-
PDF
- Split View
-
Views
-
Cite
Cite
Ilaria Maccora, Niccolò Lombardi, Giada Crescioli, Alessandra Bettiol, Roberto Bonaiuti, Ilaria Pagnini, Valerio Maniscalco, Edoardo Marrani, Maria Vincenza Mastrolia, Claudia Ravaldi, Rita Consolini, Marco Cattalini, Alfredo Vannacci, Gabriele Simonini, OBSIDIAN – real-world evidence of originator to biosimilar drug switch in juvenile idiopathic arthritis, Rheumatology, Volume 61, Issue 4, April 2022, Pages 1518–1528, https://doi.org/10.1093/rheumatology/keab572
Close - Share Icon Share
Abstract
Limited data about use of biosimilars (BIOs) are available in children with JIA. This study therefore aimed to evaluate long-term efficacy and safety of switching from etanercept (ETA) and adalimumab (ADA) originators to their biosimilars (BIOs), in children with JIA, in a real-world setting.
This is a retro-prospective non-interventional multicentre Italian comparative cohort study. Medical charts of JIA children treated with biosimilars of ETA or ADA were included. Efficacy and safety of TNF-inhibitors therapy was evaluated at last follow-up during originator and at 3, 6 and 12 months following the switch to biosimilar.
A total of 59 children (42 female, median age at onset 88 months) were treated with biosimilar of ETA (21) and ADA (38). Forty-five switched from the originator to the BIO (17 ETA, 28 ADA). At time of switch, 12/17 patients on ETA and 18/28 on ADA were in remission. No significant difference has been found at 3, 6 and 12 months after the switch. Ten patients discontinued biosimilars due to disease remission (4 ETA, 3 ADA), family willing (1 ETA), occurrence of burning at injection site (1 ETA) and persistent activity (1 ADA). No statistically significant difference was observed between originator and BIOs, nor between originator and BIOs, and between ADA and ETA in time to disease remission achievement, time to relapse and number of patients who experienced adverse event (AE).
Our real-life results seem to confirm the efficacy and safety profile of switching from originator of ADA and ETA to their respective BIOs, also in paediatric patients with JIA.
Treatment with biologics and biosimilars has dramatically improved clinical outcomes of juvenile idiopathic arthritis patients.
TNF-inhibitor biosimilars showed similar efficacy and safety in juvenile idiopathic arthritis, although further studies are required.
Introduction
JIA is the most common chronic rheumatic disease of childhood with an estimated prevalence of 3.8–400 cases every 100 000 children under 16 years of age [1]. JIA encompasses a heterogeneous group of arthritis characterized by persistent joint inflammation lasting longer than 6 weeks and beginning before 16 years with an unknown cause. According to the International League of Associations for Rheumatology (ILAR) criteria, the term JIA distinguishes seven categories with different clinical presentation, disease course and treatment response [2]. In 2019, an international consensus was performed to review this classification that is still waiting for validation [3]. Uveitis is its most frequent complication, involving up to 20% of JIA patients [4–7]. In the past, this disease was burdened by numerous joint and ocular complications.
In the last 20 years, the use of biologic drugs such as tumour necrosis factor α inhibitors (TNFα-inhibitors) has dramatically improved JIA outcomes with a good safety profile [8–25], even if their high cost has partially hampered a broader use. While many biologics are still covered by patents, some others, such as etanercept (ETA) and adalimumab (ADA) are now available as biosimilars (BIOs) because their patents have expired. With the placing on the market of the first BIO TNFα-inhibitors, a new era has started. As defined by the European Medicines Agency and Food and Drugs Administration, a BIO is a biologic product that is similar to an already approved reference biologic drug in terms of efficacy, potency, purity, quality and immunogenicity [26–31]. However, a BIO is not completely equivalent to its originator, because biologics are complex molecules, very sensitive to any slight change in the manufacturing process [28].
To date, large-scale paediatric trials are lacking, and only some observational studies in paediatric IBD are available for children [32–36]. In Italy, since 2018, ETA and ADA BIOs have been available for JIA as part of routine clinical practice. Healthcare services have the chance to switch JIA patients treated with originators to BIOs for economic reasons defined as ‘nonmedical switching’. Data about the BIO efficacy and safety in JIA patients were reported only in small cohorts presented in conference abstracts, and in two papers that include adult JIA [37, 38]. In this context, data from larger cohorts with a longer follow-up are needed. It appears reasonable that physicians need cumulative evidence about BIO efficacy and safety over time, including the effects of switching and the ‘naïve’ use.
Assuming that ETA and ADA BIOs have comparable properties to the originators, we aimed to perform a retro-prospective cohort study on the efficacy and safety of BIOs compared with originators in a representative JIA paediatric sample over a long-term follow-up in a real-world setting.
Methods
Study design, setting and population
This was a retro-prospective non-interventional multicentre comparative cohort study involving the rheumatology unit of the following hospitals: Meyer Children’s University Hospital, Spedali Civili di Brescia and Santa Chiara University Hospital of Pisa. Outpatients of these rheumatology units have been enrolled from December 2019 to August 2020 if they fulfilled the following inclusion criteria: (i) diagnosis of JIA according to ILAR criteria [2]; (ii) age under 18 years old during treatment; (iii) treatment with BIOs of ETA (Benepali, Erelzi) or ADA (Amgevita, Imraldi, Solymbic), that were the biosimilars available at the time of the study in to the different Italian recruiting centres; (iv) negativity of infectious screening; (v) follow-up of at least 3 months after the starting of the BIOs. We considered the following exclusion criteria: (i) systemic JIA with persistent systemic features; (ii) other diagnosis than JIA.
Data collection and outcomes
Data were retrieved by the revision of the medical records of JIA patients treated with BIOs and collected in an ad-hoc Excel customized database. For each included patient, the following data have been collected: demographic data (gender, age at onset); characteristics of the disease (JIA category and presence of uveitis); autoantibody positivity (ANA, RF, ANCA, and positivity for other antibodies); HLA typing; comorbidities (i.e. autoimmune thyroiditis, coeliac disease, IBD); previous treatments before TNFα-inhibitors.
We defined ‘biologic naïve’ those patients who received biosimilar as first biologic, without being pre-exposed to other originator biologics.
In order to compare the outcome of originators vs BIOs, medical records of JIA patients who previously received originator and then have switched to BIO were retrieved. Data have been collected at the start of TNFα-inhibitors (originator and/or BIOs), after at least 3, 6, 12 months and at the last available follow-up on treatment. The following information has been collected at the above mentioned time points: number of active joints, presence of active uveitis, inflammatory markers (ESR in mm/h and CRP in mg/dl), parent/patient global assessment (PPGA) measured on a 10-cm Visual Analogue Scale (VAS) and Physician Global Assessment (PGA) measured on a 10-cm VAS, Childhood Health Assessment questionnaire (CHAQ), the 10-joint Juvenile Arthritis Disease Activity Score (JADAS10) [39], data on concomitant treatments (topical and systemic corticosteroid, DMARDs), safety data [in particular the number of adverse drug events (ADEs), number of serious ADEs, and ADEs description], date and reason of TNFα-inhibitors discontinuation. We also reported the reason for switching from originator to BIOs.
Definition of outcomes
Treatment response has been evaluated considering the following definitions: complete response, as inactive disease, defined by JADAS10 <0.7, ESR <20 mm/h, CRP<0.5 mg/dl, N of active joints <1, PPGA <2, PGA <2 and absence of uveitis. Failure was defined as the presence of one of the following conditions: worsening or no improvement of the aforementioned parameters compared with baseline, discontinuation of BIOs for severe adverse event (AE) and/or for significant laboratory alterations, relapse of arthritis, any intermittent or continuous suspension of BIOs for a cumulative period of >4 weeks.
