-
PDF
- Split View
-
Views
-
Cite
Cite
Manil Subesinghe, Shaheel Bhuva, Nikita Arumalla, Andrew Cope, David D’Cruz, Sujith Subesinghe, 2-deoxy-2[18F]fluoro-D-glucose positron emission tomography–computed tomography in rheumatological diseases, Rheumatology, Volume 61, Issue 5, May 2022, Pages 1769–1782, https://doi.org/10.1093/rheumatology/keab675
Close - Share Icon Share
Abstract
2-deoxy-2[18F]fluoro-D-glucose (FDG) PET-CT has revolutionized oncological imaging. The cellular processes that make cancer cells visible on FDG PET-CT also occur in a number of inflammatory cells. Exploiting this phenomenon has led to a growth of evidence supporting the use of FDG PET-CT in a wide range of infective and inflammatory diseases. Rheumatological diseases can affect multiple sites within the musculoskeletal system alongside multi-organ extra-articular disease manifestations. Inflammation is central to these diseases, making FDG PET-CT a logical choice. In this review article we describe the various applications of FDG PET-CT in rheumatological diseases using illustrative examples to highlight the beneficial role of FDG PET-CT in each case.
The distribution and localisation of inflammatory uptake on FDG PET-CT can suggest specific rheumatological diagnoses.
FDG PET-CT demonstrates the true extent of disease and identifies suitable targets for biopsy.
FDG PET-CT has an emerging role in the assessment of response to therapy.
Introduction
2-deoxy-2[18F]fluoro-D-glucose (FDG) PET-CT is a hybrid imaging technique that provides simultaneous functional and anatomical information. Its superiority in demonstrating the presence and distribution of cancer throughout the human body has revolutionized oncological imaging. Cancer cells metabolize glucose via lower-efficiency aerobic glycolysis, in preference to oxidative phosphorylation (Warburg effect), upregulating their cellular glycolytic processes to compensate for this [1]. These are visualized as areas of increased accumulation of the radiopharmaceutical FDG, which is a glucose analogue that enters cells and is phosphorylated like glucose but cannot be further metabolized, thus becoming trapped intracellularly and visible on PET-CT.
Increased cellular glycolysis is not specific to cancer cells. Increased FDG uptake has been demonstrated in relation to a number of inflammatory cells, including neutrophils, macrophages, lymphocytes and fibroblasts in several animal models [2–4], including a murine model of pannus formation in RA [5]. Hyperaemia and increased vascular permeability increase delivery of FDG to inflamed tissues while cytokines mediate upregulation of glycolytic processes within these inflammatory cells. In combination, these manifest as areas of increased FDG uptake on PET-CT [6]. Exploiting this phenomenon, in conjunction with standardization of FDG PET-CT imaging procedures [7, 8], has led to the growth of evidence supporting the use of FDG PET-CT in a wide range of infective and inflammatory diseases.
Rheumatological diseases can affect multiple sites within the musculoskeletal system, including joints, tendons, ligaments, muscles and cartilage, alongside multi-organ extra-articular disease manifestations. Inflammation is central to these diseases, which makes FDG PET-CT a logical choice. In this review article we describe the various applications of FDG PET-CT in rheumatological diseases as recommended by the intercollegiate evidence-based PET-CT guidelines published by the Royal College of Physicians and Royal College of Radiologists in 2016 [9], in addition to emerging applications, using illustrative examples to highlight the beneficial role of FDG PET-CT in each case.
Established indications
Large vessel vasculitis
A middle-aged man presented with anorexia, fever and weight loss. Blood tests revealed a normocytic anaemia, hypoalbuminaemia and elevated inflammatory markers (ESR and CRP). Upper and lower gastrointestinal tract endoscopy and contrast-enhanced CT (CECT) of the chest, abdomen and pelvis were negative for malignancy. FDG PET-CT demonstrated abnormal increased murally based uptake (>liver uptake) in the aorta and its major branches, in keeping with an active large vessel vasculitis (LVV) (Fig. 1).
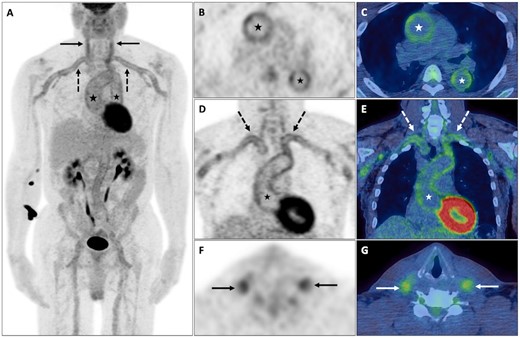
Large vessel vasculitis
Abnormal increased FDG uptake (>liver uptake) within the walls of the aorta (black and white *) and its major branches, including the subclavian (dashed black and white arrows), carotid (solid black and white arrows), iliac and femoral arteries, in keeping with an active LVV on PET maximum-intensity projection (MIP), axial PET and fused PET-CT (B–G).
