-
PDF
- Split View
-
Views
-
Cite
Cite
Tugba Izci Duran, Murat Torgutalp, Valeria Rios Rodriguez, Fabian Proft, Clementina López-Medina, Maxime Dougados, Denis Poddubnyy, The impact of psoriasis on the clinical characteristics, disease burden and treatment patterns of peripheral spondyloarthritis, Rheumatology, Volume 62, Issue 1, January 2023, Pages 135–146, https://doi.org/10.1093/rheumatology/keac235
Close - Share Icon Share
Abstract
To evaluate the clinical characteristics, disease burden, and treatment patterns of peripheral spondyloarthritis (pSpA) patients with and without psoriasis using data from the ASAS-perSpA study.
We included 433 patients who had a diagnosis of pSpA according to the rheumatologist’s diagnosis from the ASAS-PerSpA study. The presence of a personal history of psoriasis was defined as the presence of signs of psoriasis at physical examination or the presence of psoriatic nail dystrophy, including onycholysis, pitting and hyperkeratosis, or a history of psoriasis diagnosed by a physician. Clinical characteristics, patient-reported outcomes and treatment pattern were compared between subgroups with and without psoriasis.
A total of 83 patients (19.2%) had a personal history of psoriasis. Patients with psoriasis were older (48.4 vs 43.2 years) and had a longer diagnostic delay (7.4 vs 3.5 years), a higher frequency of dactylitis (36.1 vs 20.0%) and enthesitis (65.1 vs 55.4%) than patients without psoriasis. A longer diagnostic delay (odds ratio [OR] = 1.06 [95% CI 1.01, 1.11]), lower odds for HLA-B27 positivity (OR = 0.31 [95% CI 0.15, 0.65]) and higher odds for enthesitis (OR = 2.39 [95% CI 1.16, 4.93]) were associated with the presence of psoriasis in a multivariable regression analysis. While patient-reported outcomes were comparable between groups, a higher use of biologic DMARDs was observed in patients with vs without psoriasis.
The presence of psoriasis has an impact on clinical characteristics of pSpA. pSpA patients without psoriasis were less frequently treated with biologic DMARDs despite similar disease burden as compared with patients with psoriasis.
The presence of psoriasis has an impact on clinical characteristics of peripheral spondyloarthritis (pSpA).
Higher frequency of enthesitis and lower frequency of HLA-B27 are associated with psoriasis in pSpA.
pSpA patients without psoriasis are less frequently treated with bDMARDs despite comparable disease burden.
Introduction
Peripheral spondyloarthritis (pSpA) is the term used to classify patients with SpA manifesting predominantly or entirely peripherally (arthritis, enthesitis, dactylitis) rather than axially. In 2011, the Assessment of Spondyloarthritis International Society (ASAS) developed a set of classification criteria capturing SpA patients presenting without clinical signs of axial involvement [1]. The classification criteria for peripheral and axial SpA (axSpA) are mutually exclusive—in the current presence of back pain, axSpA criteria should be applied, while patients with peripheral manifestations of SpA without current back pain can be classified as pSpA. In clinical practice, however, the leading manifestation often defines the wording of the diagnosis. Furthermore, access to biologic DMARDs (bDMARDs) might play a role: there are a number of treatment options available for axSpA, while there are no formally approved drugs for pSpA. Since psoriasis is one of the common extra-musculoskeletal SpA manifestations, there is also a natural overlap between pSpA and PsA. This is true for both diagnosis in daily clinical practice and classification in clinical studies: patients presenting with arthritis, enthesitis, and/or dactylitis and with a personal or family history of psoriasis may meet both ASAS pSpA and the Classification Criteria for Psoriatic Arthritis (CASPAR) criteria [2]. While polyarticular forms of PsA, arthritis mutilans, do not fit into the SpA concept, in patients with SpA-compatible arthritis (mono- or oligoarthritis with predominant involvement of the lower extremities), differentiation between pSpA with psoriasis and PsA is practically impossible; the labelling of the disease is often impacted by a larger number of approved drugs in PsA than in pSpA. There is, however, a large unmet need for patients with pSpA who cannot be classified otherwise (i.e. patients without psoriasis and without axial involvement) due to a lack of formally approved treatment options and lack of evidence of efficacy of different drug classes in this population. In this study, we aimed to evaluate the clinical characteristics, disease burden and current treatment patterns of pSpA patients without and with psoriasis using data from the ASAS-PerSpA (peripheral involvement in SpA) study.
Method
Patient selection
The details of the study design and the description of the entire study population have been reported elsewhere [3]. Briefly, ASAS-PerSpA was a multicentre, international, cross-sectional study with 24 participating countries in four geographical regions that recruited from July 2018 to February 2020. For the ASAS-PerSpA study, a national lead investigator (ASAS members) was appointed by the study scientific committee for each participating country. Local rheumatologists representing qualified rheumatology centres were invited to participate by national lead investigators. Adult patients (i.e. at least 18 years old) with SpA, including axSpA, pSpA, PsA, IBD associated SpA (IBD-SpA), reactive arthritis or juvenile SpA (Juv-SpA) diagnoses by their rheumatologists, who were able to understand and complete questionnaires, were included.
Among the 4465 patients belonging to the ASAS-PerSpA study, we included in the present ancillary analysis 433 patients diagnosed with pSpA according to their rheumatologists (Fig. 1). The study was approved by the ethical committees in all countries (Supplementary Data S1, available at Rheumatology online) and written informed consent was obtained from participants prior to inclusion.
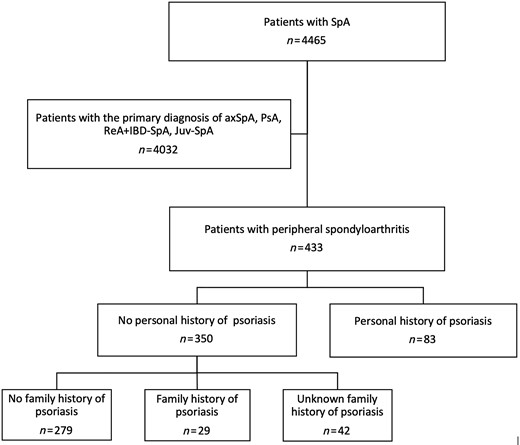
Flow-chart of the patient selection for the present analysis
AxSpA: axial spondyloarthritis; Juv-SpA: juvenile spondyloarthritis; ReA: reactive arthritis; SpA: spondyloarthritis.
Collected data
The following data were collected by the rheumatologist at each centre using a case report form during a single routine patient visit:
Demographic and clinical characteristics: age, sex, BMI (kg/m2), smoking (ever), alcohol intake (ever), level of education, marital status, employment status and country of residence. Symptom duration since symptom onset, diagnostic delay and first- or second-degree relatives with SpA (uveitis, IBD, reactive arthritis [ReA], psoriasis or ankylosing spondylitis) were collected.
Extra-musculoskeletal involvement: uveitis, IBD confirmed by endoscopy and psoriasis confirmed by a dermatologist.
Musculoskeletal involvement: included axial involvement, chronic back pain, HLA-B27 status, information concerning the presence of sacroiliitis on radiographs and MRI; peripheral articular disease (excluding root joints) ever, a monoarticular, oligoarticular or polyarticular pattern, the presence of objective signs of synovitis (i.e. physical examination by a rheumatologist or confirmed by ultrasonography) and localization; midfoot arthritis (tarsitis) ever as well as confirmation by specific investigations; ‘root-joint’ (i.e. shoulder and hip joints) involvement ever according to the rheumatologist; enthesitis ever confirmed by specific tests (i.e. sonography, radiographs, MRI or bone scintigraphy), localization and natural history (single episode, intermittent, continuous or progressive) and information about dactylitis ever and localization of dactylitis (fingers or toes) were collected. In addition, the presence of existing peripheral musculoskeletal findings (except dactylitis) was evaluated during the study visit based on physical examination.
Disease activity, function and patient-reported outcomes (PROs): current disease activity was measured at the single study visit by the BASDAI [4] and the Ankylosing Spondylitis Disease Activity Score-CRP (ASDAS-CRP) [5]. Moreover, the tender joint count (TJC), 66 swollen joint count (SJC) [6], Mander enthesitis index (MEI) [7], Leeds Enthesitis Index (LEI) [8] and Spondyloarthritis Research Consortium of Canada enthesitis score (SPARCC) [9] were assessed. While the BASFI was used to evaluate function [10], the ASAS Health Index (ASAS-HI) was used to evaluate health [11]. Patient Global Assessment of Well-being (PGA) (0–10) and Euro quality of life (QoL)-5D (EQ-5D) were collected [12]. In addition, the self-reported Fibromyalgia Rapid Screening Tool (FiRST) [13] was completed, and the presence of secondary fibromyalgia according to the rheumatologist’s opinion was collected.
Laboratory analysis: CRP and rheumatoid factor.
Treatments (ever and current): NSAIDs, oral and local corticosteroids, conventional synthetic DMARDs (csDMARDs) and bDMARDs.
Statistical analysis
In the main analysis, we performed a comparison of clinical features, disease burden and treatment modalities of pSpA patient groups with and without personal history of psoriasis that was defined as the presence of signs of psoriasis at physical examination or the presence of psoriatic nail dystrophy, including onycholysis, pitting and hyperkeratosis, or a personal history of psoriasis diagnosed by a physician. In addition, we compared pSpA presenting with personal (as defined above) or family history (first- or second-degree relatives) of psoriasis with patients without personal and family history of psoriasis.
Descriptive data are presented as the mean (s.d.) for continuous variables and as frequencies and percentages for categorical variables. Univariate pairwise comparisons were performed using the χ2/Fisher’s exact test for categorical variables or Mann–Whitney test for continuous variables. The Benjamini–Hochberg method was used to adjust for multiple comparisons [14].
Finally, we conducted a logistic regression analysis to identify factors independently associated with the presence of psoriasis in patients with pSpA. The following variables were selected for multivariable analysis based on their clinical relevance and identified differences in the univariable analysis: age, sex, HLA-B27 positivity, diagnostic delay, family history of SpA except psoriasis, treatment with bDMARDs, dactylitis, enthesitis, arthritis, CRP and PGA. Odds ratios and 95% CIs were calculated. Data were analysed using SPSS Statistics v.25 (IBM Corp., Armonk, NY, USA).
Results
Of 433 patients with pSpA, 83 patients (19.2%) had a personal history of psoriasis, and further 29 patients had a family history of psoriasis but no personal history (Fig. 1). Table 1 shows the sociodemographic and disease characteristics of the overall population and of the subgroups with and without a personal history of psoriasis.