Statistical analysis
Statistical analysis has been performed using STATA version 14. Only subjects with available data for a given variable at a considered follow-up time point were accounted for in the analysis, and no imputation of missing data was performed.
Continuous variables have been with medians and interquartile ranges (IQR), while categorical variables were summarized with frequencies and percentages. Demographic and clinical features have been compared between the ADA and the ETN groups using the Mann–Whitney test or the Fisher exact test for unpaired data. The efficacy of each treatment has been assessed by comparing the parameters related to disease activity at baseline, with those reported at 3, 6 and 12 months of follow-up using the Wilcoxon signed-rank test or the McNemars’ test for paired data. Relapse-free survival was assessed using Kaplan–Meier curves and hazard ratios from univariate and multivariate Cox regression models, adjusted by sex, age and disease duration. For each subject, the total number of ADEs and serious ADEs per year was calculated. A P-value ≤0.05 was considered to indicate significance. The study obtained approval from Meyer Children’s Hospital IRB (250/2019) and patients signed informed consent for study participation.
Results
Fifty-nine patients were enrolled during the observation period (42 females 71.2%, and 17 males 28.8%). Among them, 21 were treated with ETA and 38 with ADA. Forty-five patients (76.3%) switched from the originator to the biosimilar (17 patients among those treated with ETA, and 28 among those treated with ADA), while 14 were biologic-naïve (four patients among those treated with ETA, and 10 among those treated with ADA). Population characteristics are summarized in Table 1. Overall, the median age at disease onset of the disease was 7.33 years (IQR2.83–10.58), while the median age at the start of the BIOs was 13.7 years (IQR 10.9–15.5). Enrolled patients included 15 persistent oligoarticular JIA (25.4%), 21 polyarticular JIA (35.6%), eight extended oligoarticular JIA (13.6%), seven psoriatic JIA (11.9%), and eight enthesitis-related arthritis (13.6%). Eleven patients (18.6%) had uveitis. Among laboratory characteristics, ANA positivity was found in 36 patients (61%) and HLA B27 in five patients (8.5%). Eleven patients (18.6%) reported comorbidities. Patients on ETA and ADA were comparable in terms of all demographic and clinical features, with the only exception of uveitis, which was significantly more frequent in patients on ADA (29.0% vs 0%, P = 0.005).
Patients’ clinical and demographic characteristics. Previous and concomitant treatments
| . | Overall n = 59 (%) . | Etanercept n = 21 (%) . | Adalimumab n = 38 (%) . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Males | 17 (28.81) | 4 (19.05) | 13 (34.21) | |||
| Females | 42 (71.19) | 17 (80.85) | 25 (65.79) | |||
| Age | ||||||
| At the onset of the disease, months (median, IQR) | 7.33 (2.83–10.58) | 4 (2.58–8.91) | 7.41 (2.91–10.83) | |||
| At the start of therapy, years (median, IQR) | 9.53 (7.32–12.59) | 8.39 (7.22–12.26) | 9.83 (7.33–12.77) | |||
| At the therapy switch, years (median, IQR) | 13.67 (10.86–15.46) | 13.96 (10.55–15.94) | 13.50 (10.88–15.40) | |||
| Category of JIA | ||||||
| Oligoarticular JIA | 23 (38.98) | 9 (42.85) | 14 (36.85) | |||
| Persistent oligoarticular JIA | 15 (25.42) | 5 (23.81) | 10 (26.32) | |||
| Extended oligoarticular JIA | 8 (13.56) | 4 (19.05) | 4 (10.53) | |||
| Polyarticular JIA | 21 (35.59) | 7 (33.33) | 14 (36.84) | |||
| Psoriatic JIA | 7 (11.86) | 4 (19.05) | 3 (7.89) | |||
| Enthesitis-related JIA | 8 (13.56) | 1 (3.9) | 7 (18.42) | |||
| Presence of uveitis | ||||||
| No | 48 (81.36) | 21 (100) | 27 (71.05) | |||
| Yes | 11 (18.64) | 0 | 11 (28.95) | |||
| HLA | ||||||
| HLA1- B27 | 15 (25.42) | 3 (14.28) | 12 (31.58) | |||
| HLA2 | 5 (8.47)a | 3 (14.28) | 2 (5.26) | |||
| ANA | ||||||
| Negative | 23 (38.98) | 8 (38.10) | 15 (39.47) | |||
| Positive | 36 (61.02) | 13 (61.90) | 23 (60.53) | |||
| Other antibodies | ||||||
| Negative | 51 (86.44) | 20 (95.24) | 31 (81.58) | |||
| Positiveb | 8 (13.56) | 1 (4.76) | 7 (18.42) | |||
| Comorbiditiesc | ||||||
| No | 48 (81.36) | 16 (76.19) | 32 (84.21) | |||
| Yes | 11 (18.64)# | 5 (23.81) | 6 (15.79) | |||
| Biologic-naïve | ||||||
| No | 45 (76.27) | 17 (80.95) | 28 (73.68) | |||
| Yes | 14 (23.73) | 4 (19.05) | 10 (26.32) | |||
| Previous treatments | ||||||
| Non-biologics | 58 (98.31) | 21 (100) | 37 (97.37) | |||
| Methotrexate | 52 (88.14) | 18 (85.71) | 34 (89.47) | |||
| Sulfasalazine | 8 (13.56) | 3 (14.29) | 5 (13.16) | |||
| Ciclosporin | 1 (1.4) | 0 | 1 (2.2) | |||
| NSAIDs | 51 (86.44) | 18 (85.71) | 33 (86.84) | |||
| Prednisone daily dosage, mg (median, IQR) | 0 (0–10) | 0 (0–5) | 0 (0–15) | |||
| Biologics | 2 (3.39) | 0 | 2 (5.26) | |||
| Canakinumab | 1 (1.69) | 0 | 1 (2.63) | |||
| Abatacept | 1 (1.69) | 0 | 1 (2.63) | |||
| Number of previous therapies, non-biologics and biologics (median, IQR) | 2 (2–4) | 2 (2–4) | 2 (2–4) | |||
| Concomitant therapies at the start of the first anti-TNFα | ||||||
| Steroids | 9 (15.25) | 4 (19.05) | 5 (13.16) | |||
| Methotrexate | 37 (62.71) | 10 (47.62) | 27 (71.05) | |||
| Other DMARDs | 9 (15.25) | 6 (28.57) | 3 (7.89) | |||
| Concomitant therapies at the time of switch | ||||||