LVV is characterized by chronic granulomatous inflammation of the walls of the aorta and its branches and comprises two main subtypes: GCA, which can co-exist with PMR [10] (Supplementary Fig. S1, available at Rheumatology online), and Takayasu’s arteritis, associated with arterial stenoses and aneurysms [11]. Smooth linear pattern uptake involving the walls of the aorta and its branches typifies active vascular inflammation, while atherosclerotic uptake, an important pitfall and cause of false-positive uptake, is of lower intensity, more heterogeneous and non-contiguous in appearance and increases in frequency with age [12, 13]. FDG PET-CT can reliably detect extracranial arterial involvement by GCA (Supplementary Fig. S2, available at Rheumatology online) and could provide a suitable alternative to temporal artery biopsy [14]. FDG PET-CT can also identify chronic complications of LVV, including ascending aortic dilatation and aneurysm formation (Supplementary Fig. S3, available at Rheumatology online) or diffuse aortic wall calcification and stenosis (Supplementary Fig. S4, available at Rheumatology online), both of which can adversely affect patient outcome [15].
Meta-analyses report good diagnostic performance of FDG PET-CT for the detection of LVV [16, 17], with slightly higher sensitivity (90% vs 84%) and specificity (98% vs 84%) in patients with GCA vs Takayasu’s arteritis [16], but which can be affected by the imaging criteria used to define a positive scan and concurrent/recent use of glucocorticoids (GCs); a recent position paper addresses these issues [18]. A 4-point visual ordinal scale is recommended for categorizing vascular uptake, with grade 2 (intermediate-grade uptake = liver) possibly indicative and grade 3 (high-grade uptake >liver) considered positive for active LVV; FDG avid arthropathy and enthesopathy/bursitis related to coexistent PMR should be reported using the same scale. GCs reduce the intensity of vascular inflammation-related FDG uptake [19], while conversely increasing the level of background liver uptake [20], which can individually and in combination reduce the sensitivity of FDG PET-CT. If stopping GCs before scanning is not possible, performing an FDG PET-CT examination within 3 days of starting GCs can limit the negative effects on diagnostic accuracy [18, 19].
Data regarding the use of FDG PET-CT in response assessment in LVV are emerging, but its clinical value remains unclear. Treatment of LVV using GCs, MTX or tocilizumab leads to a reduction in the intensity and extent of vascular uptake [21]. A recent systematic review and meta-analysis while confirming the above noted that difficulty remains in differentiating post-treatment low-grade FDG uptake related to residual active disease from clinical remission; image interpretation should always be in the context of the clinical suspicion of disease activity [22].
Sarcoidosis
A middle-aged woman with quiescent cutaneous and pulmonary sarcoidosis re-presented with a new cough and fatigue. Clinical examination and blood tests including serum angiotensin-converting enzyme (ACE) were normal. CECT of the chest, abdomen and pelvis demonstrated new perilymphatic pulmonary nodularity, intrathoracic lymphadenopathy and several low-attenuation hepatic foci. FDG PET-CT demonstrated FDG avid disease in the lungs, intrathoracic nodes, liver and skeleton, the latter inconspicuous on CECT (Fig. 2).
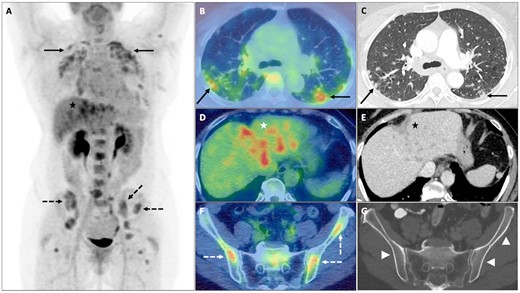
Sarcoidosis
Multifocal sites of FDG avid nodal and extranodal sarcoidosis on PET maximum-intensity projection (A), including; lungs with upper-midzone predominant FDG avid perilymphatic nodularity (solid black arrows) on axial fused PET-CT (B) and CECT (C); left lobe of the liver (black and white *) with FDG avid low-attenuation foci on axial fused PET-CT (D) and CECT (E); and skeleton with FDG avid foci in the iliac bones (dashed black and white arrows) on axial fused PET-CT (F), which are without a morphological correlate (white arrowheads) on axial CECT (G).