Socio-demographics and clinical characteristics, disease activity and treatment of patients with peripheral spondyloarthritis stratified according to the presence or absence of personal history of psoriasis
| Characteristic . | Total (N = 433) . | Patients without personal history of psoriasis (N = 350) . | Patients with personal history of psoriasis (N = 83) . | P . | B–H Adj. P . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (s.d.), years | 44.2 (14.4) | 43.2 (14.2) | 48.4 (14.5) | 0.005 | 0.033 |
| Sex (men), n/N (%) | 203/433 (46.9) | 167/350 (47.7) | 36/83 (43.4) | 0.541 | 0.776 |
| BMI, mean (s.d.), kg/m2 | 26.3 (5.4) | 26.2 (5.2) | 27.0 (6.0) | 0.350 | 0.550 |
| Ever smoker, n/N (%) | 128/432 (29.6) | 99/350 (28.3) | 29/82 (35.4) | 0.227 | 0.428 |
| Region, n/N (%) | 0.820 | >0.999 | |||
| Latin America | 35/433 (8.1) | 30/350 (8.6) | 5/83 (6.0) | ||
| Europe and North America | 102/433 (23.5) | 82/350 (23.4) | 20/83 (24.1) | ||
| Asia | 138/433 (31.9) | 109/350 (31.1) | 29/83 (34.9) | ||
| Middle East and North Africa | 158/433 (36.5) | 129/350 (36.9) | 29/83 (34.9) | ||
| Symptom duration of SpA, mean (s.d.), years | 10.1 (9.5) | 9.0 (8.8) | 14.4 (10.8) | <0.001 | <0.001 |
| Diagnosis delay of SpA, mean (s.d.), years | 4.3 (6.6) | 3.5 (5.9) | 7.4 (8.4) | <0.001 | <0.001 |
| Fibromyalgia (rheumatologist’s opinion), n/N (%) | 48/433 (11.1) | 34/350 (9.7) | 14/83 (16.9) | 0.079 | 0.217 |
| First- or second-degree relatives with SpA except psoriasisa, n/N (%) | 74/433 (17.1) | 61/350 (17.4) | 13/83 (15.7) | 0.871 | >0.999 |
| First- or second-degree relatives with psoriasis, n/N (%) | 63/391 (16.1) | 29/308 (9.4) | 34/83 (41.0) | <0.001 | <0.001 |
| Patients who fulfilled peripheral ASAS criteria | 95/433 (21.9) | 74/350 (21.1) | 21/83 (25.3) | 0.461 | 0.692 |
| Patients who fulfilled CASPAR criteria | 81/433 (18.7) | 12/350 (3.4) | 69/83 (83.1) | <0.001 | <0.001 |
| Extramusculoskeletal involvement | |||||
| Uveitis ever, n/N (%) | 75/433 (17.3) | 64/350 (18.3) | 11/83 (13.3) | 0.334 | 0.538 |
| Number of uveitis ever, mean (s.d.) | 5.9 (7.7) | 6.1 (8.0) | 4.9 (5.6) | 0.867 | >0.999 |
| History of IBD confirmed by endoscopy, n/N (%) | 19/433 (4.4) | 14/350 (4.0) | 5/83 (6.0) | 0.289 | 0.502 |
| Urethritis or cervicitis or diarrhoea within 1 month before onset of arthritis/entesitis/dactylitis, n/N (%) | 28/433 (6.5) | 20/350 (5.7) | 8/83 (9.6) | 0.216 | 0.419 |
| Musculoskeletal involvement | |||||
| Peripheral articular disease (peripheral arthritis) | |||||
| Peripheral articular disease ever, n/N (%) | 410/433 (94.7) | 335/350 (95.7) | 75/83 (90.4) | 0.059 | 0.177 |
| Peripheral articular disease in the past confirmed by specific investigations, n/N (%) | 385/410 (88.9) | 313/335 (93.4) | 72/75 (96.0) | 0.594 | 0.800 |
| Any current tender joint, n/N (%) | 271/433 (62.6) | 220/350 (62.9) | 51/83 (61.4) | 0.802 | >0.999 |
| Tender joint count at current examination, mean (s.d.) | 3.3 (6.2) | 3.3 (6.2) | 3.5 (5.8) | 0.900 | >0.999 |
| Any current swollen joint, n/N (%) | 180/433 (41.6) | 146/350 (41.7) | 34/83 (41.0) | >0.999 | >0.999 |
| Swollen joint count at current examination, mean (s.d.) | 1.2 (2.9) | 1.1 (2.8) | 1.3 (3.2) | 0.885 | >0.999 |
| Localization of affected peripheral joints, n/N (%) | 0.282 | 0.503 | |||
| Predominantly lower limbs | 11/71 (15.5) | 6/50 (12.0) | 5/21 (23.8) | ||
| Predominantly hands | 60/71 (84.5) | 44/50 (88.0) | 16/21 (76.2) | ||
| Root jointb disease ever, n/N (%) | 192/433 (44.3) | 162/350 (46.3) | 30/83 (36.1) | 0.110 | 0.259 |
| Number of affected joints excluding root joints ever, mean (s.d.) | 8.3 (9.5) | 7.7 (8.4) | 10.7 (13.1) | 0.085 | 0.224 |
| Number of affected joints excluding root joints ever, n/N (%) | |||||
| Monoarthritis | 45/410 (11.0) | 42/335 (12.5) | 3/75 (4.0) | 0.039 | 0.135 |
| Oligoarthritis | 184/410 (44.9) | 148/335 (44.2) | 36/75 (48.0) | 0.608 | 0.803 |
| Polyarthritis | 181/410 (44.1) | 145/335 (43.3) | 36/75 (48.0) | 0.520 | 0.763 |
| Midfoot arthritis (tarsitis) ever, n/N (%) | 59/433 (13.6) | 52/350 (14.9) | 7/83 (8.4) | 0.155 | 0.330 |
| Radiographic evidence of juxta-articular new bone formation, n/N (%) | 42/433 (9.7) | 29/350 (8.3) | 13/83 (15.7) | 0.061 | 0.175 |
| Destructive arthropathy of the distal interphalangeal joints, n/N (%) | 27/433 (6.2) | 15/350 (4.3) | 12/83 (14.5) | 0.002 | 0.017 |
| Enthesitis | |||||
| Enthesitis ever, n/N (%) | 248/433 (57.3) | 194/350 (55.4) | 54/83 (65.1) | 0.138 | 0.304 |
| Any enthesitis in the past confirmed by specific investigations, n/N (%) | 112/433 (25.9) | 81/350 (23.1) | 31/83 (37.3) | 0.045 | 0.149 |
| Heel enthesitis, ever, n/N (%) | 233/433 (53.9) | 189/350 (54.2) | 44/83 (53.0) | 0.903 | >0.999 |
| Heel enthesitis, current, n/N (%) | 50/433 (11.5) | 35/350 (10) | 15/83 (18.1) | 0.054 | 0.170 |
| Current MEI score, mean (s.d.) | 2.4 (5.7) | 2.0 (4.2) | 4.0 (9.6) | 0.210 | 0.420 |
| Current SPARCC Enthesitis Index score, mean (s.d.) | 0.4 (1.1) | 0.3 (0.9) | 0.6 (1.6) | 0.013 | 0.061 |
| Current LEI score, mean (s.d.) | 0.2 (0.6) | 0.2 (0.6) | 0.3 (0.8) | 0.031 | 0.114 |
| Dactylitis | |||||
| Dactylitis ever, n/N (%) | 100/433 (23.1) | 70/350 (20.0) | 30/83 (36.1) | 0.003 | 0.022 |
| Localization of affected dactylitis, n/N (%) | 0.011 | 0.061 | |||
| Predominantly finger | 52/94 (55.3) | 32/68 (47.1) | 20/26 (76.9) | ||
| Predominantly toe | 42/94 (44.7) | 36/68 (52.9) | 6/26 (23.1) | ||
| Axial involvement | |||||
| Axial involvement ever according to the rheumatologist, n/N (%) | 238/433 (55.0) | 193/350 (55.1) | 45/83 (54.2) | 0.903 | >0.999 |
| Back pain, n/N (%) | 325/433 (75.1) | 263/350 (75.1) | 62/83 (74.7) | >0.999 | >0.999 |
| Back pain ≥3 months duration | 282/433 (65.1) | 229/350 (65.4) | 53/83 (63.9) | 0.799 | >0.999 |
| Back pain with age at onset <45 years | 261/433 (60.3) | 214/350 (61.1) | 47/83 (56.6) | 0.457 | 0.701 |
| Inflammatory back pain according to the ASAS definition | 240/433 (55.4) | 198/350 (56.6) | 42/83 (50.6) | 0.329 | 0.543 |
| Sacroiliitis on X-ray, n/N (%) | 146/433 (33.7) | 121/350 (34.6) | 25/83 (30.1) | 0.019 | 0.078 |
| Sacroiliitis on MRI, n/N (%) | 126/276 (45.7) | 102/226 (45.1) | 24/50 (48.0) | 0.755 | 0.977 |
| Laboratory assessment | |||||
| HLA-B27 positive, n/N (%) | 197/316 (62.3) | 179/269 (66.5) | 18/47 (38.3) | <0.001 | 0.005 |
| Rheumatoid factor positive, n/N (%) | 10/395 (2.5) | 4/317 (1.3) | 6/78 (7.7) | 0.005 | 0.030 |
| CRP, mean (s.d.), mg/l | 13.9 (25.4) | 15.2 (26.9) | 8.5 (16.5) | 0.019 | 0.074 |
| CRP ≥6 mg/l, n/N (%) | 208/433 (48.0) | 174/350 (49.7) | 34/83 (41.0) | 0.179 | 0.369 |
| Disease activity, function, PROs | |||||
| ASDAS-CRP, mean (s.d.) | 2.6 (1.2) | 2.7 (1.2) | 2.4 (1.1) | 0.107 | 0.262 |
| BASDAI, mean (s.d.) | 4.0 (2.4) | 4.0 (2.4) | 3.9 (2.3) | 0.592 | 0.814 |
| PGA, mean (s.d.) | 4.5 (2.7) | 4.7 (2.7) | 3.9 (2.5) | 0.018 | 0.079 |
| BASFI, mean (s.d.) | 2.8 (2.6) | 2.8 (2.6) | 2.6 (2.5) | 0.278 | 0.510 |
| ASAS-HI, mean (s.d.) | 6.6 (4.4) | 6.7 (4.5) | 6.3 (4.2) | 0.569 | 0.799 |
| EQ-5D, mean (s.d.) | 0.6 (0.2) | 0.6 (0.2) | 0.7 (0.2) | 0.129 | 0.294 |
| Fibromyalgia (according to FiRST score), n/N (%) | 69/391 (17.6) | 56/312 (17.9) | 13/79 (16.5) | 0.869 | >0.999 |
| Treatment | |||||
| NSAIDs, n/N (%) | 421/421 (100.0) | 339/339 (100.0) | 82/82 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids ever, n/N (%) | 212/213 (99.5) | 179/180 (99.4) | 33/33 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids current, n/N (%) | 89/433 (20.6) | 76/350 (21.7) | 13/83 (15.7) | 0.290 | 0.491 |
| Local injection of glucocorticoids for peripheral musculoskeletal involvement ever, n/N (%) | 183/193 (94.8) | 156/159 (98.1) | 27/34 (79.4) | <0.001 | 0.003 |
| csDMARDs ever, n/N (%) | 384/433 (88.7) | 310/350 (88.6) | 74/83 (89.2) | >0.999 | >0.999 |
| csDMARDs current, n/N (%) | 230/433 (53.1) | 192/350 (54.9) | 38/83 (45.8) | 0.086 | 0.218 |
| bDMARDs ever, n/N (%) | 223/433 (51.5) | 164/350 (46.9) | 59/83 (71.1) | <0.001 | 0.001 |
| bDMARDs current, n/N (%) | 160/433 (37.0) | 119/350 (34.0) | 41/83 (49.4) | 0.011 | 0.056 |