| Steroids | 3 (6.67) | 2 (11.76) | 1 (3.57) | |||
| Methotrexate | 17 (37.78) | 5 (29.41) | 12 (42.86) | |||
| Other DMARDs | 4 (8.89) | 2 (11.76) | 2 (7.14) | |||
| Years of therapy with the originator before the switch (median, IQR) | 3.36 (1.70–6.06) | 5.37 (2.44–7.74) | 3.02 (1.50–4.73) | |||
| . | Overall n = 59 (%) . | Etanercept n = 21 (%) . | Adalimumab n = 38 (%) . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Males | 17 (28.81) | 4 (19.05) | 13 (34.21) | |||
| Females | 42 (71.19) | 17 (80.85) | 25 (65.79) | |||
| Age | ||||||
| At the onset of the disease, months (median, IQR) | 7.33 (2.83–10.58) | 4 (2.58–8.91) | 7.41 (2.91–10.83) | |||
| At the start of therapy, years (median, IQR) | 9.53 (7.32–12.59) | 8.39 (7.22–12.26) | 9.83 (7.33–12.77) | |||
| At the therapy switch, years (median, IQR) | 13.67 (10.86–15.46) | 13.96 (10.55–15.94) | 13.50 (10.88–15.40) | |||
| Category of JIA | ||||||
| Oligoarticular JIA | 23 (38.98) | 9 (42.85) | 14 (36.85) | |||
| Persistent oligoarticular JIA | 15 (25.42) | 5 (23.81) | 10 (26.32) | |||
| Extended oligoarticular JIA | 8 (13.56) | 4 (19.05) | 4 (10.53) | |||
| Polyarticular JIA | 21 (35.59) | 7 (33.33) | 14 (36.84) | |||
| Psoriatic JIA | 7 (11.86) | 4 (19.05) | 3 (7.89) | |||
| Enthesitis-related JIA | 8 (13.56) | 1 (3.9) | 7 (18.42) | |||
| Presence of uveitis | ||||||
| No | 48 (81.36) | 21 (100) | 27 (71.05) | |||
| Yes | 11 (18.64) | 0 | 11 (28.95) | |||
| HLA | ||||||
| HLA1- B27 | 15 (25.42) | 3 (14.28) | 12 (31.58) | |||
| HLA2 | 5 (8.47)a | 3 (14.28) | 2 (5.26) | |||
| ANA | ||||||
| Negative | 23 (38.98) | 8 (38.10) | 15 (39.47) | |||
| Positive | 36 (61.02) | 13 (61.90) | 23 (60.53) | |||
| Other antibodies | ||||||
| Negative | 51 (86.44) | 20 (95.24) | 31 (81.58) | |||
| Positiveb | 8 (13.56) | 1 (4.76) | 7 (18.42) | |||
| Comorbiditiesc | ||||||
| No | 48 (81.36) | 16 (76.19) | 32 (84.21) | |||
| Yes | 11 (18.64)# | 5 (23.81) | 6 (15.79) | |||
| Biologic-naïve | ||||||
| No | 45 (76.27) | 17 (80.95) | 28 (73.68) | |||
| Yes | 14 (23.73) | 4 (19.05) | 10 (26.32) | |||
| Previous treatments | ||||||
| Non-biologics | 58 (98.31) | 21 (100) | 37 (97.37) | |||
| Methotrexate | 52 (88.14) | 18 (85.71) | 34 (89.47) | |||
| Sulfasalazine | 8 (13.56) | 3 (14.29) | 5 (13.16) | |||
| Ciclosporin | 1 (1.4) | 0 | 1 (2.2) | |||
| NSAIDs | 51 (86.44) | 18 (85.71) | 33 (86.84) | |||
| Prednisone daily dosage, mg (median, IQR) | 0 (0–10) | 0 (0–5) | 0 (0–15) | |||
| Biologics | 2 (3.39) | 0 | 2 (5.26) | |||
| Canakinumab | 1 (1.69) | 0 | 1 (2.63) | |||
| Abatacept | 1 (1.69) | 0 | 1 (2.63) | |||
| Number of previous therapies, non-biologics and biologics (median, IQR) | 2 (2–4) | 2 (2–4) | 2 (2–4) | |||
| Concomitant therapies at the start of the first anti-TNFα | ||||||
| Steroids | 9 (15.25) | 4 (19.05) | 5 (13.16) | |||
| Methotrexate | 37 (62.71) | 10 (47.62) | 27 (71.05) | |||
| Other DMARDs | 9 (15.25) | 6 (28.57) | 3 (7.89) | |||
| Concomitant therapies at the time of switch | ||||||
| Steroids | 3 (6.67) | 2 (11.76) | 1 (3.57) | |||
| Methotrexate | 17 (37.78) | 5 (29.41) | 12 (42.86) | |||
| Other DMARDs | 4 (8.89) | 2 (11.76) | 2 (7.14) | |||
| Years of therapy with the originator before the switch (median, IQR) | 3.36 (1.70–6.06) | 5.37 (2.44–7.74) | 3.02 (1.50–4.73) | |||
One patient positive for DQ5 and DR1; one patient positive for DR11 and DQ2; three patients positive for DR5. bOne patient with anti-gliadin antibodies; one patient with antimicrosomal antibodies; one patient with anti-mitochondrial antibodies; one patient positive for ANCA (p-ANCA). cOne patient with Prader–Willi syndrome; two patients allergic to inhalant agents; three coeliac patients; two patients with psoriasis; one patient with Hashimoto thyroiditis; one patient with growth hormone deficiency; one patient with osteochondritis; one patient with cerebral paralysis; one patient with anxiety-depressive disorder. IQR: interquartile range; IL1: interleukine 1; IL6: interleukine 6.
Patients’ clinical and demographic characteristics. Previous and concomitant treatments
| . | Overall n = 59 (%) . | Etanercept n = 21 (%) . | Adalimumab n = 38 (%) . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Males | 17 (28.81) | 4 (19.05) | 13 (34.21) | |||
| Females | 42 (71.19) | 17 (80.85) | 25 (65.79) | |||
| Age | ||||||
| At the onset of the disease, months (median, IQR) | 7.33 (2.83–10.58) | 4 (2.58–8.91) | 7.41 (2.91–10.83) | |||
| At the start of therapy, years (median, IQR) | 9.53 (7.32–12.59) | 8.39 (7.22–12.26) | 9.83 (7.33–12.77) | |||
| At the therapy switch, years (median, IQR) | 13.67 (10.86–15.46) | 13.96 (10.55–15.94) | 13.50 (10.88–15.40) | |||
| Category of JIA | ||||||
| Oligoarticular JIA | 23 (38.98) | 9 (42.85) | 14 (36.85) | |||
| Persistent oligoarticular JIA | 15 (25.42) | 5 (23.81) | 10 (26.32) | |||
| Extended oligoarticular JIA | 8 (13.56) | 4 (19.05) | 4 (10.53) | |||
| Polyarticular JIA | 21 (35.59) | 7 (33.33) | 14 (36.84) | |||
| Psoriatic JIA | 7 (11.86) | 4 (19.05) | 3 (7.89) | |||
| Enthesitis-related JIA | 8 (13.56) | 1 (3.9) | 7 (18.42) | |||
| Presence of uveitis | ||||||
| No | 48 (81.36) | 21 (100) | 27 (71.05) | |||
| Yes | 11 (18.64) | 0 | 11 (28.95) | |||
| HLA | ||||||
| HLA1- B27 | 15 (25.42) | 3 (14.28) | 12 (31.58) | |||
| HLA2 | 5 (8.47)a | 3 (14.28) | 2 (5.26) | |||
| ANA | ||||||
| Negative | 23 (38.98) | 8 (38.10) | 15 (39.47) | |||
