-
PDF
- Split View
-
Views
-
Cite
Cite
Katharina Ziegeler, Sevtap Tugce Ulas, Denis Poddubnyy, Fabian Proft, Valeria Rios Rodriguez, Judith Rademacher, Kay Geert A Hermann, Torsten Diekhoff, Anatomical variation of the sacroiliac joint carries an increased risk for erosion and bone marrow oedema in axial spondyloarthritis, Rheumatology, Volume 62, Issue 3, March 2023, Pages 1117–1123, https://doi.org/10.1093/rheumatology/keac282
Close - Share Icon Share
Abstract
To assess the impact of joint shape variations on inflammatory lesions on SI joint MRIs in patients with axial spondyloarthritis (axSpA).
A total of 1194 patients from four different prospective cohorts were evaluated, with 684 (57.3%) having sufficient imaging data for inclusion (379 axSpA, 305 controls). All images were evaluated for joint form, erosion, sclerosis, fat metaplasia and bone marrow oedema (BMO) by two independent readers. Logistic regression analyses were used to assess the association of joint form and lesions on imaging for axSpA patients and controls.
Atypical joint forms were common in both axSpA (43.5% [154/354]) and control patients (44.2% [134/303]); both intra-articular variants and a crescent joint shape were significantly more common in axSpA patients (18.4% vs 11.6% and 11.0% vs 5.3.%, respectively; P < 0.001). The axSpA patients with intra-articular joint form variants had 2-fold higher odds of exhibiting erosions [odds ratio (OR) 2.09 (95% CI 1.18, 3.69)] and BMO [OR 1.79 (95% CI 1.13, 2.82)]; this association was not observed in controls. Accessory joints increased the odds for sclerosis in axSpA patients [OR 2.54 (95% CI 1.10, 5.84)] and for sclerosis [OR 17.91 (95% CI 6.92, 46.37)] and BMO [OR 2.05 (95% CI 1.03, 4.07)] in controls.
Joint form variations are associated with the presence of inflammatory lesions on SI joint MRIs of axSpA patients. This should be taken into consideration in future research on the interplay of mechanical strain and inflammation in axSpA.
Atypical joint forms are common in axSpA and non-axSpA back pain.
Odds for erosion and osteitis are doubled in axSpA patients with atypical joints.
Accessory joints in non-axSpA back pain increase the chances for osteitis and sclerosis.
Introduction
Mechanical stress has long been discussed as a contributing factor in both the development and progression of axial spondyloarthritis (axSpA) [1, 2]. As a primary site of disease manifestation and as the main transductor of force from the legs to the torso [3], the biophysical properties of the SI joints deserve special attention. The main function of the SI joint is stabilization against axial load, which is achieved by the wedge-like shape of the sacrum as well as an uneven joint surface that leads to friction [3]. Further stabilization is provided by a firm joint capsule and strong ligaments, which also strictly limit the range of motion [4]. One possible cause of increased mechanical stress to the SI joint that has recently gained attention from the scientific community is anatomical variations of the joints [5–9]. An increase in mechanical stress or decreased physiological distribution within the joint may be significant in the imaging of axSpA in different ways. First, inflammation itself may be triggered or maintained by microdamage to the joint cartilage [10]. Furthermore, lesions such as sclerosis and bone marrow oedema (BMO), which are regularly observed in mechanical joint conditions, may be induced, thus mimicking inflammatory joint disease. It is important to note, however, that opinions among experts regarding the importance of mechanical stress in axSpA are not unequivocal and, for example, limited stress during exercise is considered beneficial in the disease course [11, 12].
To date, no study has investigated the relationship between joint form variation and the presence of inflammatory lesions on MRI. The purpose of this investigation was to assess the impact of joint shape variation on inflammatory lesions, separately for patients with axSpA and controls with mechanical or degenerative conditions of the SI joint.
