-
PDF
- Split View
-
Views
-
Cite
Cite
Melek Yalcin Mutlu, Jochen Wacker, Koray Tascilar, Jule Taubmann, Bernhard Manger, Gerhard Krönke, Georg Schett, David Simon, Effective and safe treatment of anti-CD38 therapy in systemic lupus erythematosus–associated refractory cerebral vasculitis induces immune tolerance, Rheumatology, Volume 62, Issue 2, February 2023, Pages e21–e23, https://doi.org/10.1093/rheumatology/keac393
Close - Share Icon Share
• This case underscores the fundamental considerations of targeting plasma cells in autoimmune diseases such as SLE.
Dear Editor, Autoimmunity is central to the pathogenesis of SLE. Autoantibodies, produced by short- and long-lived plasma cells, form immune complexes, deposit in target tissues, and drive systemic and organ-specific inflammation [1]. A major unmet need in SLE is to establish a curative treatment concept that would allow the elimination of autoreactive antibody-producing cells, the (re-)induction of immune tolerance and the achievement of a drug-free remission. CD20-targeted therapies may not affect all classes of autoantibodies, suggesting that SLE-specific autoantibodies derive from different sources, i.e. short- and long-lived plasma cells [2, 3]. Of note, long-lived plasma cells usually lack standard B cell markers such as CD20 and are therefore not affected by B cell–depleting therapy [4]. Hence long-lived plasma cells be of key importance in the treatment of patients with SLE who are refractory to conventional therapies. Daratumumab, a human monoclonal anti-CD38 antibody approved for the treatment of multiple myeloma [5], has been shown to reduce plasma cells in the bone marrow. In vitro studies and a case report in SLE have shown that anti-CD38 treatment with daratumumab can effectively target plasma cells and reduce disease activity in SLE [1, 6]. Here, we present a patient with SLE manifesting with cerebral vasculitis, who was successfully treated with daratumumab and in whom autoreactive antibody-producing cells seem to have been reduced.
In September 2020, a 39-year-old female was referred to our clinic. The woman was first diagnosed with SLE in 1992 at the age of 13 years when she developed rash, dry eyes and mouth, and RP. She tested positive for ANA (titer 1:3200) with homogeneous pattern and positive anti-dsDNA antibodies. The patient had a long history of SLE [skin, musculoskeletal and neurological involvement (in 2014 Broca’s motor aphasia, hemiplegia)] with multiple prior immunomodulatory therapies that were either ineffective or not tolerated (glucocorticoids, HCQ, rituximab, belimumab, upadacitinib). Throughout all these treatments the patient remained serologically active, with positive anti-dsDNA and persistent hypocomplementemia. She was left-hand dominant. At follow-up, her motor aphasia had improved without residue.
In September 2020 the patient reported paresis of the left hand with limitations in fine motor skills accompanied by severe headache. On her baseline physical examination she had finger spasticity (sequel of previous neurologic event) and impaired fine motor skills. In her left upper and lower extremity distal muscle strengths were 4+/5. She had a transcortical sensory aphasia. MRI showed multiple acute/subacute infarcts and chronic ischaemic changes, MR angiography (MRA) demonstrated vasculitis of internal carotid artery (Fig. 1A and B).
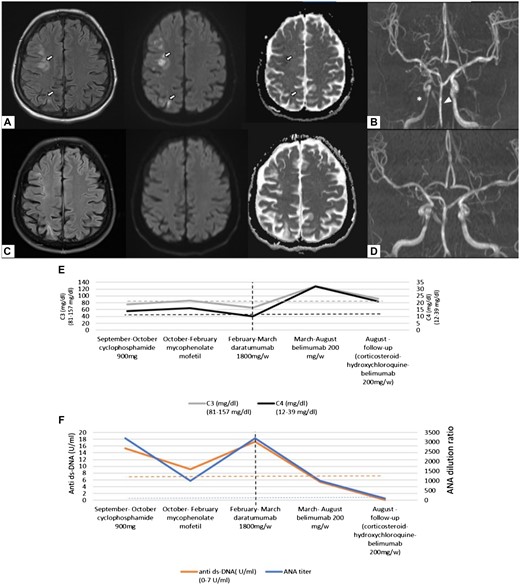
Cranial MR imaging and serological changes after plasma cell targeting of SLE
Cranial MRI showing multiple acute/early subacute infarcts in the right hemisphere affecting the medial and posterior subcortical area on T2-FLAIR, diffusion and ADC sequences, respectively (white arrows) (A); MR angiography showing vasculitis of the right internal carotid artery (ICA) (asterisk) and irregularities in basilar artery wall (arrow head) (B). MR images after treatment with daratumumab (C), showing no new ischaemic lesion. Control MR angiographic images showing regression of ICA and basilar artery vasculitis (D). Changes of serum complement factor levels C3 and C4 (E) and ANA and anti-dsDNA antibodies (anti-ds-DNA-Ab); (F) during different immunomodulatory treatments, the vertical dashed line depicts the time of daratumumab therapy.