Sarcoidosis is a multi-organ chronic granulomatous inflammatory disease most often affecting the respiratory and lymphatic systems, with symptoms that can include a persistent cough, palpable peripheral lymphadenopathy and other manifestations of extrathoracic disease (e.g. erythema nodosum) on the background of generalized constitutional upset. Diagnosis is based on compatible clinical and radiological findings, histological evidence of non-caseating granulomas and an absence of alternative diagnoses (e.g. infection or malignancy) [23]. Serum ACE levels can be elevated in 30–80% of patients, but its role in the diagnosis and management of sarcoidosis remains debateable owing to its variable sensitivity and lack of specificity [24].
FDG PET-CT has a high sensitivity (89–100%) for the detection of active sarcoidosis [25]. It helps identify safe and accessible biopsy sites to obtain histological confirmation of sarcoidosis, particularly when none are apparent on clinical examination or conventional imaging or when suspected organ involvement is considered high-risk for biopsy (e.g. cardiac sarcoidosis) [26]. It accurately demonstrates the extent of active disease, especially extrathoracic disease (present in 30–50% cases) [27]. Musculoskeletal manifestations including arthritis, myopathy and skeletal involvement (i.e. bone and bone marrow involvement) [28] are largely occult on CECT but are readily apparent on FDG PET-CT [29], while demonstration of active cardiac disease requires dedicated patient preparation [e.g. prolonged fasting (>12 h) and a high-fat, low-carbohydrate diet] to suppress physiological myocardial glucose utilization and optimize diagnostic accuracy [30]. This is of importance, as cardiac, in addition to hepatic, splenic and neurological involvement is associated with increased mortality [31]. FDG PET-CT is beneficial in assessing disease activity, particularly when patients are symptomatic in the setting of acute or chronic disease but with normal biomarkers of disease activity [e.g. ACE or soluble IL-2 receptor (sIL-2R)] [32–34]. The ability to provide additional information over and above conventional imaging (e.g. demonstration of unsuspected but clinically important sites of extrapulmonary disease) results in a significant impact on patient management [34, 35].
Sparse data regarding the use of FDG PET-CT to assess response to treatment (Supplementary Fig. S5, available at Rheumatology online) suggest that a reduction in FDG uptake following treatment corresponds to an improvement in symptoms following GC [36–39] or biologic therapy [40, 41], while an absence of a favourable metabolic response is associated with lower levels of clinical remission and high relapse rates [38, 39].
Pyrexia of unknown origin and/or concern for malignancy
PMR
A middle-aged woman with a presumptive diagnosis of SLE was clinically and serologically reassessed. The diagnosis was refuted and maintenance GCs were gradually tapered. One month later she reported new-onset pain and stiffness in the neck, shoulder and pelvic girdles with weight loss, which, despite normal ESR and CRP, was suspicious for PMR. FDG PET-CT demonstrated FDG avid arthropathy around the shoulders and hips and widespread FDG-avid enthesopathy/bursitis around the proximal femurs, bony pelvis and vertebral column (Fig. 3).
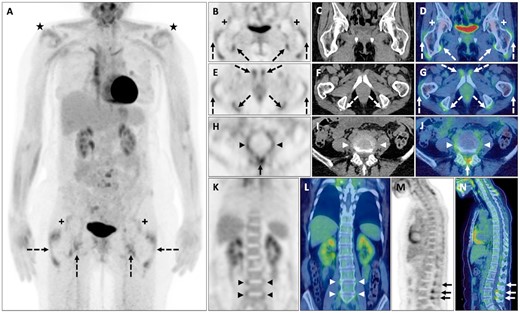
PMR
FDG avid (>liver uptake) shoulder (black *) and hip (black and white +) arthropathy and FDG avid enthesopathy/bursitis around the greater trochanters of femurs and iliopsoas, pectineal and hamstring insertions (with enthesophytes on CT) into the bony pelvis (dashed black and white arrows). Further FDG avid enthesopathy/bursitis involving the ligamentum flavum, interspinous ligament (solid black and white arrows) and annulus fibrosus of several intervertebral discs (black and white arrowheads) in keeping with PMR on PET maximum-intensity projection (A); axial PET, CT and fused PET-CT (B–J) and coronal (K and L) and sagittal (M and N) PET and fused PET-CT.