Materials and methods
Patients
Included in this analysis were patients from four different prospective cohorts: the GErman Spondyloarthritis Inception Cohort (GESPIC; n = 448) [13–15], the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study (n = 564) [16, 17], the SacroIliac joint MAgnetic resonance imaging and Computed Tomography (SIMACT) study (n = 110) [18–20] and the Virtual Non-contrast Susceptibility-Weighted Imaging (VNC-SWI) study (n = 72) [21]. The GESPIC study includes patients from three different arms: GESPIC-AS has patients with active radiographic axSpA; GESPIC-Crohn has patients with Crohn’s disease with or without chronic low back pain (LBP) and GESPIC-Uveitis has patients with non-infectious acute anterior uveitis with or without chronic LBP. OptiRef, SIMACT and VNC-SWI prospectively enrolled patients with chronic LBP and possible axSpA. A more detailed description of the respective cohorts can be obtained from the cited references. For the purpose of this investigation, only baseline data were used. Patients with missing or incomplete imaging data or a missing clinical diagnosis were excluded from the analysis. A total of 1194 patients were evaluated and 684 patients were included in the analysis after application of exclusion criteria. Of these, 379 were clinically diagnosed with axSpA. Fig. 1 depicts patient flow and clinical characteristics.
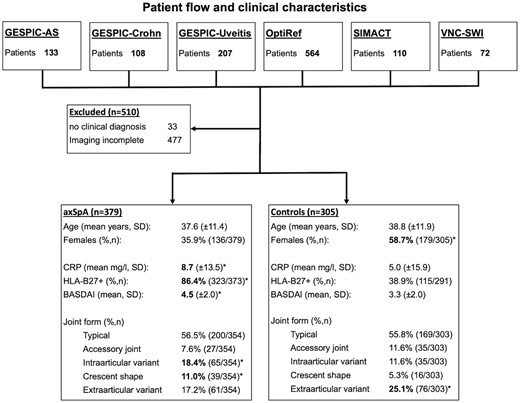
Patient flow and clinical characteristics
Significantly higher mean values or frequencies, as detected by unpaired t-tests or chi-squared tests are printed in bold and denoted with an asterisk. Percentages for joint form do not add up to 100 because joint form could not be assessed due to ankylosis in some patients and other patients exhibited more than one variant.
Informed consent, ethical approval and data availability
All included studies were approved by the ethics review board of the Charité – Universitätsmedizin Berlin prior to study commencement (EA4/161/15, EA4/170/16, EA1/0886/16, EA1/073/10, NCT01277419). All patients gave written informed consent before enrolment in the respective study. The study was conducted in compliance with the Declaration of Helsinki and local legislation and ethical standards. The data underlying this article will be shared by the corresponding author upon reasonable request.
Scoring system and lesion definition
All images were analysed using a simplified semi-quantitative scoring system, which was adapted from previous studies [19, 21]. The SI joint was divided into three portions (ventral, middle and dorsal third of the joint) and in each portion the iliac and sacral parts on both sides were assessed separately. For each joint, the shape was classified as typical (type 1), small nudge within the joint (type 2), overall concave shape of the ilium (type 3), accessory joint facet (type 4) and shape variation outside the joint itself, i.e. bipartite ilium or lumbo-sacral transitional anomaly (type 5). The presence of erosion, sclerosis, fat metaplasia and BMO were assessed for each region; a pictorial guide to shape assessment is provided in Fig. 2. All images were read by two radiology residents: K.Z., with 6 years of experience in musculoskeletal (MSK) imaging, and S.T.U., with 2 years of experience. Cases of disagreement were resolved in a consensus reading under the supervision of a senior expert MSK radiologist (T.D.).
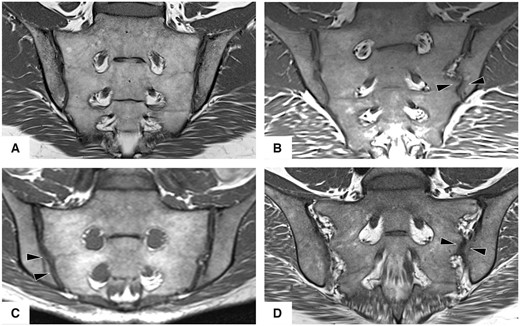
Joint forms
T1-weighted images in an oblique coronal orientation: (A) typical joint, (B) intra-articular variant with dysmorphic joint surface (arrowheads), (C) crescent joint shape with concave iliac surface (arrowheads) and (D) accessory joint facet (left joint) in the ligamentous portion (arrowheads).
