| Necrosis/ulcerations (unknown =30) | 13 (12.7%) | |
| Pharyngeal involvement (unknown =10) | 53 (43.4%) | |
| Interstitial lung disease (unknown n = 5) | 14 (11.0%) | |
| Presence of cancer | 72 (54.5%) | |
| Histology (unknown n = 4) | ||
| Breast | 19 (27.9%) | |
| Ovaries | 15 (22.1%) | |
| Lungs | 8 (11.8%) | |
| ENT | 5 (7.4%) | |
| Lymphoma | 4 (5.9%) | |
| Colon | 3 (4.4%) | |
| Cervical | 2 (2.9%) | |
| Liver | 1 (1.5%) | |
| Leukaemia | 1 (1.5%) | |
| Myeloma | 1 (1.5%) | |
| Esophageal | 1 (1.5%) | |
| Peritoneum | 1 (1.5%) | |
| Lungs/Kidney | 1 (1.5%) | |
| Prostate | 1 (1.5%) | |
| Thymoma | 1 (1.5%) | |
| Uterus | 1 (1.5%) | |
| Vagina | 1 (1.5%) | |
| Bladder | 1 (1.5%) | |
| Bladder/Ovaries | 1 (1.5%) | |
| Timing DM/Cancer (uknown n = 10) | ||
| Cancer before DM | 20 (32.3%) | |
| Cancer concomitant to DM (+/- 30 days) | 12 (19.3%) | |
| Cancer after DM | 30 (48.4%) | |
| Metastatic cancer (unknown n = 39) | 21 (63.6%) | |
| Cancer in remission at the latest news (unknown n = 19) | 15 (28.3%) | |
Anti-TIF1γ IgG2 aAb ALBIA values and cancer
Fifty-one (38.6%) patients were positive for anti-TIF1γ IgG2 aAb with ALBIA, 34 (66.7%) of whom had cancer: n = 8 (26.7%) before DM diagnosis, n = 8 (26.7%) concomitantly, n = 14 (46.6%) during follow-up, n = 4 not determined (11.8%) and 17 (33.3%) of whom had anti-TIF1γ IgG2 aAb without cancer. The association between the presence of cancer and the presence of anti-TIF1γ IgG2 aAb was statistically significant (P = 0.026), with an OR of 2.26 (95% CI: 1.10, 4.76). Patients with cancer displayed significantly higher anti-TIF1γ IgG2 aAb ALBIA values with a median value of 1.15 AU/ml (IQR: 0.14–9.76) compared with 0.50 AU/ml (IQR: 0.14–1.46) for patients without cancer (P = 0.042) (Fig. 1A).
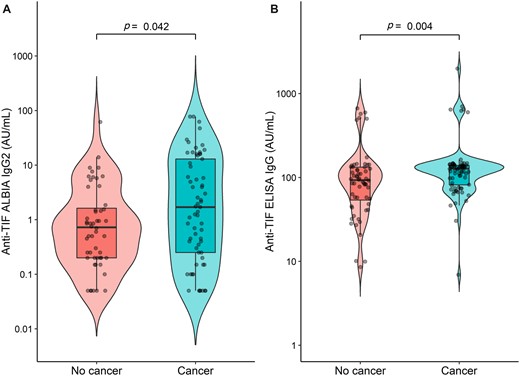
Anti-TIF1 γ IgG2 ALBIA (A) and Anti-TIF1 γ IgG ELISA (B) values according to the presence of cancer in adult patients with DM
Anti-TIF1γ IgG aAb ELISA values and cancer
One hundred and thirty patients (98.5%) were positive for anti-TIF1 γ IgG aAb with ELISA, 71 (54.6%) of whom had cancer. Two patients displayed ELISA values close to the threshold determined for this study (6.9 and 8.6 AU/ml for a threshold of 9.2 AU/ml). Patients with cancer displayed significantly higher anti-TIF1γ IgG aAb ELISA values with a median value of 127.5 AU/ml (IQR: 81.5–139.6) compared with 93.0 AU/ml (IQR: 54.0–132.9) for patients without cancer (P = 0.004) (Fig. 1B.). Pearson’s correlation coefficient between anti-TIF1γ IgG aAb ELISA values and anti-TIF1γ IgG2 aAb ALBIA values was 0.37 (P = 0.002).
Anti-TIF1γ aAb detected by line blot and cancer
One hundred and nineteen patients (90.2%), 66 (55.5%) of whom had cancer were positive with line blot. However, the median band intensity was not significantly different between patients with cancer (i.e., 46; IQR: 22–90) and those without cancer (i.e., 42; IQR: 20–63; P = 0.12).
Anti-TIF1γ aAb and IIF on HEp2 cells
Regarding the serums of the 132 adult DM patients, 81 patients (61.4%), 43 (53.1%) of whom had cancer, had a finely speckled nuclear fluorescence pattern evocative of anti-TIF1γ aAb.
Discussion
The results of our study demonstrate an association between the anti-TIF1γ aAb IgG2 isotype and cancer in anti-TIF1γ aAb-positive adult DM patients. Therefore, these results confirm in a large series of 132 patients and at an international level those of our previous study conducted in a cohort of 51 patients from a single European country [7].