PMR is an inflammatory syndrome characterized by pain and stiffness around the shoulder and hip girdles, elevated inflammatory markers and constitutional upset. Although frequently diagnosed on clinical grounds, FDG PET-CT can be beneficial in cases of diagnostic uncertainty [42]. PMR is associated with a distinct pattern of symmetrical synovial FDG uptake around the shoulders, hips and sternoclavicular joints and extrasynovial uptake related to enthesopathy/bursitis around the bony pelvis and vertebral column [43].
FDG uptake (≥liver uptake) around the ischial tuberosities (hamstring insertions/bursae) in combination with similar-intensity uptake around the shoulders or vertebral interspinous bursae can diagnose PMR with high sensitivity and specificity (>90%) [44] and differentiate it from elderly onset RA [45, 46]. A systematic review and meta-analysis [47] confirmed that FDG uptake around the ischial tuberosities and greater trochanters, and FDG uptake in relation to the vertebral interspinous bursae, demonstrated the highest pooled sensitivity and specificity (>80%) for diagnosing PMR and that high pooled positive likelihood ratios (> 2.3) were associated with FDG uptake at these sites as well as the hips, shoulders and sternoclavicular joints.
FDG PET-CT is most useful at diagnosis when targeted imaging of the joints to identify synovitis (e.g. US or MRI) are non-contributory. In patients presenting with non-specific systemic upset, FDG PET-CT can also exclude potential infective or malignant aetiologies while identifying coexistent GCA in up to 40% of PMR cases (Supplementary Fig. S1, available at Rheumatology online) [47]. FDG PET-CT response assessment remains exploratory, but studies have demonstrated a reduction in arthropathic/enthesopathic uptake following treatment with GCs [48] and biologic agents [49], the latter study demonstrating a correlation between FDG uptake and bioclinical markers of disease activity.
DM/PM
A middle-aged woman with biopsy-proven anti-transcriptional intermediary factor 1γ (anti-TIF1-γ) antibody-positive DM underwent both MRI and FDG PET-CT assessment at diagnosis, which revealed features consistent with myositis but no evidence of malignancy. Five months later she developed rapid-onset painful enlargement of her left breast and left upper arm. Repeat FDG PET-CT demonstrated interval development of a new FDG avid multicentric left breast cancer with locoregional nodal involvement (Fig. 4).
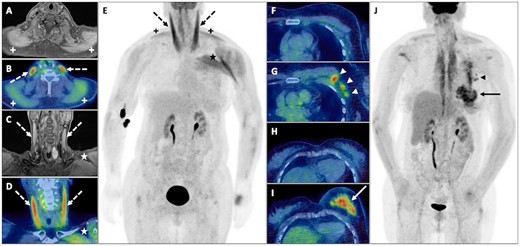
DM
Abnormal oedema and increased FDG uptake in both the sternocleidomastoid (dashed black and white arrows) and trapezius muscles (black and white +), left pectoral and deltoid muscles (black and white *) consistent with myositis but without evidence of malignancy on axial MRI and fused PET-CT (A and B), coronal MRI and fused PET-CT (C and D) and PET MIP (E). FDG PET-CT 5 months later demonstrates a multicentric breast cancer (solid black and white arrows) with involved left axillary nodes (black and white arrowheads) on axial fused PET-CT (G and I) and PET maximum-intensity projection (J) not present previously on axial fused PET-CT (F and H).
DM and PM are inflammatory myopathies characterized by moderate to severe proximal muscle weakness, often without myalgia, developing over weeks to months. DM is characterized by a typical rash that either accompanies or precedes muscle weakness, with additional symptoms that can include fever, weight loss, arthralgia and system-specific symptoms [e.g. dyspnoea secondary to interstitial lung disease (ILD)] [50].
DM and PM are both associated with increased cancer risk. A systematic review and meta-analysis of five population-based cohorts (>4500 patients) reported a cancer incidence of 12.1% and higher relative risk of cancer in DM than PM, with the highest risk for DM patients in the first year after diagnosis, persisting for 5 years post-diagnosis [51]. Cancer risk in DM patients increases 27-fold if the anti-TIF1-γ antibody is present [52]. CT and whole-body MRI are effective in detecting unsuspected cancer in DM/PM patients [53, 54], while FDG PET-CT has equivalent accuracy (92.7%) to multiple conventional cancer screening tests (e.g. tumour markers and imaging) [55] while being advantageous as a single imaging investigation requiring only a single hospital visit. The best use of imaging is in a complementary role to a thorough history and clinical examination and complete autoantibody profile [56].