Statistical analysis
The association of different types of joint form variation with the presence of erosion, sclerosis, fat metaplasia and BMO was assessed in logistic regression models with age and sex as additional covariables; as joint form variation is regularly asymmetric, these analyses were carried out at the joint rather than on a patient level, separately for axSpA patients and controls. Interreader agreement was investigated using Cohen’s κ and intraclass correlation coefficients (ICCs) with a two-way mixed model. All analyses were carried out using SPSS version 27 (IBM, Armonk, NY, USA) with a two-tailed significance level of α = 0.05.
Results
Patients and frequency of joint forms and lesions
Clinical characteristics, as well as frequencies of joint forms per group on a patient level, including comparisons between groups, are given in Fig. 1. Overall, only slightly more than half of axSpA patients (200/354) and controls (169/303) displayed a typical joint form. Two variants were significantly more prevalent in axSpA patients: intra-articular variants (18.4% vs 11.6.%; P < 0.001) and a crescent shape (11.0% vs 5.3%; P < 0.001). Extra-articular variants were more prevalent in controls (17.2% vs 25.1%; P < 0.001). A summary of frequencies of lesions is given in Table 1, where results for the control group are additionally given for HLA-B27-positive and negative individuals separately. All joint lesions were significantly (P < 0.001) more prevalent in axSpA patients and the difference was most pronounced for erosion (69.1% vs 14.8%) and fat metaplasia (53.0% vs 9.2%). Imaging examples of atypical joints with lesions are given in Fig. 3. Frequencies of joint lesions did not differ between HLA-B27-positive and negative individuals.
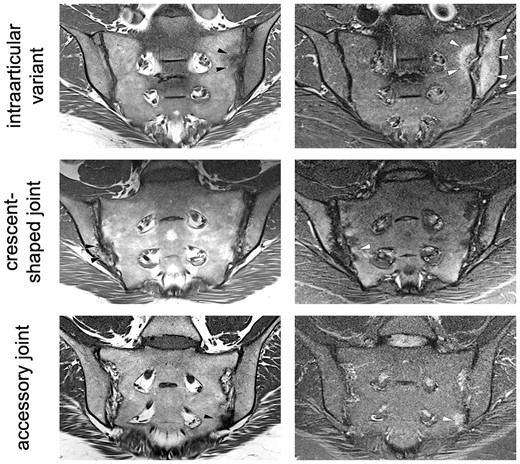
Imaging examples
Oblique coronal orientation T1-weighted images (left) and STIR images (right). First line: intra-articular variant (left SI joint) with erosions (black arrowheads) and BMO (white arrowheads). Second line: crescent-shaped joint with sclerosis (black arrowheads) and very little BMO (white arrowheads). Third line: accessory joint (left SI joint) with sclerosis (black arrowheads) and BMO (white arrowheads).
| Type . | axSpA, % (n/N) . | Controls, % (n/N) . | ||
|---|---|---|---|---|
| HLA-B27 positive . | HLA-B27 negative . | Overall . | ||
| Erosion | 69.1 (262/379) | 12.2 (14/115) | 17.1 (31/181) | 14.8 (45/305) |
| Sclerosis | 63.9 (242/379) | 40.0 (46/115) | 45.9 (83/181) | 43.6 (133/305) |
| Fat metaplasia | 53.0 (201/379) | 10.4 (12/115) | 8.8 (16/181) | 9.2 (28/305) |
| BMO | 57.8 (219/379) | 28.7 (33/115) | 23.2 (42/181) | 24.9 (76/305) |
| Type . | axSpA, % (n/N) . | Controls, % (n/N) . | ||
|---|---|---|---|---|
| HLA-B27 positive . | HLA-B27 negative . | Overall . | ||
| Erosion | 69.1 (262/379) | 12.2 (14/115) | 17.1 (31/181) | 14.8 (45/305) |
| Sclerosis | 63.9 (242/379) | 40.0 (46/115) | 45.9 (83/181) | 43.6 (133/305) |
| Fat metaplasia | 53.0 (201/379) | 10.4 (12/115) | 8.8 (16/181) | 9.2 (28/305) |
| BMO | 57.8 (219/379) | 28.7 (33/115) | 23.2 (42/181) | 24.9 (76/305) |
The frequencies of all lesions was significantly (P < 0.05) higher in axSpA patients than controls (chi-squared tests). HLA status does not add up to the total because status was not known in nine patients. Frequencies of lesions did not differ between HLA-B27-positive and negative individuals (P > 0.05, using chi-squared tests).