| Characteristic . | Total (N = 433) . | Patients without personal history of psoriasis (N = 350) . | Patients with personal history of psoriasis (N = 83) . | P . | B–H Adj. P . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (s.d.), years | 44.2 (14.4) | 43.2 (14.2) | 48.4 (14.5) | 0.005 | 0.033 |
| Sex (men), n/N (%) | 203/433 (46.9) | 167/350 (47.7) | 36/83 (43.4) | 0.541 | 0.776 |
| BMI, mean (s.d.), kg/m2 | 26.3 (5.4) | 26.2 (5.2) | 27.0 (6.0) | 0.350 | 0.550 |
| Ever smoker, n/N (%) | 128/432 (29.6) | 99/350 (28.3) | 29/82 (35.4) | 0.227 | 0.428 |
| Region, n/N (%) | 0.820 | >0.999 | |||
| Latin America | 35/433 (8.1) | 30/350 (8.6) | 5/83 (6.0) | ||
| Europe and North America | 102/433 (23.5) | 82/350 (23.4) | 20/83 (24.1) | ||
| Asia | 138/433 (31.9) | 109/350 (31.1) | 29/83 (34.9) | ||
| Middle East and North Africa | 158/433 (36.5) | 129/350 (36.9) | 29/83 (34.9) | ||
| Symptom duration of SpA, mean (s.d.), years | 10.1 (9.5) | 9.0 (8.8) | 14.4 (10.8) | <0.001 | <0.001 |
| Diagnosis delay of SpA, mean (s.d.), years | 4.3 (6.6) | 3.5 (5.9) | 7.4 (8.4) | <0.001 | <0.001 |
| Fibromyalgia (rheumatologist’s opinion), n/N (%) | 48/433 (11.1) | 34/350 (9.7) | 14/83 (16.9) | 0.079 | 0.217 |
| First- or second-degree relatives with SpA except psoriasisa, n/N (%) | 74/433 (17.1) | 61/350 (17.4) | 13/83 (15.7) | 0.871 | >0.999 |
| First- or second-degree relatives with psoriasis, n/N (%) | 63/391 (16.1) | 29/308 (9.4) | 34/83 (41.0) | <0.001 | <0.001 |
| Patients who fulfilled peripheral ASAS criteria | 95/433 (21.9) | 74/350 (21.1) | 21/83 (25.3) | 0.461 | 0.692 |
| Patients who fulfilled CASPAR criteria | 81/433 (18.7) | 12/350 (3.4) | 69/83 (83.1) | <0.001 | <0.001 |
| Extramusculoskeletal involvement | |||||
| Uveitis ever, n/N (%) | 75/433 (17.3) | 64/350 (18.3) | 11/83 (13.3) | 0.334 | 0.538 |
| Number of uveitis ever, mean (s.d.) | 5.9 (7.7) | 6.1 (8.0) | 4.9 (5.6) | 0.867 | >0.999 |
| History of IBD confirmed by endoscopy, n/N (%) | 19/433 (4.4) | 14/350 (4.0) | 5/83 (6.0) | 0.289 | 0.502 |
| Urethritis or cervicitis or diarrhoea within 1 month before onset of arthritis/entesitis/dactylitis, n/N (%) | 28/433 (6.5) | 20/350 (5.7) | 8/83 (9.6) | 0.216 | 0.419 |
| Musculoskeletal involvement | |||||
| Peripheral articular disease (peripheral arthritis) | |||||
| Peripheral articular disease ever, n/N (%) | 410/433 (94.7) | 335/350 (95.7) | 75/83 (90.4) | 0.059 | 0.177 |
| Peripheral articular disease in the past confirmed by specific investigations, n/N (%) | 385/410 (88.9) | 313/335 (93.4) | 72/75 (96.0) | 0.594 | 0.800 |
| Any current tender joint, n/N (%) | 271/433 (62.6) | 220/350 (62.9) | 51/83 (61.4) | 0.802 | >0.999 |
| Tender joint count at current examination, mean (s.d.) | 3.3 (6.2) | 3.3 (6.2) | 3.5 (5.8) | 0.900 | >0.999 |
| Any current swollen joint, n/N (%) | 180/433 (41.6) | 146/350 (41.7) | 34/83 (41.0) | >0.999 | >0.999 |
| Swollen joint count at current examination, mean (s.d.) | 1.2 (2.9) | 1.1 (2.8) | 1.3 (3.2) | 0.885 | >0.999 |
| Localization of affected peripheral joints, n/N (%) | 0.282 | 0.503 | |||
| Predominantly lower limbs | 11/71 (15.5) | 6/50 (12.0) | 5/21 (23.8) | ||
| Predominantly hands | 60/71 (84.5) | 44/50 (88.0) | 16/21 (76.2) | ||
| Root jointb disease ever, n/N (%) | 192/433 (44.3) | 162/350 (46.3) | 30/83 (36.1) | 0.110 | 0.259 |
| Number of affected joints excluding root joints ever, mean (s.d.) | 8.3 (9.5) | 7.7 (8.4) | 10.7 (13.1) | 0.085 | 0.224 |
| Number of affected joints excluding root joints ever, n/N (%) | |||||
| Monoarthritis | 45/410 (11.0) | 42/335 (12.5) | 3/75 (4.0) | 0.039 | 0.135 |
| Oligoarthritis | 184/410 (44.9) | 148/335 (44.2) | 36/75 (48.0) | 0.608 | 0.803 |
| Polyarthritis | 181/410 (44.1) | 145/335 (43.3) | 36/75 (48.0) | 0.520 | 0.763 |
| Midfoot arthritis (tarsitis) ever, n/N (%) | 59/433 (13.6) | 52/350 (14.9) | 7/83 (8.4) | 0.155 | 0.330 |
| Radiographic evidence of juxta-articular new bone formation, n/N (%) | 42/433 (9.7) | 29/350 (8.3) | 13/83 (15.7) | 0.061 | 0.175 |
| Destructive arthropathy of the distal interphalangeal joints, n/N (%) | 27/433 (6.2) | 15/350 (4.3) | 12/83 (14.5) | 0.002 | 0.017 |
| Enthesitis | |||||
| Enthesitis ever, n/N (%) | 248/433 (57.3) | 194/350 (55.4) | 54/83 (65.1) | 0.138 | 0.304 |
| Any enthesitis in the past confirmed by specific investigations, n/N (%) | 112/433 (25.9) | 81/350 (23.1) | 31/83 (37.3) | 0.045 | 0.149 |
| Heel enthesitis, ever, n/N (%) | 233/433 (53.9) | 189/350 (54.2) | 44/83 (53.0) | 0.903 | >0.999 |
| Heel enthesitis, current, n/N (%) | 50/433 (11.5) | 35/350 (10) | 15/83 (18.1) | 0.054 | 0.170 |
| Current MEI score, mean (s.d.) | 2.4 (5.7) | 2.0 (4.2) | 4.0 (9.6) | 0.210 | 0.420 |
| Current SPARCC Enthesitis Index score, mean (s.d.) | 0.4 (1.1) | 0.3 (0.9) | 0.6 (1.6) | 0.013 | 0.061 |
| Current LEI score, mean (s.d.) | 0.2 (0.6) | 0.2 (0.6) | 0.3 (0.8) | 0.031 | 0.114 |
| Dactylitis | |||||
| Dactylitis ever, n/N (%) | 100/433 (23.1) | 70/350 (20.0) | 30/83 (36.1) | 0.003 | 0.022 |
| Localization of affected dactylitis, n/N (%) | 0.011 | 0.061 | |||
| Predominantly finger | 52/94 (55.3) | 32/68 (47.1) | 20/26 (76.9) | ||
| Predominantly toe | 42/94 (44.7) | 36/68 (52.9) | 6/26 (23.1) | ||
| Axial involvement | |||||
| Axial involvement ever according to the rheumatologist, n/N (%) | 238/433 (55.0) | 193/350 (55.1) | 45/83 (54.2) | 0.903 | >0.999 |
| Back pain, n/N (%) | 325/433 (75.1) | 263/350 (75.1) | 62/83 (74.7) | >0.999 | >0.999 |
| Back pain ≥3 months duration | 282/433 (65.1) | 229/350 (65.4) | 53/83 (63.9) | 0.799 | >0.999 |
| Back pain with age at onset <45 years | 261/433 (60.3) | 214/350 (61.1) | 47/83 (56.6) | 0.457 | 0.701 |
| Inflammatory back pain according to the ASAS definition | 240/433 (55.4) | 198/350 (56.6) | 42/83 (50.6) | 0.329 | 0.543 |
| Sacroiliitis on X-ray, n/N (%) | 146/433 (33.7) | 121/350 (34.6) | 25/83 (30.1) | 0.019 | 0.078 |
| Sacroiliitis on MRI, n/N (%) | 126/276 (45.7) | 102/226 (45.1) | 24/50 (48.0) | 0.755 | 0.977 |
| Laboratory assessment | |||||
| HLA-B27 positive, n/N (%) | 197/316 (62.3) | 179/269 (66.5) | 18/47 (38.3) | <0.001 | 0.005 |
| Rheumatoid factor positive, n/N (%) | 10/395 (2.5) | 4/317 (1.3) | 6/78 (7.7) | 0.005 | 0.030 |
| CRP, mean (s.d.), mg/l | 13.9 (25.4) | 15.2 (26.9) | 8.5 (16.5) | 0.019 | 0.074 |
| CRP ≥6 mg/l, n/N (%) | 208/433 (48.0) | 174/350 (49.7) | 34/83 (41.0) | 0.179 | 0.369 |
| Disease activity, function, PROs | |||||
| ASDAS-CRP, mean (s.d.) | 2.6 (1.2) | 2.7 (1.2) | 2.4 (1.1) | 0.107 | 0.262 |
| BASDAI, mean (s.d.) | 4.0 (2.4) | 4.0 (2.4) | 3.9 (2.3) | 0.592 | 0.814 |
| PGA, mean (s.d.) | 4.5 (2.7) | 4.7 (2.7) | 3.9 (2.5) | 0.018 | 0.079 |
| BASFI, mean (s.d.) | 2.8 (2.6) | 2.8 (2.6) | 2.6 (2.5) | 0.278 | 0.510 |
| ASAS-HI, mean (s.d.) | 6.6 (4.4) | 6.7 (4.5) | 6.3 (4.2) | 0.569 | 0.799 |
| EQ-5D, mean (s.d.) | 0.6 (0.2) | 0.6 (0.2) | 0.7 (0.2) | 0.129 | 0.294 |
| Fibromyalgia (according to FiRST score), n/N (%) | 69/391 (17.6) | 56/312 (17.9) | 13/79 (16.5) | 0.869 | >0.999 |
| Treatment | |||||
| NSAIDs, n/N (%) | 421/421 (100.0) | 339/339 (100.0) | 82/82 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids ever, n/N (%) | 212/213 (99.5) | 179/180 (99.4) | 33/33 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids current, n/N (%) | 89/433 (20.6) | 76/350 (21.7) | 13/83 (15.7) | 0.290 | 0.491 |
| Local injection of glucocorticoids for peripheral musculoskeletal involvement ever, n/N (%) | 183/193 (94.8) | 156/159 (98.1) | 27/34 (79.4) | <0.001 | 0.003 |
| csDMARDs ever, n/N (%) | 384/433 (88.7) | 310/350 (88.6) | 74/83 (89.2) | >0.999 | >0.999 |
| csDMARDs current, n/N (%) | 230/433 (53.1) | 192/350 (54.9) | 38/83 (45.8) | 0.086 | 0.218 |
| bDMARDs ever, n/N (%) | 223/433 (51.5) | 164/350 (46.9) | 59/83 (71.1) | <0.001 | 0.001 |
| bDMARDs current, n/N (%) | 160/433 (37.0) | 119/350 (34.0) | 41/83 (49.4) | 0.011 | 0.056 |
All results are presented as mean and s.d. and percentages for continuous and categorical variables, respectively.