| Positive | 36 (61.02) | 13 (61.90) | 23 (60.53) | |||
| Other antibodies | ||||||
| Negative | 51 (86.44) | 20 (95.24) | 31 (81.58) | |||
| Positiveb | 8 (13.56) | 1 (4.76) | 7 (18.42) | |||
| Comorbiditiesc | ||||||
| No | 48 (81.36) | 16 (76.19) | 32 (84.21) | |||
| Yes | 11 (18.64)# | 5 (23.81) | 6 (15.79) | |||
| Biologic-naïve | ||||||
| No | 45 (76.27) | 17 (80.95) | 28 (73.68) | |||
| Yes | 14 (23.73) | 4 (19.05) | 10 (26.32) | |||
| Previous treatments | ||||||
| Non-biologics | 58 (98.31) | 21 (100) | 37 (97.37) | |||
| Methotrexate | 52 (88.14) | 18 (85.71) | 34 (89.47) | |||
| Sulfasalazine | 8 (13.56) | 3 (14.29) | 5 (13.16) | |||
| Ciclosporin | 1 (1.4) | 0 | 1 (2.2) | |||
| NSAIDs | 51 (86.44) | 18 (85.71) | 33 (86.84) | |||
| Prednisone daily dosage, mg (median, IQR) | 0 (0–10) | 0 (0–5) | 0 (0–15) | |||
| Biologics | 2 (3.39) | 0 | 2 (5.26) | |||
| Canakinumab | 1 (1.69) | 0 | 1 (2.63) | |||
| Abatacept | 1 (1.69) | 0 | 1 (2.63) | |||
| Number of previous therapies, non-biologics and biologics (median, IQR) | 2 (2–4) | 2 (2–4) | 2 (2–4) | |||
| Concomitant therapies at the start of the first anti-TNFα | ||||||
| Steroids | 9 (15.25) | 4 (19.05) | 5 (13.16) | |||
| Methotrexate | 37 (62.71) | 10 (47.62) | 27 (71.05) | |||
| Other DMARDs | 9 (15.25) | 6 (28.57) | 3 (7.89) | |||
| Concomitant therapies at the time of switch | ||||||
| Steroids | 3 (6.67) | 2 (11.76) | 1 (3.57) | |||
| Methotrexate | 17 (37.78) | 5 (29.41) | 12 (42.86) | |||
| Other DMARDs | 4 (8.89) | 2 (11.76) | 2 (7.14) | |||
| Years of therapy with the originator before the switch (median, IQR) | 3.36 (1.70–6.06) | 5.37 (2.44–7.74) | 3.02 (1.50–4.73) | |||
| . | Overall n = 59 (%) . | Etanercept n = 21 (%) . | Adalimumab n = 38 (%) . | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Males | 17 (28.81) | 4 (19.05) | 13 (34.21) | |||
| Females | 42 (71.19) | 17 (80.85) | 25 (65.79) | |||
| Age | ||||||
| At the onset of the disease, months (median, IQR) | 7.33 (2.83–10.58) | 4 (2.58–8.91) | 7.41 (2.91–10.83) | |||
| At the start of therapy, years (median, IQR) | 9.53 (7.32–12.59) | 8.39 (7.22–12.26) | 9.83 (7.33–12.77) | |||
| At the therapy switch, years (median, IQR) | 13.67 (10.86–15.46) | 13.96 (10.55–15.94) | 13.50 (10.88–15.40) | |||
| Category of JIA | ||||||
| Oligoarticular JIA | 23 (38.98) | 9 (42.85) | 14 (36.85) | |||
| Persistent oligoarticular JIA | 15 (25.42) | 5 (23.81) | 10 (26.32) | |||
| Extended oligoarticular JIA | 8 (13.56) | 4 (19.05) | 4 (10.53) | |||
| Polyarticular JIA | 21 (35.59) | 7 (33.33) | 14 (36.84) | |||
| Psoriatic JIA | 7 (11.86) | 4 (19.05) | 3 (7.89) | |||
| Enthesitis-related JIA | 8 (13.56) | 1 (3.9) | 7 (18.42) | |||
| Presence of uveitis | ||||||
| No | 48 (81.36) | 21 (100) | 27 (71.05) | |||
| Yes | 11 (18.64) | 0 | 11 (28.95) | |||
| HLA | ||||||
| HLA1- B27 | 15 (25.42) | 3 (14.28) | 12 (31.58) | |||
| HLA2 | 5 (8.47)a | 3 (14.28) | 2 (5.26) | |||
| ANA | ||||||
| Negative | 23 (38.98) | 8 (38.10) | 15 (39.47) | |||
| Positive | 36 (61.02) | 13 (61.90) | 23 (60.53) | |||
| Other antibodies | ||||||
| Negative | 51 (86.44) | 20 (95.24) | 31 (81.58) | |||
| Positiveb | 8 (13.56) | 1 (4.76) | 7 (18.42) | |||
| Comorbiditiesc | ||||||
| No | 48 (81.36) | 16 (76.19) | 32 (84.21) | |||
| Yes | 11 (18.64)# | 5 (23.81) | 6 (15.79) | |||
| Biologic-naïve | ||||||
| No | 45 (76.27) | 17 (80.95) | 28 (73.68) | |||
| Yes | 14 (23.73) | 4 (19.05) | 10 (26.32) | |||
| Previous treatments | ||||||
| Non-biologics | 58 (98.31) | 21 (100) | 37 (97.37) | |||
| Methotrexate | 52 (88.14) | 18 (85.71) | 34 (89.47) | |||
| Sulfasalazine | 8 (13.56) | 3 (14.29) | 5 (13.16) | |||
| Ciclosporin | 1 (1.4) | 0 | 1 (2.2) | |||
| NSAIDs | 51 (86.44) | 18 (85.71) | 33 (86.84) | |||
| Prednisone daily dosage, mg (median, IQR) | 0 (0–10) | 0 (0–5) | 0 (0–15) | |||
| Biologics | 2 (3.39) | 0 | 2 (5.26) | |||
| Canakinumab | 1 (1.69) | 0 | 1 (2.63) | |||
| Abatacept | 1 (1.69) | 0 | 1 (2.63) | |||
| Number of previous therapies, non-biologics and biologics (median, IQR) | 2 (2–4) | 2 (2–4) | 2 (2–4) | |||
| Concomitant therapies at the start of the first anti-TNFα | ||||||
| Steroids | 9 (15.25) | 4 (19.05) | 5 (13.16) | |||
| Methotrexate | 37 (62.71) | 10 (47.62) | 27 (71.05) | |||
| Other DMARDs | 9 (15.25) | 6 (28.57) | 3 (7.89) | |||
| Concomitant therapies at the time of switch | ||||||
| Steroids | 3 (6.67) | 2 (11.76) | 1 (3.57) | |||
| Methotrexate | 17 (37.78) | 5 (29.41) | 12 (42.86) | |||
| Other DMARDs | 4 (8.89) | 2 (11.76) | 2 (7.14) | |||
| Years of therapy with the originator before the switch (median, IQR) | 3.36 (1.70–6.06) | 5.37 (2.44–7.74) | 3.02 (1.50–4.73) | |||
One patient positive for DQ5 and DR1; one patient positive for DR11 and DQ2; three patients positive for DR5. bOne patient with anti-gliadin antibodies; one patient with antimicrosomal antibodies; one patient with anti-mitochondrial antibodies; one patient positive for ANCA (p-ANCA). cOne patient with Prader–Willi syndrome; two patients allergic to inhalant agents; three coeliac patients; two patients with psoriasis; one patient with Hashimoto thyroiditis; one patient with growth hormone deficiency; one patient with osteochondritis; one patient with cerebral paralysis; one patient with anxiety-depressive disorder. IQR: interquartile range; IL1: interleukine 1; IL6: interleukine 6.