| Type . | axSpA, % (n/N) . | Controls, % (n/N) . | ||
|---|---|---|---|---|
| HLA-B27 positive . | HLA-B27 negative . | Overall . | ||
| Erosion | 69.1 (262/379) | 12.2 (14/115) | 17.1 (31/181) | 14.8 (45/305) |
| Sclerosis | 63.9 (242/379) | 40.0 (46/115) | 45.9 (83/181) | 43.6 (133/305) |
| Fat metaplasia | 53.0 (201/379) | 10.4 (12/115) | 8.8 (16/181) | 9.2 (28/305) |
| BMO | 57.8 (219/379) | 28.7 (33/115) | 23.2 (42/181) | 24.9 (76/305) |
| Type . | axSpA, % (n/N) . | Controls, % (n/N) . | ||
|---|---|---|---|---|
| HLA-B27 positive . | HLA-B27 negative . | Overall . | ||
| Erosion | 69.1 (262/379) | 12.2 (14/115) | 17.1 (31/181) | 14.8 (45/305) |
| Sclerosis | 63.9 (242/379) | 40.0 (46/115) | 45.9 (83/181) | 43.6 (133/305) |
| Fat metaplasia | 53.0 (201/379) | 10.4 (12/115) | 8.8 (16/181) | 9.2 (28/305) |
| BMO | 57.8 (219/379) | 28.7 (33/115) | 23.2 (42/181) | 24.9 (76/305) |
The frequencies of all lesions was significantly (P < 0.05) higher in axSpA patients than controls (chi-squared tests). HLA status does not add up to the total because status was not known in nine patients. Frequencies of lesions did not differ between HLA-B27-positive and negative individuals (P > 0.05, using chi-squared tests).
Influence of joint form on different lesions and disease activity
Odds ratios (ORs) from regression analyses are visualized as forest plots in Fig. 4. The axSpA patients with intra-articular variants had ∼2-fold greater odds of exhibiting erosion [OR 2.09 (95% CI 1.18, 3.69)] and BMO [OR 1.79 (95% CI 1.13, 2.82)], while this association was not observed in controls. Both axSpA and control patients with accessory joints had markedly increased chances of exhibiting sclerosis [OR 2.54 (95% CI 1.10, 5.84) and 17.91 (95% CI 6.92, 46.37), respectively]. This constellation was similar for BMO in controls [OR 2.05 (95% CI 1.03, 4.07)] but not in axSpA patients [OR 1.37 (95% CI 0.66, 2.86)]. There was no difference in disease activity at baseline (expressed as BASDAI) between axSpA patients with typical and atypical joint forms: female axSpA patients with typical joints had a mean BASDAI of 4.27 (s.d. 2.04) vs 4.83 (s.d. 2.00) in patients with atypical joints (P = 0.120); similar results were seen in males [4.41 (s.d. 2.00) vs 4.37 (s.d. 2.04); P = 0.894].
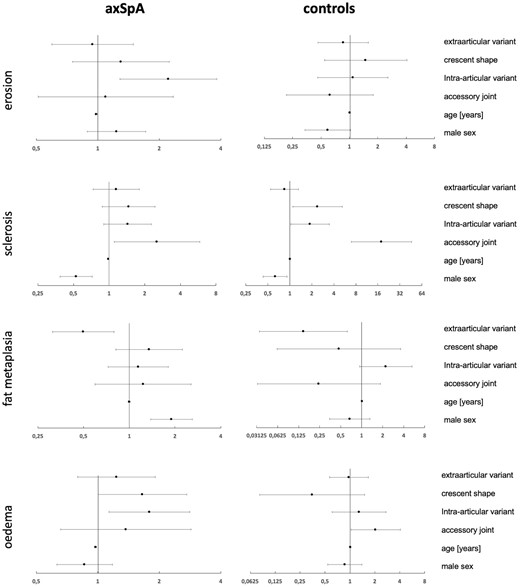
Forest plots of ORs
ORs as dots with 95% CIs as whiskers. Logarithmic x-axis. Vertical reference line at 1.0. Horizontal lines intersecting the vertical reference line denote non-significant variables.