First-degree or second-degree relatives with ankylosing spondylitis, uveitis, reactive arthritis or IBD.
Shoulder and hip joints. ASAS: Assessment of SpondyloArthritis international Society; ASAS-HI: ASAS Health Index; ASDAS: Ankylosing Spondylitis Disease Activity Score; bDMARD: biologic DMARD; B–H Adj. P: the Benjamini–Hochberg adjusted P-value; CASPAR: Classification Criteria for Psoriatic Arthritis; csDMARD: conventional synthetic DMARD; EQ-5D: Euro quality of life (QoL)-5D; FiRST: Fibromyalgia Rapid Screening Tool; HLA-B27: human leucocyte antigen B27; LEI: Leeds Enthesitis Index; MEI: Mander Enthesitis Index; PGA: patient’s global assessment; PRO: patient-reported outcome; SpA: Spondyloarthritis; SPARCC Enthesitis Index: Spondyloarthritis Research Consortium of Canada Enthesitis Index.
Socio-demographics and clinical characteristics, disease activity and treatment of patients with peripheral spondyloarthritis stratified according to the presence or absence of personal history of psoriasis
| Characteristic . | Total (N = 433) . | Patients without personal history of psoriasis (N = 350) . | Patients with personal history of psoriasis (N = 83) . | P . | B–H Adj. P . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (s.d.), years | 44.2 (14.4) | 43.2 (14.2) | 48.4 (14.5) | 0.005 | 0.033 |
| Sex (men), n/N (%) | 203/433 (46.9) | 167/350 (47.7) | 36/83 (43.4) | 0.541 | 0.776 |
| BMI, mean (s.d.), kg/m2 | 26.3 (5.4) | 26.2 (5.2) | 27.0 (6.0) | 0.350 | 0.550 |
| Ever smoker, n/N (%) | 128/432 (29.6) | 99/350 (28.3) | 29/82 (35.4) | 0.227 | 0.428 |
| Region, n/N (%) | 0.820 | >0.999 | |||
| Latin America | 35/433 (8.1) | 30/350 (8.6) | 5/83 (6.0) | ||
| Europe and North America | 102/433 (23.5) | 82/350 (23.4) | 20/83 (24.1) | ||
| Asia | 138/433 (31.9) | 109/350 (31.1) | 29/83 (34.9) | ||
| Middle East and North Africa | 158/433 (36.5) | 129/350 (36.9) | 29/83 (34.9) | ||
| Symptom duration of SpA, mean (s.d.), years | 10.1 (9.5) | 9.0 (8.8) | 14.4 (10.8) | <0.001 | <0.001 |
| Diagnosis delay of SpA, mean (s.d.), years | 4.3 (6.6) | 3.5 (5.9) | 7.4 (8.4) | <0.001 | <0.001 |
| Fibromyalgia (rheumatologist’s opinion), n/N (%) | 48/433 (11.1) | 34/350 (9.7) | 14/83 (16.9) | 0.079 | 0.217 |
| First- or second-degree relatives with SpA except psoriasisa, n/N (%) | 74/433 (17.1) | 61/350 (17.4) | 13/83 (15.7) | 0.871 | >0.999 |
| First- or second-degree relatives with psoriasis, n/N (%) | 63/391 (16.1) | 29/308 (9.4) | 34/83 (41.0) | <0.001 | <0.001 |
| Patients who fulfilled peripheral ASAS criteria | 95/433 (21.9) | 74/350 (21.1) | 21/83 (25.3) | 0.461 | 0.692 |
| Patients who fulfilled CASPAR criteria | 81/433 (18.7) | 12/350 (3.4) | 69/83 (83.1) | <0.001 | <0.001 |
| Extramusculoskeletal involvement | |||||
| Uveitis ever, n/N (%) | 75/433 (17.3) | 64/350 (18.3) | 11/83 (13.3) | 0.334 | 0.538 |
| Number of uveitis ever, mean (s.d.) | 5.9 (7.7) | 6.1 (8.0) | 4.9 (5.6) | 0.867 | >0.999 |
| History of IBD confirmed by endoscopy, n/N (%) | 19/433 (4.4) | 14/350 (4.0) | 5/83 (6.0) | 0.289 | 0.502 |
| Urethritis or cervicitis or diarrhoea within 1 month before onset of arthritis/entesitis/dactylitis, n/N (%) | 28/433 (6.5) | 20/350 (5.7) | 8/83 (9.6) | 0.216 | 0.419 |
| Musculoskeletal involvement | |||||
| Peripheral articular disease (peripheral arthritis) | |||||
| Peripheral articular disease ever, n/N (%) | 410/433 (94.7) | 335/350 (95.7) | 75/83 (90.4) | 0.059 | 0.177 |
| Peripheral articular disease in the past confirmed by specific investigations, n/N (%) | 385/410 (88.9) | 313/335 (93.4) | 72/75 (96.0) | 0.594 | 0.800 |
| Any current tender joint, n/N (%) | 271/433 (62.6) | 220/350 (62.9) | 51/83 (61.4) | 0.802 | >0.999 |
| Tender joint count at current examination, mean (s.d.) | 3.3 (6.2) | 3.3 (6.2) | 3.5 (5.8) | 0.900 | >0.999 |
| Any current swollen joint, n/N (%) | 180/433 (41.6) | 146/350 (41.7) | 34/83 (41.0) | >0.999 | >0.999 |
| Swollen joint count at current examination, mean (s.d.) | 1.2 (2.9) | 1.1 (2.8) | 1.3 (3.2) | 0.885 | >0.999 |
| Localization of affected peripheral joints, n/N (%) | 0.282 | 0.503 | |||
| Predominantly lower limbs | 11/71 (15.5) | 6/50 (12.0) | 5/21 (23.8) | ||
| Predominantly hands | 60/71 (84.5) | 44/50 (88.0) | 16/21 (76.2) | ||
| Root jointb disease ever, n/N (%) | 192/433 (44.3) | 162/350 (46.3) | 30/83 (36.1) | 0.110 | 0.259 |
| Number of affected joints excluding root joints ever, mean (s.d.) | 8.3 (9.5) | 7.7 (8.4) | 10.7 (13.1) | 0.085 | 0.224 |
| Number of affected joints excluding root joints ever, n/N (%) | |||||
| Monoarthritis | 45/410 (11.0) | 42/335 (12.5) | 3/75 (4.0) | 0.039 | 0.135 |
| Oligoarthritis | 184/410 (44.9) | 148/335 (44.2) | 36/75 (48.0) | 0.608 | 0.803 |
| Polyarthritis | 181/410 (44.1) | 145/335 (43.3) | 36/75 (48.0) | 0.520 | 0.763 |
| Midfoot arthritis (tarsitis) ever, n/N (%) | 59/433 (13.6) | 52/350 (14.9) | 7/83 (8.4) | 0.155 | 0.330 |
| Radiographic evidence of juxta-articular new bone formation, n/N (%) | 42/433 (9.7) | 29/350 (8.3) | 13/83 (15.7) | 0.061 | 0.175 |
| Destructive arthropathy of the distal interphalangeal joints, n/N (%) | 27/433 (6.2) | 15/350 (4.3) | 12/83 (14.5) | 0.002 | 0.017 |
| Enthesitis | |||||
| Enthesitis ever, n/N (%) | 248/433 (57.3) | 194/350 (55.4) | 54/83 (65.1) | 0.138 | 0.304 |
| Any enthesitis in the past confirmed by specific investigations, n/N (%) | 112/433 (25.9) | 81/350 (23.1) | 31/83 (37.3) | 0.045 | 0.149 |
| Heel enthesitis, ever, n/N (%) | 233/433 (53.9) | 189/350 (54.2) | 44/83 (53.0) | 0.903 | >0.999 |
| Heel enthesitis, current, n/N (%) | 50/433 (11.5) | 35/350 (10) | 15/83 (18.1) | 0.054 | 0.170 |
| Current MEI score, mean (s.d.) | 2.4 (5.7) | 2.0 (4.2) | 4.0 (9.6) | 0.210 | 0.420 |
| Current SPARCC Enthesitis Index score, mean (s.d.) | 0.4 (1.1) | 0.3 (0.9) | 0.6 (1.6) | 0.013 | 0.061 |
| Current LEI score, mean (s.d.) | 0.2 (0.6) | 0.2 (0.6) | 0.3 (0.8) | 0.031 | 0.114 |
| Dactylitis | |||||
| Dactylitis ever, n/N (%) | 100/433 (23.1) | 70/350 (20.0) | 30/83 (36.1) | 0.003 | 0.022 |
| Localization of affected dactylitis, n/N (%) | 0.011 | 0.061 | |||
| Predominantly finger | 52/94 (55.3) | 32/68 (47.1) | 20/26 (76.9) | ||
| Predominantly toe | 42/94 (44.7) | 36/68 (52.9) | 6/26 (23.1) | ||
| Axial involvement | |||||
| Axial involvement ever according to the rheumatologist, n/N (%) | 238/433 (55.0) | 193/350 (55.1) | 45/83 (54.2) | 0.903 | >0.999 |
| Back pain, n/N (%) | 325/433 (75.1) | 263/350 (75.1) | 62/83 (74.7) | >0.999 | >0.999 |
| Back pain ≥3 months duration | 282/433 (65.1) | 229/350 (65.4) | 53/83 (63.9) | 0.799 | >0.999 |
| Back pain with age at onset <45 years | 261/433 (60.3) | 214/350 (61.1) | 47/83 (56.6) | 0.457 | 0.701 |
| Inflammatory back pain according to the ASAS definition | 240/433 (55.4) | 198/350 (56.6) | 42/83 (50.6) | 0.329 | 0.543 |
| Sacroiliitis on X-ray, n/N (%) | 146/433 (33.7) | 121/350 (34.6) | 25/83 (30.1) | 0.019 | 0.078 |
| Sacroiliitis on MRI, n/N (%) | 126/276 (45.7) | 102/226 (45.1) | 24/50 (48.0) | 0.755 | 0.977 |
| Laboratory assessment | |||||
| HLA-B27 positive, n/N (%) | 197/316 (62.3) | 179/269 (66.5) | 18/47 (38.3) | <0.001 | 0.005 |
| Rheumatoid factor positive, n/N (%) | 10/395 (2.5) | 4/317 (1.3) | 6/78 (7.7) | 0.005 | 0.030 |
| CRP, mean (s.d.), mg/l | 13.9 (25.4) | 15.2 (26.9) | 8.5 (16.5) | 0.019 | 0.074 |
| CRP ≥6 mg/l, n/N (%) | 208/433 (48.0) | 174/350 (49.7) | 34/83 (41.0) | 0.179 | 0.369 |
| Disease activity, function, PROs | |||||
| ASDAS-CRP, mean (s.d.) | 2.6 (1.2) | 2.7 (1.2) | 2.4 (1.1) | 0.107 | 0.262 |
| BASDAI, mean (s.d.) | 4.0 (2.4) | 4.0 (2.4) | 3.9 (2.3) | 0.592 | 0.814 |
| PGA, mean (s.d.) | 4.5 (2.7) | 4.7 (2.7) | 3.9 (2.5) | 0.018 | 0.079 |
| BASFI, mean (s.d.) | 2.8 (2.6) | 2.8 (2.6) | 2.6 (2.5) | 0.278 | 0.510 |
| ASAS-HI, mean (s.d.) | 6.6 (4.4) | 6.7 (4.5) | 6.3 (4.2) | 0.569 | 0.799 |
| EQ-5D, mean (s.d.) | 0.6 (0.2) | 0.6 (0.2) | 0.7 (0.2) | 0.129 | 0.294 |
| Fibromyalgia (according to FiRST score), n/N (%) | 69/391 (17.6) | 56/312 (17.9) | 13/79 (16.5) | 0.869 | >0.999 |
| Treatment | |||||
| NSAIDs, n/N (%) | 421/421 (100.0) | 339/339 (100.0) | 82/82 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids ever, n/N (%) | 212/213 (99.5) | 179/180 (99.4) | 33/33 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids current, n/N (%) | 89/433 (20.6) | 76/350 (21.7) | 13/83 (15.7) | 0.290 | 0.491 |
| Local injection of glucocorticoids for peripheral musculoskeletal involvement ever, n/N (%) | 183/193 (94.8) | 156/159 (98.1) | 27/34 (79.4) | <0.001 | 0.003 |