Moreover, Table 1 shows patients’ previous and concomitant treatments. Fifty-eight patients (98.3%) received methotrexate as previous treatment, while eight (13.6%) received sulfasalazine, one (1.4%) ciclosporin, and 51 (86.4%) NSAIDs as previous non-biologic treatments. Among previous treatments, two patients received other biologics (3.4%), one anti-IL 1 and one abatacept. Overall, the median number of previous therapies, non-biologics and biologics was two (IQR 2–4). At the beginning of the first TNFα-inhibitors, nine patients (15.2%) were concomitantly treated with corticosteroids, 37 (62.7%) with methotrexate, and nine (15.2%) with other DMARDs. Overall, patients received the originator for a median time of 3.4 years (IQR 1.7–6.1). At the time of the switch from the originator to the biosimilar, concomitant therapies were reported therapies in three patients (6.7%) as corticosteroids, in 17 (37.8%) as methotrexate, and in four (8.9%) as other DMARDs.
Inactive diseases, evaluation of the disease, relapses and discontinuations of patients who switched from the originator to BIOs of ETA are reported in Table 2. Among the 17 patients who switched from the originator to the BIOs, 12 (70.6%) had an inactive disease at the moment of the switch. Three months after the switch, all patients maintained the remission and one new inactive disease was reported, with a statistically significant improvement in CRP (mg/dl), JADAS10, PGA compared with the time of switching (P =0.034, 0.015 and 0.026, respectively). Only one patient discontinued the therapy for parents’ choice. At the second time point (6 months), inactive disease maintenance was reported for eight patients (80.0%), three patients discontinued the therapy for persistent inactive disease, and no significant difference was observed among the parameters for the evaluation of the disease. Twelve months after the switch, nine patients (81.8%) maintained inactive disease, and no significant difference was observed in terms of efficacy. Two patients discontinued the therapy, one for persistent inactive disease and one for burning at the injection site. No new relapse was observed during the 12 months of follow-up after the switch to the BIOs of ETA.
JIA remission maintenance after the switch from the originator to the biosimilar in patients treated with etanercept
| . | Switch (n = 17) . | 3 months after the switch (n = 16) . | P-valuea . | 6 months after the switch (n = 10) . | P-valuea . | 12 months after the switch (n = 11) . | P-valuea . | |
|---|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 12 (70.59) | 13 (81.25) | 0.157 | 8 (80.00) | 0.564 | 9 (81.82) | 0.317 | |
| Inactive disease maintenance (%) | — | 12 (75.00) | 8 (80.00) | 9 (81.82) | ||||
| New remission (%) | — | 1 (6.25) | — | — | ||||
| Evaluation of the disease | ||||||||
| N of articulations median ( IQR) | 0 (0–1.0) | 0 (0–0) | 0.172 | 0 (0–0.5) | 0.089 | 0 (0–0) | 0.181 | |
| Uveitis, n (%) | 0 | 0 | 1 | 0 | 1 | 0 | 1 | |
| CRP (mg/dl) median ( IQR) | 0.29 (0.29–0.60) | 0.29 (0.10–0.29) | 0.034 | 0.29 (0.1–0.29) | 0.816 | 0.29 (0.29–0.29) | 0.883 | |
| ESR (mm/h) median ( IQR) | 7.0 (2–25) | 8.0 (2.0–16.0) | 0.114 | 8.0 (4–20) | 0.457 | 12.0 (5.0–18.0) | 0.374 | |
| JADAS10 median ( IQR) | 0.10 (0–12.10) | 0 (0–0.80) | 0.015 | 0 (0–5.3) | 0.151 | 0 (0–3.0) | 0.228 | |
| PGA median ( IQR) | 0 (0–3.0) | 0 (0–0) | 0.026 | 0 (0–2) | 0.098 | 0 (0–1.0) | 0.119 | |
| PPGA median ( IQR) | 0 (0–3.50) | 0 (0–3) | 0.075 | 0 (0–3) | 0.139 | 0 (0–1.0) | 0.082 | |
| CHAQ median ( IQR) | 0 (0–1.0) | 0 (0–0.2) | 0.095 | 0 (0–0.2) | 0.186 | 0 (0–0) | 0.073 | |
| Relapse n (%) | — | 0 | 0 | 0 | ||||
| Discontinuation | — | 1/16 | 3/10 | 2/11 | ||||
| . | Switch (n = 17) . | 3 months after the switch (n = 16) . | P-valuea . | 6 months after the switch (n = 10) . | P-valuea . | 12 months after the switch (n = 11) . | P-valuea . | |
|---|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 12 (70.59) | 13 (81.25) | 0.157 | 8 (80.00) | 0.564 | 9 (81.82) | 0.317 | |
| Inactive disease maintenance (%) | — | 12 (75.00) | 8 (80.00) | 9 (81.82) | ||||
| New remission (%) | — | 1 (6.25) | — | — | ||||
| Evaluation of the disease | ||||||||
| N of articulations median ( IQR) | 0 (0–1.0) | 0 (0–0) | 0.172 | 0 (0–0.5) | 0.089 | 0 (0–0) | 0.181 | |
| Uveitis, n (%) | 0 | 0 | 1 | 0 | 1 | 0 | 1 | |
| CRP (mg/dl) median ( IQR) | 0.29 (0.29–0.60) | 0.29 (0.10–0.29) | 0.034 | 0.29 (0.1–0.29) | 0.816 | 0.29 (0.29–0.29) | 0.883 | |
| ESR (mm/h) median ( IQR) | 7.0 (2–25) | 8.0 (2.0–16.0) | 0.114 | 8.0 (4–20) | 0.457 | 12.0 (5.0–18.0) | 0.374 | |
| JADAS10 median ( IQR) | 0.10 (0–12.10) | 0 (0–0.80) | 0.015 | 0 (0–5.3) | 0.151 | 0 (0–3.0) | 0.228 | |
| PGA median ( IQR) | 0 (0–3.0) | 0 (0–0) | 0.026 | 0 (0–2) | 0.098 | 0 (0–1.0) | 0.119 | |
| PPGA median ( IQR) | 0 (0–3.50) | 0 (0–3) | 0.075 | 0 (0–3) | 0.139 | 0 (0–1.0) | 0.082 | |
| CHAQ median ( IQR) | 0 (0–1.0) | 0 (0–0.2) | 0.095 | 0 (0–0.2) | 0.186 | 0 (0–0) | 0.073 | |
| Relapse n (%) | — | 0 | 0 | 0 | ||||
| Discontinuation | — | 1/16 | 3/10 | 2/11 | ||||
Reason for discontinuation: 0–3 months: parents’ choice; 3–6 months: persistent remission; 6–12 months: one for remission, one for burning at the injection site. aMcNemar’s test for categorical variables, Wilcoxon’s test for continuous variables. CHAQ: childhood health assessment questionnaire; IQR: interquartile range; JADAS10: juvenile arthritis disease activity score10; n: number; PGA: physician global assessment; PPGA: parent/patient global assessment. Bold values represent the % or the IQR.