Interreader agreement
Agreement regarding joint forms was overall moderate with Cohen’s κ ranging from 0.539 (intra-articular variants) to 0.668 (extra-articular variants). For joint lesions, agreement was moderate for sclerosis [ICC 0.591 (95% CI 0.525, 0.647); P < 0.001], good for fat metaplasia [ICC 0.899 (95% CI 0.883, 0.913); P < 0.001] and excellent for erosions [ICC 0.906 (95% CI 0.891, 0.919); P < 0.001] and BMO [ICC 0.917 (95% CI 0.903, 0.928); P < 0.001].
Discussion
To the best of our knowledge, this is the first large-scale study to investigate the impact of anatomical variation of the SI joint on inflammatory MRI lesions in the context of axSpA. We found a 2-fold increased risk for erosions and BMO in axSpA patients with intra-articular variants. In controls, this relationship was not significant, indicating a role for intra-articular variants in inflammation itself.
Our results indicate that joint form variations should be considered when diagnosing axSpA on imaging. The fact that we detected lower proportions of atypical joints (37.2 for axSpA and 38.0% for controls) than in previous estimations (44.1% and 80.3%, respectively) [4] places an emphasis on the imaging modality best suited for this assessment—the latter investigation used CT, which provides direct bone imaging and higher spatial resolution and thus might be superior to MRI for this purpose. Notably, the authors are not aware of any method for detecting joint variations in radiography, which has been viewed as increasingly critically in recent years [22].
We deliberately adjusted the original classification of SI joint form by Prassopoulos et al. [23], because it inadequately captures smaller groves and nudges and places emphasis on extra-articular variation. We found an increased chance for control patients with accessory joints to exhibit BMO, in line with El Rafei et al. [24]. The moderate interrater reliability in our study, which is lower than in current publications [25], stresses the need for a more quantitative method to capture joint geometry, expanding on the work of Jesse et al. [26], but must also be stressed as a limitation for the interpretation of our results. As limitations for discussion, data on physical activity, number of deliveries in women and precise body weight were not available for all patients, which somewhat limits the precision of the regression models. Furthermore, we did not investigate longitudinal data. Additionally, our regression analyses were carried out on the joint level rather than the patient level. This approach was chosen because of significant asymmetry of joint anatomy, but it limits the generalizability of our results. Lastly, imaging was included in the diagnostic process. This limitation, although very common in studies on axSpA patients, is important, as it carries the risk of circular reasoning. An alternative explanation for our findings may therefore be that a subset of axSpA patients was misclassified because their joint form mimicked inflammatory lesions.
In conclusion, our results show that joint form variations have an impact on the presence of inflammatory lesions on SI joint MRI in axSpA. In future research on the interplay of mechanical strain and inflammation in axSpA, considerations of joint shape should be included, even if their final clinical significance remains uncertain as of now. Further longitudinal clinical data as well as biomechanical investigations are needed to better understand the interplay of joint shape variation and disease progression in axSpA.
Acknowledgements
The authors thank the Berlin Institute of Health for providing essential infrastructure for data collection.
Funding: This research was funded by the Assessment of SpondyloArthritis international Society (ASAS) (research grant to K.Z.) and the Berlin Institute of Health (personal funding for J.R.).
Disclosure statement: K.Z. reports funding (research grant) from the ASAS during the conduct of this study. D.P. reports grants and personal fees from AbbVie, Eli Lilly, MSD, Novartis and Pfizer and personal fees from Bristol-Myers Squibb, Roche, UCB, Biocad, GlaxoSmithKline and Gilead outside the submitted work. F.P. reports grants and personal fees from Novartis, Eli Lilly and UCB, as well as personal fees from AbbVie, Amgen, Bristol-Myers Squibb, Hexal, Janssen, MSD, Pfizer and Roche. V.R.R. reports personal fees from AbbVie and the Falk Foundation. J.R. reports funding from the Berlin Institute of Health during the conduct of this study (Clinician Scientist Programme). K.G.A.H. is co-owner of BerlinFlame and reports lecture fees from AbbVie, MSD and Novartis outside the submitted work. T.D. reports personal fees from MSD, Novartis and Eli Lilly. All other authors report no conflicts of interest.




Comments