| csDMARDs ever, n/N (%) | 384/433 (88.7) | 310/350 (88.6) | 74/83 (89.2) | >0.999 | >0.999 |
| csDMARDs current, n/N (%) | 230/433 (53.1) | 192/350 (54.9) | 38/83 (45.8) | 0.086 | 0.218 |
| bDMARDs ever, n/N (%) | 223/433 (51.5) | 164/350 (46.9) | 59/83 (71.1) | <0.001 | 0.001 |
| bDMARDs current, n/N (%) | 160/433 (37.0) | 119/350 (34.0) | 41/83 (49.4) | 0.011 | 0.056 |
| Characteristic . | Total (N = 433) . | Patients without personal history of psoriasis (N = 350) . | Patients with personal history of psoriasis (N = 83) . | P . | B–H Adj. P . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (s.d.), years | 44.2 (14.4) | 43.2 (14.2) | 48.4 (14.5) | 0.005 | 0.033 |
| Sex (men), n/N (%) | 203/433 (46.9) | 167/350 (47.7) | 36/83 (43.4) | 0.541 | 0.776 |
| BMI, mean (s.d.), kg/m2 | 26.3 (5.4) | 26.2 (5.2) | 27.0 (6.0) | 0.350 | 0.550 |
| Ever smoker, n/N (%) | 128/432 (29.6) | 99/350 (28.3) | 29/82 (35.4) | 0.227 | 0.428 |
| Region, n/N (%) | 0.820 | >0.999 | |||
| Latin America | 35/433 (8.1) | 30/350 (8.6) | 5/83 (6.0) | ||
| Europe and North America | 102/433 (23.5) | 82/350 (23.4) | 20/83 (24.1) | ||
| Asia | 138/433 (31.9) | 109/350 (31.1) | 29/83 (34.9) | ||
| Middle East and North Africa | 158/433 (36.5) | 129/350 (36.9) | 29/83 (34.9) | ||
| Symptom duration of SpA, mean (s.d.), years | 10.1 (9.5) | 9.0 (8.8) | 14.4 (10.8) | <0.001 | <0.001 |
| Diagnosis delay of SpA, mean (s.d.), years | 4.3 (6.6) | 3.5 (5.9) | 7.4 (8.4) | <0.001 | <0.001 |
| Fibromyalgia (rheumatologist’s opinion), n/N (%) | 48/433 (11.1) | 34/350 (9.7) | 14/83 (16.9) | 0.079 | 0.217 |
| First- or second-degree relatives with SpA except psoriasisa, n/N (%) | 74/433 (17.1) | 61/350 (17.4) | 13/83 (15.7) | 0.871 | >0.999 |
| First- or second-degree relatives with psoriasis, n/N (%) | 63/391 (16.1) | 29/308 (9.4) | 34/83 (41.0) | <0.001 | <0.001 |
| Patients who fulfilled peripheral ASAS criteria | 95/433 (21.9) | 74/350 (21.1) | 21/83 (25.3) | 0.461 | 0.692 |
| Patients who fulfilled CASPAR criteria | 81/433 (18.7) | 12/350 (3.4) | 69/83 (83.1) | <0.001 | <0.001 |
| Extramusculoskeletal involvement | |||||
| Uveitis ever, n/N (%) | 75/433 (17.3) | 64/350 (18.3) | 11/83 (13.3) | 0.334 | 0.538 |
| Number of uveitis ever, mean (s.d.) | 5.9 (7.7) | 6.1 (8.0) | 4.9 (5.6) | 0.867 | >0.999 |
| History of IBD confirmed by endoscopy, n/N (%) | 19/433 (4.4) | 14/350 (4.0) | 5/83 (6.0) | 0.289 | 0.502 |
| Urethritis or cervicitis or diarrhoea within 1 month before onset of arthritis/entesitis/dactylitis, n/N (%) | 28/433 (6.5) | 20/350 (5.7) | 8/83 (9.6) | 0.216 | 0.419 |
| Musculoskeletal involvement | |||||
| Peripheral articular disease (peripheral arthritis) | |||||
| Peripheral articular disease ever, n/N (%) | 410/433 (94.7) | 335/350 (95.7) | 75/83 (90.4) | 0.059 | 0.177 |
| Peripheral articular disease in the past confirmed by specific investigations, n/N (%) | 385/410 (88.9) | 313/335 (93.4) | 72/75 (96.0) | 0.594 | 0.800 |
| Any current tender joint, n/N (%) | 271/433 (62.6) | 220/350 (62.9) | 51/83 (61.4) | 0.802 | >0.999 |
| Tender joint count at current examination, mean (s.d.) | 3.3 (6.2) | 3.3 (6.2) | 3.5 (5.8) | 0.900 | >0.999 |
| Any current swollen joint, n/N (%) | 180/433 (41.6) | 146/350 (41.7) | 34/83 (41.0) | >0.999 | >0.999 |
| Swollen joint count at current examination, mean (s.d.) | 1.2 (2.9) | 1.1 (2.8) | 1.3 (3.2) | 0.885 | >0.999 |
| Localization of affected peripheral joints, n/N (%) | 0.282 | 0.503 | |||
| Predominantly lower limbs | 11/71 (15.5) | 6/50 (12.0) | 5/21 (23.8) | ||
| Predominantly hands | 60/71 (84.5) | 44/50 (88.0) | 16/21 (76.2) | ||
| Root jointb disease ever, n/N (%) | 192/433 (44.3) | 162/350 (46.3) | 30/83 (36.1) | 0.110 | 0.259 |
| Number of affected joints excluding root joints ever, mean (s.d.) | 8.3 (9.5) | 7.7 (8.4) | 10.7 (13.1) | 0.085 | 0.224 |
| Number of affected joints excluding root joints ever, n/N (%) | |||||
| Monoarthritis | 45/410 (11.0) | 42/335 (12.5) | 3/75 (4.0) | 0.039 | 0.135 |
| Oligoarthritis | 184/410 (44.9) | 148/335 (44.2) | 36/75 (48.0) | 0.608 | 0.803 |
| Polyarthritis | 181/410 (44.1) | 145/335 (43.3) | 36/75 (48.0) | 0.520 | 0.763 |
| Midfoot arthritis (tarsitis) ever, n/N (%) | 59/433 (13.6) | 52/350 (14.9) | 7/83 (8.4) | 0.155 | 0.330 |
| Radiographic evidence of juxta-articular new bone formation, n/N (%) | 42/433 (9.7) | 29/350 (8.3) | 13/83 (15.7) | 0.061 | 0.175 |
| Destructive arthropathy of the distal interphalangeal joints, n/N (%) | 27/433 (6.2) | 15/350 (4.3) | 12/83 (14.5) | 0.002 | 0.017 |
| Enthesitis | |||||
| Enthesitis ever, n/N (%) | 248/433 (57.3) | 194/350 (55.4) | 54/83 (65.1) | 0.138 | 0.304 |
| Any enthesitis in the past confirmed by specific investigations, n/N (%) | 112/433 (25.9) | 81/350 (23.1) | 31/83 (37.3) | 0.045 | 0.149 |
| Heel enthesitis, ever, n/N (%) | 233/433 (53.9) | 189/350 (54.2) | 44/83 (53.0) | 0.903 | >0.999 |
| Heel enthesitis, current, n/N (%) | 50/433 (11.5) | 35/350 (10) | 15/83 (18.1) | 0.054 | 0.170 |
| Current MEI score, mean (s.d.) | 2.4 (5.7) | 2.0 (4.2) | 4.0 (9.6) | 0.210 | 0.420 |
| Current SPARCC Enthesitis Index score, mean (s.d.) | 0.4 (1.1) | 0.3 (0.9) | 0.6 (1.6) | 0.013 | 0.061 |
| Current LEI score, mean (s.d.) | 0.2 (0.6) | 0.2 (0.6) | 0.3 (0.8) | 0.031 | 0.114 |
| Dactylitis | |||||
| Dactylitis ever, n/N (%) | 100/433 (23.1) | 70/350 (20.0) | 30/83 (36.1) | 0.003 | 0.022 |
| Localization of affected dactylitis, n/N (%) | 0.011 | 0.061 | |||
| Predominantly finger | 52/94 (55.3) | 32/68 (47.1) | 20/26 (76.9) | ||
| Predominantly toe | 42/94 (44.7) | 36/68 (52.9) | 6/26 (23.1) | ||
| Axial involvement | |||||
| Axial involvement ever according to the rheumatologist, n/N (%) | 238/433 (55.0) | 193/350 (55.1) | 45/83 (54.2) | 0.903 | >0.999 |
| Back pain, n/N (%) | 325/433 (75.1) | 263/350 (75.1) | 62/83 (74.7) | >0.999 | >0.999 |
| Back pain ≥3 months duration | 282/433 (65.1) | 229/350 (65.4) | 53/83 (63.9) | 0.799 | >0.999 |
| Back pain with age at onset <45 years | 261/433 (60.3) | 214/350 (61.1) | 47/83 (56.6) | 0.457 | 0.701 |
| Inflammatory back pain according to the ASAS definition | 240/433 (55.4) | 198/350 (56.6) | 42/83 (50.6) | 0.329 | 0.543 |
| Sacroiliitis on X-ray, n/N (%) | 146/433 (33.7) | 121/350 (34.6) | 25/83 (30.1) | 0.019 | 0.078 |
| Sacroiliitis on MRI, n/N (%) | 126/276 (45.7) | 102/226 (45.1) | 24/50 (48.0) | 0.755 | 0.977 |
| Laboratory assessment | |||||
| HLA-B27 positive, n/N (%) | 197/316 (62.3) | 179/269 (66.5) | 18/47 (38.3) | <0.001 | 0.005 |
| Rheumatoid factor positive, n/N (%) | 10/395 (2.5) | 4/317 (1.3) | 6/78 (7.7) | 0.005 | 0.030 |
| CRP, mean (s.d.), mg/l | 13.9 (25.4) | 15.2 (26.9) | 8.5 (16.5) | 0.019 | 0.074 |
| CRP ≥6 mg/l, n/N (%) | 208/433 (48.0) | 174/350 (49.7) | 34/83 (41.0) | 0.179 | 0.369 |
| Disease activity, function, PROs | |||||
| ASDAS-CRP, mean (s.d.) | 2.6 (1.2) | 2.7 (1.2) | 2.4 (1.1) | 0.107 | 0.262 |
| BASDAI, mean (s.d.) | 4.0 (2.4) | 4.0 (2.4) | 3.9 (2.3) | 0.592 | 0.814 |
| PGA, mean (s.d.) | 4.5 (2.7) | 4.7 (2.7) | 3.9 (2.5) | 0.018 | 0.079 |
| BASFI, mean (s.d.) | 2.8 (2.6) | 2.8 (2.6) | 2.6 (2.5) | 0.278 | 0.510 |
| ASAS-HI, mean (s.d.) | 6.6 (4.4) | 6.7 (4.5) | 6.3 (4.2) | 0.569 | 0.799 |
| EQ-5D, mean (s.d.) | 0.6 (0.2) | 0.6 (0.2) | 0.7 (0.2) | 0.129 | 0.294 |
| Fibromyalgia (according to FiRST score), n/N (%) | 69/391 (17.6) | 56/312 (17.9) | 13/79 (16.5) | 0.869 | >0.999 |
| Treatment | |||||
| NSAIDs, n/N (%) | 421/421 (100.0) | 339/339 (100.0) | 82/82 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids ever, n/N (%) | 212/213 (99.5) | 179/180 (99.4) | 33/33 (100.0) | >0.999 | >0.999 |
| Systemic glucocorticoids current, n/N (%) | 89/433 (20.6) | 76/350 (21.7) | 13/83 (15.7) | 0.290 | 0.491 |
| Local injection of glucocorticoids for peripheral musculoskeletal involvement ever, n/N (%) | 183/193 (94.8) | 156/159 (98.1) | 27/34 (79.4) | <0.001 | 0.003 |
| csDMARDs ever, n/N (%) | 384/433 (88.7) | 310/350 (88.6) | 74/83 (89.2) | >0.999 | >0.999 |
| csDMARDs current, n/N (%) | 230/433 (53.1) | 192/350 (54.9) | 38/83 (45.8) | 0.086 | 0.218 |
| bDMARDs ever, n/N (%) | 223/433 (51.5) | 164/350 (46.9) | 59/83 (71.1) | <0.001 | 0.001 |
| bDMARDs current, n/N (%) | 160/433 (37.0) | 119/350 (34.0) | 41/83 (49.4) | 0.011 | 0.056 |
All results are presented as mean and s.d. and percentages for continuous and categorical variables, respectively.