JIA remission maintenance after the switch from the originator to the biosimilar in patients treated with etanercept
| . | Switch (n = 17) . | 3 months after the switch (n = 16) . | P-valuea . | 6 months after the switch (n = 10) . | P-valuea . | 12 months after the switch (n = 11) . | P-valuea . | |
|---|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 12 (70.59) | 13 (81.25) | 0.157 | 8 (80.00) | 0.564 | 9 (81.82) | 0.317 | |
| Inactive disease maintenance (%) | — | 12 (75.00) | 8 (80.00) | 9 (81.82) | ||||
| New remission (%) | — | 1 (6.25) | — | — | ||||
| Evaluation of the disease | ||||||||
| N of articulations median ( IQR) | 0 (0–1.0) | 0 (0–0) | 0.172 | 0 (0–0.5) | 0.089 | 0 (0–0) | 0.181 | |
| Uveitis, n (%) | 0 | 0 | 1 | 0 | 1 | 0 | 1 | |
| CRP (mg/dl) median ( IQR) | 0.29 (0.29–0.60) | 0.29 (0.10–0.29) | 0.034 | 0.29 (0.1–0.29) | 0.816 | 0.29 (0.29–0.29) | 0.883 | |
| ESR (mm/h) median ( IQR) | 7.0 (2–25) | 8.0 (2.0–16.0) | 0.114 | 8.0 (4–20) | 0.457 | 12.0 (5.0–18.0) | 0.374 | |
| JADAS10 median ( IQR) | 0.10 (0–12.10) | 0 (0–0.80) | 0.015 | 0 (0–5.3) | 0.151 | 0 (0–3.0) | 0.228 | |
| PGA median ( IQR) | 0 (0–3.0) | 0 (0–0) | 0.026 | 0 (0–2) | 0.098 | 0 (0–1.0) | 0.119 | |
| PPGA median ( IQR) | 0 (0–3.50) | 0 (0–3) | 0.075 | 0 (0–3) | 0.139 | 0 (0–1.0) | 0.082 | |
| CHAQ median ( IQR) | 0 (0–1.0) | 0 (0–0.2) | 0.095 | 0 (0–0.2) | 0.186 | 0 (0–0) | 0.073 | |
| Relapse n (%) | — | 0 | 0 | 0 | ||||
| Discontinuation | — | 1/16 | 3/10 | 2/11 | ||||
| . | Switch (n = 17) . | 3 months after the switch (n = 16) . | P-valuea . | 6 months after the switch (n = 10) . | P-valuea . | 12 months after the switch (n = 11) . | P-valuea . | |
|---|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 12 (70.59) | 13 (81.25) | 0.157 | 8 (80.00) | 0.564 | 9 (81.82) | 0.317 | |
| Inactive disease maintenance (%) | — | 12 (75.00) | 8 (80.00) | 9 (81.82) | ||||
| New remission (%) | — | 1 (6.25) | — | — | ||||
| Evaluation of the disease | ||||||||
| N of articulations median ( IQR) | 0 (0–1.0) | 0 (0–0) | 0.172 | 0 (0–0.5) | 0.089 | 0 (0–0) | 0.181 | |
| Uveitis, n (%) | 0 | 0 | 1 | 0 | 1 | 0 | 1 | |
| CRP (mg/dl) median ( IQR) | 0.29 (0.29–0.60) | 0.29 (0.10–0.29) | 0.034 | 0.29 (0.1–0.29) | 0.816 | 0.29 (0.29–0.29) | 0.883 | |
| ESR (mm/h) median ( IQR) | 7.0 (2–25) | 8.0 (2.0–16.0) | 0.114 | 8.0 (4–20) | 0.457 | 12.0 (5.0–18.0) | 0.374 | |
| JADAS10 median ( IQR) | 0.10 (0–12.10) | 0 (0–0.80) | 0.015 | 0 (0–5.3) | 0.151 | 0 (0–3.0) | 0.228 | |
| PGA median ( IQR) | 0 (0–3.0) | 0 (0–0) | 0.026 | 0 (0–2) | 0.098 | 0 (0–1.0) | 0.119 | |
| PPGA median ( IQR) | 0 (0–3.50) | 0 (0–3) | 0.075 | 0 (0–3) | 0.139 | 0 (0–1.0) | 0.082 | |
| CHAQ median ( IQR) | 0 (0–1.0) | 0 (0–0.2) | 0.095 | 0 (0–0.2) | 0.186 | 0 (0–0) | 0.073 | |
| Relapse n (%) | — | 0 | 0 | 0 | ||||
| Discontinuation | — | 1/16 | 3/10 | 2/11 | ||||
Reason for discontinuation: 0–3 months: parents’ choice; 3–6 months: persistent remission; 6–12 months: one for remission, one for burning at the injection site. aMcNemar’s test for categorical variables, Wilcoxon’s test for continuous variables. CHAQ: childhood health assessment questionnaire; IQR: interquartile range; JADAS10: juvenile arthritis disease activity score10; n: number; PGA: physician global assessment; PPGA: parent/patient global assessment. Bold values represent the % or the IQR.
Inactive diseases, evaluation of the disease, relapses and discontinuations of patients who switched from the originator to BIOs of ADA are reported in Table 3. Among the 28 patients who switched from the originator to the BIOs, 18 (64.3%) had inactive disease at the moment of the switch. Three months after the switch, all patients maintained the inactive disease and one new remission was reported. No significant difference was observed among the parameters for the evaluation of the disease. At the second time-point (6 months), inactive disease maintenance was reported for 21 patients (91.3%), two patients discontinued the therapy for persistent inactive disease, with a statistically significant improvement in JADAS10 score compared with the time of switching (P-value 0.049). Twelve months after the switch, 17 patients (85.0%) maintained inactive disease, and no significant difference was observed in terms of efficacy. Two patients discontinued the therapy, of whom one for persistent inactive disease and one for persistent disease activity. New relapses were observed in two patients 6 months and 12 months after the switch to the BIOs of ADA, respectively.