Based on the clinical and imaging findings, the patient was diagnosed with cerebral vasculitis secondary to SLE and intensive immunosuppressive treatment was initiated (3 days of methylprednisolone 1000 mg/day, CYC 900 mg). The patient experienced clinical improvement of the paresis. In her follow-up MRA after 1 month, the right internal carotid stenosis showed slight improvement and no new ischaemic changes were observed (MRI). Due to cytopenia, CYC was discontinued and MMF was administered (1500 mg/day). In the following 4 months the patient was clinically stable with decrease in anti-dsDNA antibodies and normalization of complement levels. MMF treatment was discontinued due to gastrointestinal side effects. In February 2021, the patient deteriorated with fatigue, persistent headache, hand tremor, joint pain, morning stiffness and myalgia. MRA showed in addition to former vasculitic changes a newly developed stenosis of the basilar artery indicating active cerebral vasculitis. On physical examination the patient had alopecia, erythema, hand and feet fissures, oligoarthritis, and hand tremor. There were no cranial nerve abnormalities, and her cerebellar examination was also normal. She was ANA positive (1:3200, homogeneous pattern), and showed high titres of anti-SM (+++) and anti-Sm/RNP (+++) antibodies. She had a slightly increased anti-dsDNA level [15.3 U/ml (Normal range: 0–7)], reduced complement levels [C3: 65 mg/dl (81–157), C4: 10 (12.2–39.2)] and proteinuria of 750 mg protein/day. Her SLEDAI score was very high, at 53. Methylprednisolone 2000 mg/day (5 days) and daratumumab 1800 mg s.c. (once weekly for 4 weeks) were administered, followed by maintenance therapy with belimumab. Daratumumab was administered as an individual treatment attempt, the patient gave written informed consent before starting therapy.
No adverse events after daratumumab therapy were documented. After therapy, headache subsided, complement levels normalized, and anti-dsDNA antibodies and proteinuria disappeared. After 5 months of maintenance therapy, the patient had no new symptoms and was in low disease activity, with a SLEDAI score of 3 (leukopenia, alopecia). Her follow-up MRI showed no new ischaemic lesions and MRA indicated regression of vasculitis both in right internal carotid artery and basilar artery (Fig. 1C and D). Following daratumumab therapy, the patient not only converted with anti-dsDNA antibodies but also with anti-nuclear (Fig. 1E and F), anti-Sm and anti-Sm/RNP antibodies.
In this SLE patient with cranial vasculitis and very high disease activity we were able to achieve low disease activity and serological remission (seroconversion) by targeting plasma cells with daratumumab. No adverse events were observed. This patient is the first case of cerebral vasculitis associated with SLE that was treated with daratumumab and the first evidence for seroconversion of autoantibodies. A previous case report showed clinical efficacy of daratumumab in refractory SLE especially with nephritis, showing a decrease but no disappearance of anti-dsDNA antibodies [6]. Long-lived plasma cells reside in the bone marrow and in inflammatory tissue niches producing autoantibodies independent of B cell activation [1]. This process might be involved in the resistance of some SLE patients to conventional immunosuppressive treatment. Overall, this case underlines the principal rationale for plasma cell targeting in autoimmune diseases such as SLE.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: All authors have declared no conflicts of interest.
Data availability statement
No new data were generated or analysed in support of this research.




Comments