First-degree or second-degree relatives with ankylosing spondylitis, uveitis, reactive arthritis or IBD.
Shoulder and hip joints. ASAS: Assessment of SpondyloArthritis international Society; ASAS-HI: ASAS Health Index; ASDAS: Ankylosing Spondylitis Disease Activity Score; bDMARD: biologic DMARD; B–H Adj. P: the Benjamini–Hochberg adjusted P-value; CASPAR: Classification Criteria for Psoriatic Arthritis; csDMARD: conventional synthetic DMARD; EQ-5D: Euro quality of life (QoL)-5D; FiRST: Fibromyalgia Rapid Screening Tool; HLA-B27: human leucocyte antigen B27; LEI: Leeds Enthesitis Index; MEI: Mander Enthesitis Index; PGA: patient’s global assessment; PRO: patient-reported outcome; SpA: Spondyloarthritis; SPARCC Enthesitis Index: Spondyloarthritis Research Consortium of Canada Enthesitis Index.
Patients with psoriasis were older, had a longer symptom duration at the time point of study inclusion and had a longer diagnostic delay. There was no difference in the frequency of family history of SpA (except psoriasis) in the subgroups with and without personal history of psoriasis. However, the family history of psoriasis was significantly more frequent in patients with psoriasis. There was no difference in the frequency of fulfilment of the ASAS pSpA classification criteria, but the CASPAR criteria were as expected more frequently fulfilled in patients with psoriasis.
The presence of psoriasis tended to be associated with a lower frequency of monoarthritic patterns (as opposed to oligo- and polyarticular patterns) of peripheral articular involvement. Affection of distal interphalangeal joints was more frequent in patients with psoriasis. Dactylitis was more common in the presence of psoriasis, as well as enthesitis, especially confirmed by specific investigations—imaging. In patients with psoriasis, dactylitis most frequently affected fingers, while in pSpA without psoriasis, toes were the predominant localization. Additionally, the SPARCC enthesitis score was higher in patients with psoriasis. There were no differences in the frequency of axial involvement diagnosed by the rheumatologist or in the frequency of back pain, but sacroiliitis on X-rays was more frequently reported in pSpA patients without psoriasis. Patients with psoriasis had a lower prevalence of HLA-B27 and a lower level of CRP but were more frequently rheumatoid factor positive.
The PROs were largely comparable between the groups with somewhat worse global assessment of disease activity score in patients without psoriasis. At the same time, patients without psoriasis more frequently received local glucocorticoid injections but substantially less frequently received bDMARDs than patients with psoriasis. Importantly, the differences in the use of bDMARDs in pSpA patients with and without psoriasis were present across all musculoskeletal manifestations (Table 2).
Specific treatments with regard to the presence of specific musculoskeletal manifestations and the personal history of psoriasis in patients with peripheral spondyloarthritis
| . | Peripheral arthritis (n = 410) . | Enthesitis (n = 248) . | Dactylitis (n = 100) . | Root jointadisease (n = 192) . | Axial disease (n = 238) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Patients without personal history of psoriasis (n = 335) . | Patients with personal history of psoriasis (n = 75) . | P . | Patients without personal history of psoriasis (n = 194) . | Patients with personal history of psoriasis (n = 54) . | P . | Patients without personal history of psoriasis (n = 70) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 162) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 19)3 . | Patients with personal history of psoriasis (n = 45) . | P . |
| Any treatment, n (%) | 314 (93.7) | 71 (94.7) | >0.999 | 126 (64.9) | 32 (59.3) | 0.522 | 49 (70.0) | 17 (56.7) | 0.250 | 141 (87.0) | 24 (80.0) | 0.388 | 166 (86.0) | 37 (82.2) | 0.491 |
| NSAIDs, n (%) | 325 (97.0) | 73 (97.3) | >0.999 | 121 (96.0) | 31 (96.9) | >0.999 | 45 (91.8) | 15 (88.2) | 0.643 | 137 (97.2) | 21 (87.5) | 0.064 | 165 (99.4) | 37 (100.0) | >0.999 |
| Systemic GCs, n (%) | 175 (52.2) | 30 (40.0) | 0.073 | 51 (40.5) | 9 (28.1) | 0.226 | NC | NC | NC | NC | NC | NC | NC | NC | NC |
| Local injections of GCs, n (%) | 137 (40.9) | 22 (29.3) | 0.067 | 18 (14.3) | 6 (18.8) | 0.582 | 11 (22.4) | 4 (23.5) | >0.999 | 27 (19.1) | 5 (20.8) | 0.786 | NC | NC | NC |
| csDMARDs, n (%) | 286 (85.4) | 62 (82.7) | 0.593 | 83 (65.9) | 18 (56.3) | 0.312 | 30 (61.2) | 9 (52.9) | 0.578 | 106 (75.2) | 16 (66.7) | 0.451 | 103 (62.0) | 26 (70.3) | 0.450 |
| bDMARDs, n (%) | 124 (37.0) | 43 (57.3) | 0.002 | 37 (29.4) | 19(59.4) | 0.003 | 11 (22.4) | 9 (52.9) | 0.031 | 39 (27.7) | 14 (58.3) | 0.004 | 63 (38.0) | 22 (59.5) | 0.026 |
| . | Peripheral arthritis (n = 410) . | Enthesitis (n = 248) . | Dactylitis (n = 100) . | Root jointadisease (n = 192) . | Axial disease (n = 238) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Patients without personal history of psoriasis (n = 335) . | Patients with personal history of psoriasis (n = 75) . | P . | Patients without personal history of psoriasis (n = 194) . | Patients with personal history of psoriasis (n = 54) . | P . | Patients without personal history of psoriasis (n = 70) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 162) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 19)3 . | Patients with personal history of psoriasis (n = 45) . | P . |
| Any treatment, n (%) | 314 (93.7) | 71 (94.7) | >0.999 | 126 (64.9) | 32 (59.3) | 0.522 | 49 (70.0) | 17 (56.7) | 0.250 | 141 (87.0) | 24 (80.0) | 0.388 | 166 (86.0) | 37 (82.2) | 0.491 |
| NSAIDs, n (%) | 325 (97.0) | 73 (97.3) | >0.999 | 121 (96.0) | 31 (96.9) | >0.999 | 45 (91.8) | 15 (88.2) | 0.643 | 137 (97.2) | 21 (87.5) | 0.064 | 165 (99.4) | 37 (100.0) | >0.999 |
| Systemic GCs, n (%) | 175 (52.2) | 30 (40.0) | 0.073 | 51 (40.5) | 9 (28.1) | 0.226 | NC | NC | NC | NC | NC | NC | NC | NC | NC |
| Local injections of GCs, n (%) | 137 (40.9) | 22 (29.3) | 0.067 | 18 (14.3) | 6 (18.8) | 0.582 | 11 (22.4) | 4 (23.5) | >0.999 | 27 (19.1) | 5 (20.8) | 0.786 | NC | NC | NC |
| csDMARDs, n (%) | 286 (85.4) | 62 (82.7) | 0.593 | 83 (65.9) | 18 (56.3) | 0.312 | 30 (61.2) | 9 (52.9) | 0.578 | 106 (75.2) | 16 (66.7) | 0.451 | 103 (62.0) | 26 (70.3) | 0.450 |
| bDMARDs, n (%) | 124 (37.0) | 43 (57.3) | 0.002 | 37 (29.4) | 19(59.4) | 0.003 | 11 (22.4) | 9 (52.9) | 0.031 | 39 (27.7) | 14 (58.3) | 0.004 | 63 (38.0) | 22 (59.5) | 0.026 |
Shoulder and hip joints. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; GC: glucocorticoid; NC: not collected.