JIA remission maintenance after the switch from the originator to the biosimilar in patients treated with adalimumab
| . | Switch (n = 28) . | 3 months after the switch (n = 26) . | P-valuea . | 6 months after the switch (n = 23) . | P-valuea . | 12 months after the switch (n = 20) . | P-valuea . |
|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 18 (64.29) | 19 (73.08) | 0.414 | 21 (91.30) | 0.058 | 17 (85.00) | 0.479 |
| Inactive disease maintenance, n (%) | — | 18 (69.23) | 21 (91.30) | 17 (85.00) | |||
| New remission, n (%) | — | 1 (3.85) | — | — | |||
| Evaluation of the disease | |||||||
| n of articulation, median (IQR) | 0 (0–0) | 0 (0–0) | 0.395 | 0 (0–0) | 0.223 | 0 (0–0) | 0.416 |
| Uveitis, n (%) | 4 (14.29) | 1 (3.85) | 0.083 | 0 | 0.083 | 1 (5.26) | 1 |
| CRP (mg/dl) , median, (IQR) | 0.29 (0.29–0.54) | 0.29 (0.29–0.29) | 0.627 | 0.29 (0.29–0.33) | 0.923 | 0.29 (0.29–0.39) | 0.294 |
| ESR (mm/h), median (IQR) | 10.5 (5–20.5) | 8.0 (6.0–19.0) | 0.818 | 9.0 (6.0–18.0) | 0.376 | 8.0 (3.0–20.0) | 0.494 |
| JADAS10, median (IQR) | 0 (0–2.40) | 0 (0–2.80) | 0.580 | 0 (0–0) | 0.049 | 0 (0–0.20) | 0.249 |
| PGA,median (IQR) | 0 (0–0) | 0 (0–0) | 0.985 | 0 (0–0) | 0.221 | 0 (0–0) | 0.638 |
| PPGA, median (IQR) | 0 (0–1.5) | 0 (0–2.0) | 0.950 | 0 (0–0) | 0.106 | 0 (0–0) | 0.289 |
| CHAQ, median (IQR) | 0 (0–0.1) | 0 (0–0.1) | 0.617 | 0 (0–0) | 0.092 | 0 (0–0) | 0.242 |
| Relapse, n (%) | — | 2 (7.69) | 1 (4.35) | 2 (11.76) | |||
| Discontinuation, n | — | 0 | 2/23 | 2/20 |
| . | Switch (n = 28) . | 3 months after the switch (n = 26) . | P-valuea . | 6 months after the switch (n = 23) . | P-valuea . | 12 months after the switch (n = 20) . | P-valuea . |
|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 18 (64.29) | 19 (73.08) | 0.414 | 21 (91.30) | 0.058 | 17 (85.00) | 0.479 |
| Inactive disease maintenance, n (%) | — | 18 (69.23) | 21 (91.30) | 17 (85.00) | |||
| New remission, n (%) | — | 1 (3.85) | — | — | |||
| Evaluation of the disease | |||||||
| n of articulation, median (IQR) | 0 (0–0) | 0 (0–0) | 0.395 | 0 (0–0) | 0.223 | 0 (0–0) | 0.416 |
| Uveitis, n (%) | 4 (14.29) | 1 (3.85) | 0.083 | 0 | 0.083 | 1 (5.26) | 1 |
| CRP (mg/dl) , median, (IQR) | 0.29 (0.29–0.54) | 0.29 (0.29–0.29) | 0.627 | 0.29 (0.29–0.33) | 0.923 | 0.29 (0.29–0.39) | 0.294 |
| ESR (mm/h), median (IQR) | 10.5 (5–20.5) | 8.0 (6.0–19.0) | 0.818 | 9.0 (6.0–18.0) | 0.376 | 8.0 (3.0–20.0) | 0.494 |
| JADAS10, median (IQR) | 0 (0–2.40) | 0 (0–2.80) | 0.580 | 0 (0–0) | 0.049 | 0 (0–0.20) | 0.249 |
| PGA,median (IQR) | 0 (0–0) | 0 (0–0) | 0.985 | 0 (0–0) | 0.221 | 0 (0–0) | 0.638 |
| PPGA, median (IQR) | 0 (0–1.5) | 0 (0–2.0) | 0.950 | 0 (0–0) | 0.106 | 0 (0–0) | 0.289 |
| CHAQ, median (IQR) | 0 (0–0.1) | 0 (0–0.1) | 0.617 | 0 (0–0) | 0.092 | 0 (0–0) | 0.242 |
| Relapse, n (%) | — | 2 (7.69) | 1 (4.35) | 2 (11.76) | |||
| Discontinuation, n | — | 0 | 2/23 | 2/20 |
Reason for discontinuation: 3–6 months: 2 for persistent remission; 6–12 months: 1 for persistent remission and 1 for persistent disease activity. aMcNemar’s test for categorical variables, Wilcoxon’s test for continuous variables CHAQ: childhood health assessment questionnaire; IQR: interquartile range; JADAS10: juvenile arthritis disease activity score10; n: number; PGA: physician global assessment, PPGA: parent/patient global assessment. Bold values represent the % or the IQR.
JIA remission maintenance after the switch from the originator to the biosimilar in patients treated with adalimumab
| . | Switch (n = 28) . | 3 months after the switch (n = 26) . | P-valuea . | 6 months after the switch (n = 23) . | P-valuea . | 12 months after the switch (n = 20) . | P-valuea . |
|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 18 (64.29) | 19 (73.08) | 0.414 | 21 (91.30) | 0.058 | 17 (85.00) | 0.479 |
| Inactive disease maintenance, n (%) | — | 18 (69.23) | 21 (91.30) | 17 (85.00) | |||
| New remission, n (%) | — | 1 (3.85) | — | — | |||
| Evaluation of the disease | |||||||
| n of articulation, median (IQR) | 0 (0–0) | 0 (0–0) | 0.395 | 0 (0–0) | 0.223 | 0 (0–0) | 0.416 |
| Uveitis, n (%) | 4 (14.29) | 1 (3.85) | 0.083 | 0 | 0.083 | 1 (5.26) | 1 |
| CRP (mg/dl) , median, (IQR) | 0.29 (0.29–0.54) | 0.29 (0.29–0.29) | 0.627 | 0.29 (0.29–0.33) | 0.923 | 0.29 (0.29–0.39) | 0.294 |
| ESR (mm/h), median (IQR) | 10.5 (5–20.5) | 8.0 (6.0–19.0) | 0.818 | 9.0 (6.0–18.0) | 0.376 | 8.0 (3.0–20.0) | 0.494 |
| JADAS10, median (IQR) | 0 (0–2.40) | 0 (0–2.80) | 0.580 | 0 (0–0) | 0.049 | 0 (0–0.20) | 0.249 |
| PGA,median (IQR) | 0 (0–0) | 0 (0–0) | 0.985 | 0 (0–0) | 0.221 | 0 (0–0) | 0.638 |
| PPGA, median (IQR) | 0 (0–1.5) | 0 (0–2.0) | 0.950 | 0 (0–0) | 0.106 | 0 (0–0) | 0.289 |
| CHAQ, median (IQR) | 0 (0–0.1) | 0 (0–0.1) | 0.617 | 0 (0–0) | 0.092 | 0 (0–0) | 0.242 |
| Relapse, n (%) | — | 2 (7.69) | 1 (4.35) | 2 (11.76) | |||
| Discontinuation, n | — | 0 | 2/23 | 2/20 |
| . | Switch (n = 28) . | 3 months after the switch (n = 26) . | P-valuea . | 6 months after the switch (n = 23) . | P-valuea . | 12 months after the switch (n = 20) . | P-valuea . |
|---|---|---|---|---|---|---|---|
| Inactive disease, n (%) | 18 (64.29) | 19 (73.08) | 0.414 | 21 (91.30) | 0.058 | 17 (85.00) | 0.479 |
| Inactive disease maintenance, n (%) | — | 18 (69.23) | 21 (91.30) | 17 (85.00) | |||
| New remission, n (%) | — | 1 (3.85) | — | — | |||
| Evaluation of the disease | |||||||
| n of articulation, median (IQR) | 0 (0–0) | 0 (0–0) | 0.395 | 0 (0–0) | 0.223 | 0 (0–0) | 0.416 |
| Uveitis, n (%) | 4 (14.29) | 1 (3.85) | 0.083 | 0 | 0.083 | 1 (5.26) | 1 |
| CRP (mg/dl) , median, (IQR) | 0.29 (0.29–0.54) | 0.29 (0.29–0.29) | 0.627 | 0.29 (0.29–0.33) | 0.923 | 0.29 (0.29–0.39) | 0.294 |
| ESR (mm/h), median (IQR) | 10.5 (5–20.5) | 8.0 (6.0–19.0) | 0.818 | 9.0 (6.0–18.0) | 0.376 | 8.0 (3.0–20.0) | 0.494 |
| JADAS10, median (IQR) | 0 (0–2.40) | 0 (0–2.80) | 0.580 | 0 (0–0) | 0.049 | 0 (0–0.20) | 0.249 |
| PGA,median (IQR) | 0 (0–0) | 0 (0–0) | 0.985 | 0 (0–0) | 0.221 | 0 (0–0) | 0.638 |
| PPGA, median (IQR) | 0 (0–1.5) | 0 (0–2.0) | 0.950 | 0 (0–0) | 0.106 | 0 (0–0) | 0.289 |
| CHAQ, median (IQR) | 0 (0–0.1) | 0 (0–0.1) | 0.617 | 0 (0–0) | 0.092 | 0 (0–0) | 0.242 |
| Relapse, n (%) | — | 2 (7.69) | 1 (4.35) | 2 (11.76) | |||
| Discontinuation, n | — | 0 | 2/23 | 2/20 |
Reason for discontinuation: 3–6 months: 2 for persistent remission; 6–12 months: 1 for persistent remission and 1 for persistent disease activity. aMcNemar’s test for categorical variables, Wilcoxon’s test for continuous variables CHAQ: childhood health assessment questionnaire; IQR: interquartile range; JADAS10: juvenile arthritis disease activity score10; n: number; PGA: physician global assessment, PPGA: parent/patient global assessment. Bold values represent the % or the IQR.