FDG PET-CT can assess disease activity in DM/PM, although concurrent statin use resulting in a drug-induced myositis can confound interpretation. Small studies have reported higher muscular FDG uptake in a proximal symmetrical distribution in patients with DM/PM compared with controls and have shown a positive correlation with serum creatine kinase levels [57–62]. Qualitative visual analysis is associated with variable sensitivity for detecting myositis (Supplementary Fig. S6, available at Rheumatology online) [57–59]. Matuszak et al. [60] reported high accuracy using semiquantitative analysis and a muscle maximum standardized uptake value (SUVmax) cut-off of 0.66 for differentiating DM/PM patients from controls. However, normal skeletal muscle demonstrates mild homogeneous uptake (SUVmax 0.5–2.0, mean 1.0) [63]. This can be increased by several factors, including exertion, a non-fasting state and radiation therapy, while SUVmax cut-off values themselves can be problematic due to measurement variability related to biological and technical factors [64]. FDG PET-CT can confirm the presence of coexistent ILD (organizing pneumonia and non-specific interstitial pneumonia), present in 17–36% patients with DM/PM and which is a significant cause of morbidity and mortality (Supplementary Fig. S7 and S8, available at Rheumatology online). Higher levels of FDG uptake can be associated with a poorer prognosis and the development of rapidly progressive ILD [62].
Adult-onset Still’s disease
A middle-aged woman presented with cough, arthralgia, myalgia, macular rash, night sweats and weight loss. Blood tests revealed mild anaemia; elevated lactate dehydrogenase, ESR and CRP; and hyperferritinaemia (48 000 µg/l). CECT of the chest, abdomen and pelvis revealed widespread lymphadenopathy suspicious for lymphoma. FDG PET-CT confirmed widespread FDG avid lymphadenopathy, abnormal splenic and marrow uptake and symmetrical arthropathy of the elbows, wrists and small joints of both hands (Fig. 5). Lymph node biopsies showed reactive changes, while a bone marrow biopsy demonstrated myeloid hyperplasia only. Lymphoma was excluded and a diagnosis of adult-onset Still’s disease (AOSD) was confirmed.
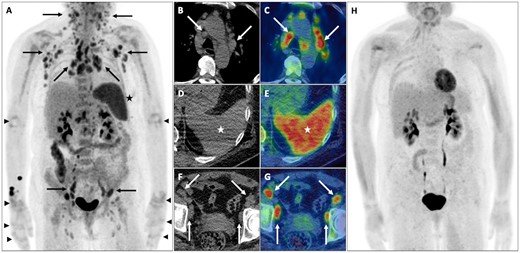
AOSD
Widespread FDG avid lymphadenopathy (solid black and white arrows) on PET maximum-intensity projection (A) and axial CT and fused PET-CT of the chest (B and C) and pelvis (F and G), abnormal diffuse splenic uptake on PET maximum-intensity projection (A), axial CT and fused PET-CT (D and E) and lower intensity diffuse increased marrow uptake. Although appearances are highly suspicious for lymphoma, the presence of FDG avid arthropathy of the elbows, wrist and small joints of both hands (black arrowheads) suggests AOSD. Response assessment FDG PET-CT following 3 months of GCs demonstrates normalization of appearances on PET maximum-intensity projection (H).
AOSD is a systemic inflammatory disease characterized by spiking fevers, arthralgia and an evanescent rash. It remains a clinical diagnosis using the Yamaguchi criteria [65] but often requires exclusion of differential diagnoses that include infection, malignancy and other systemic inflammatory diseases. Severe complications can arise with AOSD, including macrophage activation syndrome, with an associated mortality rate of 10–41%, highlighting the need for early diagnosis and treatment [66].
Small studies have reported a consistent pattern of symmetrical increased FDG uptake in cervical, axillary and inguinal lymph nodes as well as scattered nodes elsewhere in the chest, abdomen and pelvis, in conjunction with diffuse increased splenic and bone marrow uptake [67–73]. A combination of the above findings can help differentiate AOSD from other rheumatological diseases with high accuracy in the context of pyrexia of unknown origin [67]. The intensity of uptake correlates with disease severity [68, 69] and the volume of FDG avid nodal disease can be a strong predictor of macrophage activation syndrome occurrence [69]. However, appearances on FDG PET-CT are largely indistinguishable from lymphoma. The presence of symmetrical FDG avid arthropathy in up to 54% of cases [71, 72] can improve the specificity of observations, but frequently FDG PET-CT is required to identify suitable nodal or extranodal targets for biopsy with a view to confirming/refuting a diagnosis of lymphoma. Evidence regarding FDG PET-CT response assessment is scarce, although a couple of studies have reported that disease response is associated with a reduction in FDG uptake [72, 73].