Specific treatments with regard to the presence of specific musculoskeletal manifestations and the personal history of psoriasis in patients with peripheral spondyloarthritis
| . | Peripheral arthritis (n = 410) . | Enthesitis (n = 248) . | Dactylitis (n = 100) . | Root jointadisease (n = 192) . | Axial disease (n = 238) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Patients without personal history of psoriasis (n = 335) . | Patients with personal history of psoriasis (n = 75) . | P . | Patients without personal history of psoriasis (n = 194) . | Patients with personal history of psoriasis (n = 54) . | P . | Patients without personal history of psoriasis (n = 70) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 162) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 19)3 . | Patients with personal history of psoriasis (n = 45) . | P . |
| Any treatment, n (%) | 314 (93.7) | 71 (94.7) | >0.999 | 126 (64.9) | 32 (59.3) | 0.522 | 49 (70.0) | 17 (56.7) | 0.250 | 141 (87.0) | 24 (80.0) | 0.388 | 166 (86.0) | 37 (82.2) | 0.491 |
| NSAIDs, n (%) | 325 (97.0) | 73 (97.3) | >0.999 | 121 (96.0) | 31 (96.9) | >0.999 | 45 (91.8) | 15 (88.2) | 0.643 | 137 (97.2) | 21 (87.5) | 0.064 | 165 (99.4) | 37 (100.0) | >0.999 |
| Systemic GCs, n (%) | 175 (52.2) | 30 (40.0) | 0.073 | 51 (40.5) | 9 (28.1) | 0.226 | NC | NC | NC | NC | NC | NC | NC | NC | NC |
| Local injections of GCs, n (%) | 137 (40.9) | 22 (29.3) | 0.067 | 18 (14.3) | 6 (18.8) | 0.582 | 11 (22.4) | 4 (23.5) | >0.999 | 27 (19.1) | 5 (20.8) | 0.786 | NC | NC | NC |
| csDMARDs, n (%) | 286 (85.4) | 62 (82.7) | 0.593 | 83 (65.9) | 18 (56.3) | 0.312 | 30 (61.2) | 9 (52.9) | 0.578 | 106 (75.2) | 16 (66.7) | 0.451 | 103 (62.0) | 26 (70.3) | 0.450 |
| bDMARDs, n (%) | 124 (37.0) | 43 (57.3) | 0.002 | 37 (29.4) | 19(59.4) | 0.003 | 11 (22.4) | 9 (52.9) | 0.031 | 39 (27.7) | 14 (58.3) | 0.004 | 63 (38.0) | 22 (59.5) | 0.026 |
| . | Peripheral arthritis (n = 410) . | Enthesitis (n = 248) . | Dactylitis (n = 100) . | Root jointadisease (n = 192) . | Axial disease (n = 238) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Patients without personal history of psoriasis (n = 335) . | Patients with personal history of psoriasis (n = 75) . | P . | Patients without personal history of psoriasis (n = 194) . | Patients with personal history of psoriasis (n = 54) . | P . | Patients without personal history of psoriasis (n = 70) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 162) . | Patients with personal history of psoriasis (n = 30) . | P . | Patients without personal history of psoriasis (n = 19)3 . | Patients with personal history of psoriasis (n = 45) . | P . |
| Any treatment, n (%) | 314 (93.7) | 71 (94.7) | >0.999 | 126 (64.9) | 32 (59.3) | 0.522 | 49 (70.0) | 17 (56.7) | 0.250 | 141 (87.0) | 24 (80.0) | 0.388 | 166 (86.0) | 37 (82.2) | 0.491 |
| NSAIDs, n (%) | 325 (97.0) | 73 (97.3) | >0.999 | 121 (96.0) | 31 (96.9) | >0.999 | 45 (91.8) | 15 (88.2) | 0.643 | 137 (97.2) | 21 (87.5) | 0.064 | 165 (99.4) | 37 (100.0) | >0.999 |
| Systemic GCs, n (%) | 175 (52.2) | 30 (40.0) | 0.073 | 51 (40.5) | 9 (28.1) | 0.226 | NC | NC | NC | NC | NC | NC | NC | NC | NC |
| Local injections of GCs, n (%) | 137 (40.9) | 22 (29.3) | 0.067 | 18 (14.3) | 6 (18.8) | 0.582 | 11 (22.4) | 4 (23.5) | >0.999 | 27 (19.1) | 5 (20.8) | 0.786 | NC | NC | NC |
| csDMARDs, n (%) | 286 (85.4) | 62 (82.7) | 0.593 | 83 (65.9) | 18 (56.3) | 0.312 | 30 (61.2) | 9 (52.9) | 0.578 | 106 (75.2) | 16 (66.7) | 0.451 | 103 (62.0) | 26 (70.3) | 0.450 |
| bDMARDs, n (%) | 124 (37.0) | 43 (57.3) | 0.002 | 37 (29.4) | 19(59.4) | 0.003 | 11 (22.4) | 9 (52.9) | 0.031 | 39 (27.7) | 14 (58.3) | 0.004 | 63 (38.0) | 22 (59.5) | 0.026 |
Shoulder and hip joints. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; GC: glucocorticoid; NC: not collected.
In the multivariable analysis, a longer diagnostic delay, negative HLA-B27 status and the presence of enthesitis were significantly associated with the presence of psoriasis (Fig. 2).
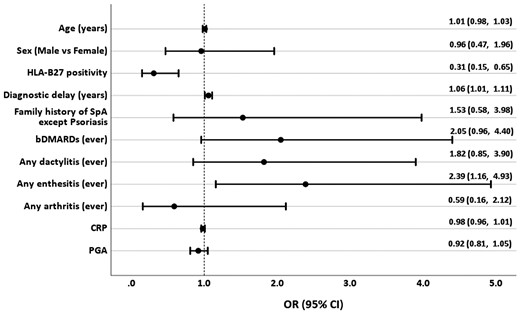
Association of demographic and clinical characteristics of peripheral spondyloarthritis with the presence of the personal history of psoriasis
bDMARD: biologic DMARD; HLA-B27: human leukocyte antigen-B27; OR: odds ratio; PGA: patient global assessment; SpA: Spondyloarthritis.
In the additional analysis that compared patients with a personal or family history of psoriasis (n = 112) with patients without such a history (after exclusion of n = 42 patients with unknown family history), the obtained results were largely comparable to the main analysis (Supplementary Table S1, available at Rheumatology online). In addition to the differences highlighted above, patients with personal or family history of psoriasis had higher BMI and were more frequently diagnosed with fibromyalgia (although there was no difference in the frequency of fibromyalgia according to the FiRST score). Interestingly, signs of preceding infection (urethritis, cervicitis or diarrhoea) were more frequently reported for patients with personal or family history of psoriasis. Peripheral arthritis was more frequently reported in patients without a personal or family history of psoriasis, although this manifestation was present in >90% of the patients in both groups. Root joints (i.e. shoulder and hip joints) tended to be more frequently affected in pSpA without a personal or family history of psoriasis, while evidence of juxta-articular new bone formation and distal interphalangeal joint involvement was more common in the presence of psoriasis. Again, enthesitis and dactylitis showed a positive association with the presence of a personal or family history of psoriasis. Laboratory findings, PROs and treatment patterns were largely consistent with the main analysis.
Discussion
In this large, international, cross-sectional study in patients with SpA, we evaluated the clinical characteristics of the subgroups of pSpA stratified according to the presence of psoriasis. We could identify a number of differences in the clinical presentation and in the applied treatment.
In general, pSpA patients (with and without psoriasis) represented only about 10% of the entire SpA population in the ASAS-PerSpA study. This might be related to several factors such as a high frequency of axial manifestations in SpA in general, focus on axial disease in research in the past decades and a higher number of approved treatment options including bDMARDs for patients who receive a diagnosis of axSpA or PsA. At the same time, in several geographic regions (such as Latin America), peripheral manifestations (which are frequent in SpA anyway) dominate in the clinical picture of SpA [3, 15].
In the ASAS-PerSpA study, the overall frequency of psoriasis in pSpA patients (19.1%) was lower than in previous studies [16–19]. The inclusion of the PsA group in the original study may have led clinicians to assign patients with psoriasis to this category.
Although the frequency of peripheral articular disease was comparable in patients with and without psoriasis, patients with psoriasis had a lower frequency of monoarthritic involvement and a higher frequency of a destructive affection of distal interphalangeal joints.
Enthesitis (and especially of enthesitis confirmed by specific investigations) was more frequent in patients with psoriasis. Also, the enthesitis scores (SPARCC and LEI) were higher in patients with psoriasis, which can reflect the severity or extent of the entheseal manifestations. This can reinforce the hypothesis of an association between the presence of psoriasis and damage in entheseal sites, which is also called the ‘deep Koebner phenomenon’ [20, 21].
Dactylitis is a less frequent but characteristic feature of pSpA and is commonly associated with psoriasis [16, 17]. In this analysis, we found a higher frequency of dactylitis in patients with psoriasis. Fingers were most commonly affected in the psoriatic group, while the toes were the predominant localization in pSpA patients without psoriasis. It is well known that dactylitis is associated with higher damage and more erosive forms of PsA [22]. To our knowledge, no studies have evaluated the clinical significance of dactylitis on damage in pSpA. For this reason, further studies of dactylitis in pSpA are warranted.
pSpA patients with psoriasis in this study showed a longer diagnostic delay, which might appear surprising. This phenomenon was also observed in axSpA patients in a recent analysis [23]. A focus on the treatment of skin condition and a neglecting of musculoskeletal manifestations might be one of the reasons for a longer diagnostic delay. This may also indicate an unmet need to improve awareness among physicians caring for patients with psoriasis.
The presence of psoriasis in pSpA was associated with a lower prevalence of HLA-B27 positivity in our study. It is known that the presence of HLA-B27 is associated with the presence of musculoskeletal manifestations of the psoriatic disease but not with skin psoriasis itself [24]. Given the genetic heterogeneity of psoriasis it could be expected that the relative contribution of HLA-B27 in pSpA patients with psoriasis is lower than in patients without.
Overall, pSpA patients with psoriasis showed a higher frequency of use of bDMARDs, while the PROs were largely comparable between the groups. On the one hand these results might indicate that patients with psoriasis were more likely to be treated with bDMARDs because of higher severity of musculoskeletal manifestations (especially, enthesitis and dactylitis). On the other hand, there are currently no approved treatment options for patients with pSpA without a personal or family history of psoriasis and no evidence of axial involvement. A few studies have suggested good efficacy of bDMARD (anti-TNF) therapy in non-axSpA and non-psoriatic pSpA [25–28]; nevertheless, bDMARD use is still off-label for this specific patient group. This status might have important implications in clinical practice since early-diagnosed non-psoriatic pSpA patients might experience a substantial delay in the introduction of effective anti-inflammatory drugs. Thus, there is an urgent need for randomized controlled trials with potentially effective bDMARDs (IL-17 and IL-23 inhibitors) and targeted synthetic DMARDs (such as Janus kinase inhibitors) in patients with non-psoriatic pSpA.
This study has several limitations. The cross-sectional nature of the study is an important limiting factor in assessing causality, as discussed above. The assignment of patients to the subgroups within the study was done based on the opinion of the local rheumatologist—this introduces some uncertainty regarding the ascertainment of patients with psoriasis to the pSpA group and not to the PsA group. Such an assignment indicates, however, that the disease phenotype was rather compatible with SpA than with PsA in the eyes of the expert who included the patient and made the diagnosis. In addition, the assignment of patients to axSpA or ReA/IBD-related SpA may cause a similar problem that could be considered as selection bias. However, since the diagnosis (and the resulting classification in this study) was made by experienced rheumatologists with expertise in SpA we accepted this expert judgement as a reference standard. Nonetheless, one should take into account a certain level of heterogeneity of patient evaluation that cannot be avoided in such a large multicentre multinational study. No central evaluation of clinical or imaging data was performed. Finally, some data related to the previous history of disease manifestation might be affected by recall bias—this applies, however, to patients with and without psoriasis.
In conclusion, we could demonstrate that the presence of psoriasis has an impact on clinical characteristics of pSpA. pSpA patients without psoriasis were less frequently treated with bDMARDs despite similar disease burden as compared with patients with psoriasis.