The clinical characteristics of this large series of anti-TIF1γ aAb-positive adult DM patients are in accordance with the literature, notably for age, unbalanced sex ratio in favour of women, high frequency of pharyngeal involvement and a low prevalence of interstitial lung disease [7, 13]. The distribution of cancers is also consistent with that reported in the literature [1, 5, 9, 10, 13].
Despite a rather low Pearson’s correlation coefficient (i.e.: 0.37, P = 0.002), we observed a good concordance between both our in-house ALBIA and the commercial kit ELISA to identify an association between DM and cancer because patients with cancer displayed significantly higher anti-TIF1γ aAb values than patients without cancer. This concordance may allow an easier access to the quantification of anti-TIF1γ aAb for DM patients because ELISA is faster, more easily available in laboratories than ALBIA and reliable as recently highlighted by Mulhearn et al. [14].
The line blot and IIF on HEp2 cells assays are frequently used in routine laboratory screening for DM aAb. Ninety percent of our serums were anti-TIF1γ aAb positive in line blot. In accordance with the previously reported poor sensitivity of line blot for anti-TIF1γ aAb detection, our results suggest that line blot performance for anti-TIF1γ aAb should be improved [15]. Moreover, we failed to find a significant difference in line blot levels between cancer and cancer-free patients probably due to the semi-quantitative nature of the assay as compared with quantitative ELISA. In addition, only 61.4% of serums had a fluorescence pattern evocative of anti-TIF1γ aAb, thus confirming that IIF on HEp2 cells cannot be used to screen for anti-TIF1γ aAb [15].
Besides the fact that IgG2 subclass switch recombination increases with age with a maximum rate at 40–65 years which corresponds to the age group of our cohort, the reason for the association of anti-TIF1γ IgG2 confirmed herein remains elusive [16]. It may be explained by the hypothesis of an anti-tumoral immune response. Indeed, the recent observation of acquired somatic mutations or loss of heterozygosity of the TIF1γ gene in the tumour of patients with cancer-associated anti-TIF1γ positive DM supports the hypothesis of the role of somatic TIF1γ gene variants as triggers of an anti-tumoral autoimmune response [17–19]. Because the majority of anti-TIF1γ aAb-positive adult DM patients had a cancer diagnosis concomitantly or after the diagnosis of DM, it may be hypothesized that isotype switching to IgG2 reflects a less efficient anti-tumoral immune response than in patients whose tumour was eliminated.
Together, the results of this study based on a large international series of patients representative of anti-TIF1γ aAb-positive European and North American adult DM patients support the role of anti-TIF1γ IgG2 ALBIA and IgG ELISA as biological predictors of cancer that could be helpful to detect anti-TIF1γ aAb-positive adult DM patients.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by funding from the Medical Research Council (MR/N003322/1), Versus Arthritis Programme Grant 18474, and Myositis UK. This report includes independent research supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Other: JV and HM acknowledge the support from the Czech Ministry of Health—Conceptual Development of Research Organization 00023728 (Institute of Rheumatology). A.S.GF. was supported by Swedish Heart and Lung foundation and Swedish Research Council 2020–01378. L.D. was supported by Börje Dahlin foundation.
Disclosure statement: The authors have declared no conflicts of interest.
Acknowledgements
Associated Investigators: Laurent Drouot, Rouen University Hospital, Department of Immunology and Biotherapy, Univ Rouen Normandie, FOCIS Center of Excellence PAn’THER, Inserm, U1234, Rouen, France. Marie Hudson, Department of Medicine, McGill University; Division of Rheumatology and Lady Davis Institute, Jewish General Hospital, Montreal, Quebec, Canada. Ingrid Lundberg, Division of Rheumatology, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden and ME Gastro, Derm, Rheuma, Karolinska University Hospital, Stockholm, Sweden. James Lilleker, Centre for Musculoskeletal Research, Faculty of Biology, Medicine and Health, The University of Manchester (Manchester, United Kingdom) and Manchester Centre for Clinical Neurosciences, Salford Royal Hospital, Northern Care Alliance NHS Foundation Trust, (Salford, United Kingdom). Anaís Mariscal, Immunology Department, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute Sant Pau (IIB Sant Pau), Barcelona, Spain. Alexander Oldroyd, Department of Rheumatology, Salford Royal Hospital, Northern Care Alliance NHS Foundation Trust, Manchester Academic Health Science Centre (Salford, United Kingdom) and Centre for Musculoskeletal Research, Faculty of Biology, Medicine and Health, The University of Manchester (Manchester, United Kingdom).
We are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript. We would like to thank the participants of the 239th European Neuromuscular Centre (ENMC) international workshop, Amsterdam, The Netherlands, 14–16 December 2018, under the auspices of which the study group was constituted. We wish to thank Marlène Thomas and Lucile Adam, Department of Immunology and Biotherapy, Rouen University Hospital, for their technical help during the immunoassays procedures.
IRB approval: This study was approved by the institutional review board of Rouen University Hospital (NO. E2021-33).
References
Author notes
Nadége Cordel and Benoit Déchelotte contributed equally to this study.




Comments