Emerging indications
Relapsing polychondritis
A young man presented with progressive breathlessness and weight loss. Blood tests revealed elevated ESR and CRP. An initial CT of the thorax was reported as normal. He was commenced on high-dose GCs for a putative diagnosis of asthma/hypersensitivity pneumonitis, but was unable to wean off GCs. Repeat CECT demonstrated an abnormally thickened trachea. FDG PET-CT performed to assess the extent and disease activity confirmed diffuse increased FDG uptake in the central airways and in relation to the costal cartilages bilaterally, consistent with relapsing polychondritis (Fig. 6). Commencement of MTX allowed for a steroid wean and discontinuation. Repeat FDG PET-CT following treatment demonstrated a reduction in central airways uptake but persistent uptake in the left main bronchus with new luminal narrowing (Supplementary Fig. S9, available at Rheumatology online). Low-dose GCs were restarted, with subsequent bronchoscopy confirming tracheomalacia and a fixed left main bronchus stenosis, which required balloon dilatation.

Relapsing polychondritis
Diffuse FDG avid circumferential soft tissue (solid black and white arrows) around the trachea and main bronchi with narrowing of the left main bronchus (black and white arrowheads) in conjunction with increased FDG uptake around the costochondral junctions bilaterally (dashed black and white arrows) in keeping with inflammation and relapsing polychondritis on PET maximum-intensity projection (A) and coronal CT, PET and fused PET-CT (B–G).
Relapsing polychondritis is a multisystem disease characterized by repeated and progressive inflammation of cartilaginous structures in the body. Clinical features include auricular, nasal and tracheobronchial cartilaginous inflammation, the latter observed in up to 50% of patients, and arthropathy [74]. Clinical criteria are used to confirm a diagnosis of relapsing polychondritis (e.g. McAdam criteria) [75]. Histology from affected cartilage may be obtained in cases of uncertainty, although biopsy results are often non-specific.
FDG PET-CT accurately demonstrates the distribution of disease, often manifesting as multifocal symmetrical increased FDG uptake involving the central airways, nasal and auricular cartilages, which can be considered pathognomic for relapsing polychondritis. FDG avid active inflammation at these locations can be associated with or precede the development of site-specific symptoms [76–79]. FDG PET-CT can assist in targeting of appropriate sites of FDG avid inflammation, improving the positive biopsy rate compared with non-targeted biopsy (e.g. bronchoscopy) and thus facilitating early diagnosis and treatment [76]. FDG PET-CT post-treatment imaging can demonstrate partial or complete resolution of previous sites of FDG avid inflammation, suggesting a role in the assessment of treatment response [76–79].
RA
An elderly man presented with new-onset hoarse voice, weight loss and arthralgia. Blood tests revealed elevated ESR and CRP with negative RF, anti-CCP and ANA. FDG PET-CT found no evidence of malignancy but revealed widespread symmetrical polyarticular FDG avid arthropathy (Supplementary Fig. S10, available at Rheumatology online). A diagnosis of seronegative RA was made and the patient was commenced on GCs, MTX and HCQ, with an excellent clinical and biochemical response.
RA is a polyarticular inflammatory disease that often presents with pain and swelling of the hands and feet and joint stiffness. Early diagnosis and treatment, the mainstay of clinical management, facilitated by the detection of subclinical synovitis on imaging (US, MRI), helps prevents progression to irreversible joint damage and its associated morbidity [80]. FDG PET-CT typically demonstrates synovial uptake involving several joints (small or large) in a symmetrical distribution with occasional reactive local nodes (e.g. axillae or groins) and can identify other extra-articular manifestations, including aortic and carotid inflammation, which is associated with higher levels of disease activity and increased risk of cardiovascular disease [81–83].
Qualitative and quantitative assessment of FDG avid arthropathy, including volumetric SUV measurements, can help distinguish RA from non-RA diseases [84], but in most instances the intensity of FDG uptake is similar [45, 46, 85] and it is the distribution of uptake that is the crucial discriminator. For example, sparing of the distal IP joints is more suggestive of RA than OA, while the absence of enthesopathy/bursitis around the femurs, bony pelvis and vertebral column reliably differentiates RA from PMR, except for polymyalgic-onset RA, which can have a combination of the above findings (Supplementary Fig. S11, available at Rheumatology online).