Acknowledgements
We would like to thank all the collaborators who participated in the study: José Maldonado-Cocco (Buenos Aires University School of Medicine, Buenos Aires, Argentina), Hernán Maldonado Ficco (Hospital San Antonio de Padua, Rio Cuarto, Argentina), Rodolfo Pérez Alamino (Hospital Dr Nicolás Avellaneda, Tucumán, Argentina), Emilio Buschiazzo (Hospital Señor del Milagro, Salta, Argentina), Romina Calvo (Hospital Provincial Dr José M. Cullen, Santa Fé, Argentina), Vanesa Duarte (Clínica Monte Grande, Buenos Aires, Argentina), Maria Victoria Martire (Instituto Médico Platense, La Plata, Argentina), Diego Baenas (Hospital Privado de Córdoba, Córdoba, Argentina), Dora Pereira (Hospital Ricardo Gutiérrez, La Plata, Argentina), Adrian Salas (Consultorio Reumatológico, La Plata, Argentina), Juan Manuel Bande (Hospital General de Agudos Dr E. Tornú, Buenos Aires, Argentina), Alberto Berman (Centro Médico Privado de Tucumán, Tucumán, Argentina), Walter P. Maksymowych (University of Alberta, Edmonton, Canada), Stephanie Belton (University of Alberta, Edmonton, Canada), Sebastián Ibáñez (Facultad de Medicina Clínica Alemana—Universdidad del Desarrollo, Santiago de Chile, Chile), María Paz Poblete (Facultad de Medicina Clínica Alemana—Universidad del Desarrollo, Santiago de Chile, Chile), Francisca Valenzuela (Facultad de Medicina Clínica Alemana—Universidad del Desarrollo, Santiago de Chile, Chile), Wilson Bautista-Molano (University Hospital Fundación Santa Fé de Bogotá, Bogotá, Colombia), Jieruo Gu (Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China), Min Xiao (Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China), C. S. Lau (Hong-Kong University, Hong Kong, China), Ho Yin Chung (Hong-Kong University, Hong Kong, China), Bassel Elzorkany (Cairo University, Cairo, Egypt), Sherif Gamal (Cairo University, Cairo, Egypt), Catherine Lebourlout (Cochin Hospital, Paris, France), Daniel Wendling (CHU Besançon, Besançon, France), Clément Prati (CHU Besançon, Besançon, France), Frank Verhoeven (CHU Besançon, Besançon, France), Martin Soubrier (CHU Clermont-Ferrand, Clermont-Ferrand, France), Carine Savel (CHU Clermont-Ferrand, Clermont-Ferrand, France), Trigui Alia (CHU Clermont-Ferrand, Clermont-Ferrand, France), Fan Angélique (CHU Clermont-Ferrand, Clermont-Ferrand, France), Pascal Claudepierre (Henri Mondor Hospital, Créteil, France), Valerie Farrenq (Henri Mondor Hospital, Créteil, France), Kamelia Faramarz (Henri Mondor Hospital, Créteil, France), Uta Kiltz (Rheumazentrum Ruhrgebiet, Herne, Germany), Isabella Sieber (Rheumazentrum Ruhrgebiet, Herne, Germany), Dories Morzeck (Rheumazentrum Ruhrgebiet, Herne, Germany), Fabian Proft (Charité University, Berlin, Germany), Pál Geher (Semmelweis University, Budapest, Hungary), Edit Toth (Flór Ferenc Hospital, Kistarcsa, Hungary), Katalin Nagy (Markhot Ferenc Hospital, Eger, Hungary), Attila Kovacs (MÁV Hospital, Szolnok, Hungary), Meghna Gavali (Nizam’s Institute of Medical Sciences, Hyderabad, India), Liza Rajasekhar (Nizam’s Institute of Medical Sciences, Hyderabad, India), Sapan Pandya (Smt NHL Medical College and Sardar Vallabhbhai Patel Hospital and Vedanta Institute of Medical Sciences, Ahmedabad, India), Bhowmik Meghnathi (Sri Sai Siri Hospital and Prathima Institue of Medical Sciences, Karimnagar, India), Carlomaurizio Montecucco (Fondazione IRCCS Policlinico San Matteo, Pavia, Italia), Sara Monti (Fondazione IRCCS Policlinico San Matteo, Pavia, Italia), Alessandro Biglia (Fondazione IRCCS Policlinico San Matteo, Pavia, Italia), Mitsumasa Kishimoto (Kyorin University School of Medicine, Tokyo, Japan), Akihiko Asahina (The Jikei University School of Medicine, Tokyo, Japan), Masato Okada (St Luke`s International University and Hospital, Tokyo, Japan), Tadashi Okano (Osaka City University, Osaka, Japan), Yuko Kaneko (Keio University School of Medicine, Tokyo, Japan), Hideto Kameda (Toho University, Tokyo, Japan), Yoshinori Taniguchi (Kochi University, Kochi, Japan), Naoto Tamura (Juntendo University School of Medicine, Tokyo, Japan), Shigeyoshi Tsuji (National Hospital Organization Osaka Minami Medical Center, Osaka, Japan), Hiroaki Dobashi (Kagawa University Faculty of Medicine, Kagawa, Japan), Yoichiro Haji (Daido Hospital, Nagoya, Japan), Akimichi Morita (Nagoya City University, Nagoya, Japan), Nelly Ziade (Saint-Joseph University, Beirut, Lebanon), Nelly Salloum (Saint-Joseph University, Beirut, Lebanon), Rubén Burgos-Vargas (Hospital General de México Eduardo Liceaga, Mexico City, Mexico), Graciela Meza (CLIDITER, Mexico City, Mexico), Julio Casasola-Vargas (Hospital General de Mexico, Mexico City, Mexico), César Pacheco-Tena (Hospital General Dr Salvador Zubirán, Chihuahua, Mexico), Greta Reyes-Cordero (Hospital General Dr Salvador Zubirán, Chihuahua, Mexico), César Ramos-Remus (Unidad de Investigación de Enfermedades Crónico Degenerativas, Jalisco, Mexico), J. Dionisio Castillo (Unidad de Investigación de Enfermedades Crónico Degenerativas, Jalisco, Mexico), Laura González-López (Universidad de Guadalajara, Jalisco, Mexico), Iván Gámez-Nava (Unidad de Investigación Biomédica 02, Hospital de Especialidades, Centro Médico Nacional de Occidente, IMSS Guadalajara, Jalisco, Mexico), Najia Hajjaj-Hassouni (International University of Rabat [UIR], Rabat, Morocco), Fadoua Allali (University Mohammed V, CHU Ibn Sina, Rabat, Morocco), Hanan Rkain (University Mohammed V, CHU Ibn Sina, Rabat, Morocco), Lahcen Achemlal (University Mohammed V, CHU Ibn Sina, Rabat, Morocco), Taoufik Harzy (University Sidi Mohammed Benabdellah, CHU Hassan II, Fès, Morocco), Fernando M. Pimentel-Santos (Universidade NOVA de Lisboa, Lisboa, Portugal), Santiago Rodrigues-Manica (Universidade NOVA de Lisboa, Lisboa, Portugal), Agna Neto (Universidade NOVA de Lisboa, Lisboa, Portugal), Jose Marona (Universidade NOVA de Lisboa, Lisboa, Portugal), Ma Joao Gonçalves (Universidade NOVA de Lisboa, Lisboa, Portugal), Ana Filipa Mourao (Universidade NOVA de Lisboa, Lisboa, Portugal), Rita Pinheiro Torres (Universidade NOVA de Lisboa, Lisboa, Portugal), Ruxandra Schiotis (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Simona Rednic (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Siao-Pin Simon (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Laura Muntean (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Ileana Filipescu (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Maria Tamas (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Laura Damian (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Ioana Felea (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Dana Fodor (Second Medical Clinic, Emergency Conty Hospital, Cluj-Napoca, Romania), Tae-Jong Kim (Chonnam National University Medical School and Hospital, Gwangju, South Korea), Hyun-Yi Kook (Chonnam National University Medical School and Hospital, Gwangju, South Korea), Hyun-Ju Jung (Chonnam National University Medical School and Hospital, Gwangju, South Korea), Tae-Hwan Kim (Hanyang University Hospital for Rheumatic Diseases, Seoul, South Korea), Victoria Navarro-Compan (University Hospital La Paz, Madrid, Spain), Mireia Moreno (Hospital Parc Taulí, Barcelona, Spain), Eduardo Collantes-Estévez (Hospital Universitario Reina Sofía de Córdoba, Córdoba, Spain), M. Carmen Castro-Villegas (Hospital Universitario Reina Sofía, Córdoba, Spain), Cristina Fernández-Carballido (Hospital Universitario San Juan de Alicante, Alicante, Spain), Elizabeth Fernández (Hospital Universtario La Paz, Madrid, Spain), Marta Arévalo (Hospital Parc Taulí, Barcelona, Spain), Shue-Fen Luo (Chang Gung Memorial Hospital-Linkou, Taoyuan, Taiwan), Yeong-Jian Jan Wu (Chang Gung Memorial Hospital, Kee-Lung, Taiwan), Tian-Tsai Cheng (Chang Gung Memorial Hospital at Kao-Hsiung, Taiwan), Cheng-Chung Wei (Chung Sun Medical University, Taiwan), Tuncay Duruöz (Marmara University School of Medicine, Istanbul, Turkey), Servet Akar (Katip Çelebi University School of Medicine, Izmir, Turkey), Ilhan Sezer (Akdeniz University School of Medicine, Antalya, Turkey), Umut Kalyoncu (Hacettepe University School of Medicine, Ankara, Turkey), Sebnem Ataman (Ankara University School of Medicine, Ankara, Turkey), Meltem Alkan Melikoglu (Erzurum Atatürk University School of Medicine, Erzurum, Turkey), Sami Hizmetli (Sivas Cumhuriyet University School of Medicine, Sivas, Turkey), Ozgur Akgul (Manisa Celal Bayar University School of Medicine, Manisa, Turkey), Nilay Sahin (Balikesir University School of Medicine, Balikesir, Turkey), Erhan Capkin (Karadeniz Teknik University School of Medicine, Trabzon, Turkey), Fatima Gluçin Ural (Ankara Yildirim Beyazit University School of Medicine, Ankara, Turkey), Figen Yilmaz (Istanbul Sisli Etfal Training and Research Hospital, Istanbul, Turkey), Ilknur Aktas (Istanbul Fatih Sultan Mehmet Training and Research Hospital, Istanbul, Turkey), Floris van Gaalen (Leiden University Medical Center, Leiden, The Netherlands), Anne Boel (Leiden University Medical Center, Leiden, The Netherlands), Sofia Ramiro (Zuyderland Medical Center, Heerlen, The Netherlands), Mirian Starmans-Kool (Zuyderland Medical Center, Heerlen, The Netherlands), Femke Hoekstra-Drost (Zuyderland Medical Center, Heerlen, The Netherlands), Maha Abdelkadir (Maasstad Hospital, Rotterdam, The Netherlands), Angelique Weel (Maasstad Hospital, Rotterdam, The Netherlands), Pedro M. Machado (University College London, London, UK), Marina Magrey (Cases Western Reserve University School of Medicine, Cleveland, OH, USA), Darerian Schueller (Cases Western Reserve University School of Medicine, Cleveland, OH, USA). S.R., E.N. and A.B. designed the study. D.C. analysed the data, and all authors were involved in the interpretation and discussion of the results. D.C. wrote the manuscript, with significant input from all co-authors. The steering committee included Joachim Sieper (Charité University, Berlin, Germany), Desirée van der Heijde (Leiden University Medical Center, Leiden, The Netherlands), Robert Landewé (Zuyderland Medical Center, Heerlen, The Netherlands), and Anna Moltó (Cochin Hospital, Paris, France).
Funding: No funding was provided for the current analysis. The ASAS-PerSpA study was conducted under the umbrella of ASAS with an unrestricted grant from Abbvie, Pfizer, Lilly, Novartis, UCB, Janssen and Merck. The funders did not have any role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data from the ASAS-PerSpA study are available to investigators on reasonable request. For information on how to access data, contact the Assessment of SpondyloArthritis international Society (www.asas-group.org).
Supplementary data
Supplementary data are available at Rheumatology online.
References
Author notes
Tugba Izci Duran and Murat Torgutalp contributed equally to this study.




Comments