Studies have confirmed a correlation between the intensity of joint uptake and clinical [e.g. 28-joint DAS (DAS-28)], biochemical (CRP, ESR) and radiological (US, MRI) measures of disease activity [86–91] and some have reported an association between the intensity of pretreatment joint uptake and the risk of developing large joint destruction [92, 93]. FDG PET-CT can demonstrate a reduction in intensity of synovial uptake that correlates with US and MRI assessment of disease [90] following several therapies [90, 91, 94, 95] with early metabolic response after 2 weeks, correlating with DAS-28 at 12 weeks following triple combination DMARD therapy [94] and DAS-28 at 14 and 24 weeks following anti-TNF-α therapy [95]. A reduction in arterial inflammation can also be observed following anti-inflammatory therapies [96].
GPA
A middle-aged woman with known GPA and recurrent subglottic stenosis requiring repeated local GC injections and balloon dilatations presented with recurrent symptoms of stridor and breathlessness. ESR and CRP were normal, as were anti-PR3 titres. FDG PET-CT showed persistent subglottic soft tissue thickening demonstrating increased FDG uptake consistent with active inflammation (Supplementary Fig. S12, available at Rheumatology online). The patient was commenced on rituximab leading to an improvement in symptoms.
GPA is a necrotizing small vessel vasculitis classically involving the respiratory tract and kidneys, associated with positive anti-PR3 ANCA. Alongside features of constitutional upset, clinical manifestations affecting the ear, nose and upper airways are common. Prompt diagnosis and commencement of immunosuppressive therapy are vital to reducing morbidity and mortality related to untreated renal disease [97, 98]. FDG PET-CT can detect multiple sites of disease, including nasal cavity, paranasal sinuses and upper aerodigestive tract (Supplementary Fig. S13, available at Rheumatology online), central airways and lungs, as well as kidneys and heart, despite their tendency for high background physiological uptake. Although individually non-specific, in combination these sites of active disease can help formulate a diagnosis of GPA while excluding alternative causes of systemic upset. FDG PET-CT can identify sites for biopsy to obtain a histological diagnosis and assist in assessment of treatment response, being able to differentiate between sites of active and inactive disease based on the intensity of residual FDG uptake [99–102].
Immune checkpoint inhibitor–induced rheumatic disease
An elderly man with metastatic prostate cancer treated with a trial combination of ipilimumab and nivolumab [i.e. immune checkpoint inhibitors (ICIs)] for 5 months reported new-onset subacute central chest pain. Blood tests revealed elevated ESR and CRP. CT pulmonary angiography was negative for pulmonary embolism. FDG PET-CT demonstrated abnormal increased murally based uptake in the aorta and its major branches (>liver uptake), in keeping with an active LVV. Response assessment FDG PET-CT demonstrated normalization of vascular uptake following 6 months of GC therapy with corresponding resolution of symptoms (Supplementary Fig. S14, available at Rheumatology online).
ICIs are a form of cancer treatment comprising monoclonal antibodies that block immune checkpoint cell surface receptors with resultant augmentation of T cell–mediated tumour destruction. The spectrum of toxicities related to ICIs, known as immune-related adverse events (irAEs), result from autoimmunity targeting normal organs [70, 71]. irAEs are common, with an estimated incidence of 66–87% [103], occur more frequently and severely with combination therapy and may be associated with other organ-specific irAEs [104]; the occurrence of an irAE can be associated with better outcomes [105].
Rheumatic irAEs can be divided into articular (36%) (Supplementary Fig. S15, available at Rheumatology online), muscular (34%), granulomatous (6%), vasculitic (12%) and systemic (12%). Although rarely reported in randomized clinical trials due to multiple factors [106], rheumatic irAEs are observed in ∼10% of patients in routine clinical practice [107] and can persist even after stopping ICIs [108]. Depending on the disease severity, and alongside continuing or pausing ICI therapy, treatment options can range from simple relief with anti-inflammatory drugs and low-dose GCs to conventional synthetic and biologic DMARDs, and even high-dose GCs and IVIG with/without plasma exchange for life-threatening manifestations of myositis [107].
Compared with conventional imaging, FDG PET-CT can provide an earlier accurate assessment of response to ICIs alongside early detection of irAEs, some of which may even precede clinical symptoms, enabling a more personalized risk-adapted approach to patient management [109, 110]. Specific to rheumatic immune-related events, FDG PET-CT allows assessment for alternative diagnoses that may present in a similar way (e.g. metastatic disease, paraneoplastic syndromes or unrelated rheumatic diseases).
IgG4-related disease
A young man presented with vomiting, diarrhoea, weight loss, night sweats and arthralgia. His past medical history included two previous episodes of unexplained acute pancreatitis and latent tuberculosis treated >10 years previously. Clinical examination revealed left cervical lymphadenopathy. Blood tests revealed a neutrophilia; elevated ESR, CRP and alkaline phosphatase (275 IU/l); and mild renal impairment. The serum IgG4 level was markedly elevated (14.95 g/l). CECT of the neck, chest, abdomen and pelvis revealed widespread lymphadenopathy, heterogeneous enhancement of the liver, bilaterally enlarged kidneys and features of acute pancreatitis. Liver biopsy demonstrated several IgG4-positive plasma cells but did not meet the histological criteria for IgG4-related disease (IgG4-RD); there was no evidence of Mycobacterium tuberculosis. FDG PET-CT demonstrated FDG avid supra- and sub-diaphragmatic lymph nodes with multi-organ diffuse FDG uptake, including the salivary glands, pancreas, kidneys and prostate. FDG PET-CT appearances in combination with the elevated serum IgG4 level and overall clinical picture were considered consistent with IgG4-RD disease. The patient was commenced on 40 mg of prednisolone daily and showed good clinical improvement with complete metabolic response to treatment on follow-up FDG PET-CT (Supplementary Fig. S16, available at Rheumatology online).
IgG4-RD is an immune-mediated disease characterized by focal or diffuse enlargement of one or more organs, elevated serum IgG4 levels (≥1.35 g/l) and marked lymphoplasmacytic infiltration, storiform fibrosis and organ infiltration by IgG4-positive plasma cells on histology [111]. Patients often present with subacute masses that are either clinically palpable (e.g. salivary or lacrimal gland enlargement) or are demonstrated on imaging, the latter often indistinguishable from cancer, but with most patients constitutionally well and without elevated inflammatory markers [112]. Serum IgG4 levels are usually but not always elevated, while the presence of IgG4-positive plasma cells on histology is not specific for IgG4-RD [113], emphasizing the requirement for careful clinico-radiological and clinico-pathological correlation in all cases.
FDG PET-CT can fulfil several roles in the management of IgG4-RD [114]. Certain patterns of FDG uptake can suggest IgG4-RD, e.g. diffuse pancreatic uptake [115, 116], symmetrical diffuse salivary and lacrimal gland uptake [116] and vascular uptake involving the iliac arteries or infrarenal aorta [117]. A combination of the above (i.e. involvement of two or more organs) is frequently associated with IgG4-RD and best demonstrated on FDG PET-CT owing to its superior sensitivity for disease detection [116, 118]. Demonstration of additional sites of disease enables consideration of alternative and potentially more accessible sites for biopsy, with a view to histological confirmation of IgG4-RD.
FDG PET-CT can demonstrate a metabolic response to GCs as well as steroid-sparing agents, with a reduction and often normalization of FDG uptake in IgG4-RD lesions. Studies have shown a correlation between derived SUV metrics and several markers of disease activity, including serum IgG4 and sIL-2R levels [119], circulating plasmablast levels [120] and the validated IgG4-responder index [121]. However, these studies remain exploratory and further research is required to better understand the role of FDG PET-CT in response assessment.
Conclusion
Non-oncological applications of FDG PET-CT continue to be developed with a growing evidence base supporting the use of FDG PET-CT in several rheumatological diseases. FDG uptake is non-specific, but the distribution and localization of inflammatory pattern uptake can help narrow differentials and suggest specific diagnoses. FDG PET-CT can demonstrate the true extent of active disease, identify sites that are occult on conventional imaging and may have prognostic significance, in addition to identifying safe and accessible sites for biopsy to obtain histological confirmation. For these reasons, it is not unreasonable to consider FDG PET-CT early in the diagnostic imaging pathway. Finally, there is an emerging role of FDG PET-CT in the assessment of response to therapy and monitoring of disease activity. However, a larger body of evidence comprising prospectively conducted multicentre trials comparing FDG PET-CT with established clinical diagnostic algorithms is required to fully understand and realize the potential of FDG PET-CT in rheumatological diseases.
Acknowledgements
This work was supported by the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z) and the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ National Health Service (NHS) Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The first draft of the manuscript was written by M.S. with assistance from S.B. and N.A. All authors commented on previous versions of the manuscript and read and approved the final manuscript. Patients signed informed consent regarding using their scan images for scientific purposes.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
Royal College of Radiologists, Royal College of Physicians of London, Royal College of Physicians and Surgeons of Glasgow, Royal College of Physicians of Edinburgh, British Nuclear Medicine Society, Administration of Radioactive Substances Advisory Committee. Evidence-based indications for the use of PET-CT in the United Kingdom. London: Royal College of Radiologists,




Comments