Similar results in terms of disease control after the switch to BIOs have been found limiting the analysis to the seven and 10 patients with polyarticular involvement who switched to ETA and ADA BIOs, respectively (P=not significant).
ADEs experienced by patients are reported in Table 4. Among patients treated with ADA, 10, nine and 11 patients exposed to the originator reported ADEs at 3, 6 and 12 months of follow-up, respectively, compared with seven, 14 and nine patients exposed to BIOs. Serious ADEs were reported in one patient. In both groups, the most frequently reported ADEs were infections (47 cases), gastrointestinal disorders (eight cases) and local reactions (six cases). Among patients treated with ETA, four, six and six patients exposed to the originator reported ADEs at 3, 6 and 12 months of follow-up, respectively, compared with one, four and three patients exposed to BIOs. No significant difference was observed between originators and BIOs of ETA and ADA as reported in Table 4. One serious ADE was reported. In both groups, the most frequently reported ADEs were infections (16 cases), and local reactions (nine cases).
Patients who experienced adverse drug events – table reports data on 44 patients naïve to originators: one JIA child had no follow-up data at 3–6-12 months, thus adverse event information was not available
| . | Follow-up . | ||
|---|---|---|---|
| 3 months | 6 months | 12 months | |
| Adalimumab | |||
| Originator | 10/27 | 9/25 | 11/25 |
| ADEs description | 1 nausea | 1 abdominal pain | 1 appendicitis |
| 7 infections | 8 infections | 1 gastroenteritis | |
| 1 loss of consciousness | 1 abdominal pain | ||
| 1 urticaria | 2 burning/pain at the injection site | ||
| 11 infections | |||
| Biosimilar | 7/33 | 14/29 | 9/23 |
| ADEs description | 1 nausea | 1 gastroenteritis | 7 infections |
| 1 burning/pain at the injection site | 2 burning/pain at the injection site | 1 gastroenteritis | |
| 5 infections | 9 infections | 1 headache | |
| 1 headache | 1 impetigo | ||
| χ 1.83 P =0.170 | χ 0.82 P =0.360 | χ 0.11 P =0.730 | |
| n of serious ADEs | — | 1/29 | — |
| Etanercept | |||
| Originator | 4/17 | 6/17 | 6/17 |
| ADEs description | 4 infections | 1 burning/pain at the injection site | 1 anaemia |
| 1 headache | 4 infections | 1 burning/pain at the injection site | |
| 1 psoriasis | 3 infections | ||
| 1 headache | |||
| 1 erythema | |||
| Biosimilar | 1/20 | 4/14 | 3/13 |
| ADEs description | 1 burning at the injection site | 1 burning/pain at the injection site | 2 burning/pain at the injection site |
| 3 infections | 2 infections | ||
| 1 cutaneous rash | |||
| 1 chondromatosis of the knee | |||
| χ 2.69 P =0.100 | χ 0.15 P =0.690 | χ 0.52 P =0.460 | |
| n of serious ADEs | — | — | — |
| . | Follow-up . | ||
|---|---|---|---|
| 3 months | 6 months | 12 months | |
| Adalimumab | |||
| Originator | 10/27 | 9/25 | 11/25 |
| ADEs description | 1 nausea | 1 abdominal pain | 1 appendicitis |
| 7 infections | 8 infections | 1 gastroenteritis | |
| 1 loss of consciousness | 1 abdominal pain | ||
| 1 urticaria | 2 burning/pain at the injection site | ||
| 11 infections | |||
| Biosimilar | 7/33 | 14/29 | 9/23 |
| ADEs description | 1 nausea | 1 gastroenteritis | 7 infections |
| 1 burning/pain at the injection site | 2 burning/pain at the injection site | 1 gastroenteritis | |
| 5 infections | 9 infections | 1 headache | |
| 1 headache | 1 impetigo | ||
| χ 1.83 P =0.170 | χ 0.82 P =0.360 | χ 0.11 P =0.730 | |
| n of serious ADEs | — | 1/29 | — |
| Etanercept | |||
| Originator | 4/17 | 6/17 | 6/17 |
| ADEs description | 4 infections | 1 burning/pain at the injection site | 1 anaemia |
| 1 headache | 4 infections | 1 burning/pain at the injection site | |
| 1 psoriasis | 3 infections | ||
| 1 headache | |||
| 1 erythema | |||
| Biosimilar | 1/20 | 4/14 | 3/13 |
| ADEs description | 1 burning at the injection site | 1 burning/pain at the injection site | 2 burning/pain at the injection site |
| 3 infections | 2 infections | ||
| 1 cutaneous rash | |||
| 1 chondromatosis of the knee | |||
| χ 2.69 P =0.100 | χ 0.15 P =0.690 | χ 0.52 P =0.460 | |
| n of serious ADEs | — | — | — |
ADE: adverse drug event.
Patients who experienced adverse drug events – table reports data on 44 patients naïve to originators: one JIA child had no follow-up data at 3–6-12 months, thus adverse event information was not available
| . | Follow-up . | ||
|---|---|---|---|
| 3 months | 6 months | 12 months | |
| Adalimumab | |||
| Originator | 10/27 | 9/25 | 11/25 |
| ADEs description | 1 nausea | 1 abdominal pain | 1 appendicitis |
| 7 infections | 8 infections | 1 gastroenteritis | |
| 1 loss of consciousness | 1 abdominal pain | ||
| 1 urticaria | 2 burning/pain at the injection site | ||
| 11 infections | |||
| Biosimilar | 7/33 | 14/29 | 9/23 |
| ADEs description | 1 nausea | 1 gastroenteritis | 7 infections |
| 1 burning/pain at the injection site | 2 burning/pain at the injection site | 1 gastroenteritis | |
| 5 infections | 9 infections | 1 headache | |
| 1 headache | 1 impetigo | ||
| χ 1.83 P =0.170 | χ 0.82 P =0.360 | χ 0.11 P =0.730 | |
| n of serious ADEs | — | 1/29 | — |
| Etanercept | |||
| Originator | 4/17 | 6/17 | 6/17 |
| ADEs description | 4 infections | 1 burning/pain at the injection site | 1 anaemia |
| 1 headache | 4 infections | 1 burning/pain at the injection site | |
| 1 psoriasis | 3 infections | ||
| 1 headache | |||
| 1 erythema | |||
| Biosimilar | 1/20 | 4/14 | 3/13 |
| ADEs description | 1 burning at the injection site | 1 burning/pain at the injection site | 2 burning/pain at the injection site |
| 3 infections | 2 infections | ||
| 1 cutaneous rash | |||
| 1 chondromatosis of the knee | |||
| χ 2.69 P =0.100 | χ 0.15 P =0.690 | χ 0.52 P =0.460 | |
| n of serious ADEs | — | — | — |
| . | Follow-up . | ||
|---|---|---|---|
| 3 months | 6 months | 12 months | |
| Adalimumab | |||
| Originator | 10/27 | 9/25 | 11/25 |
| ADEs description | 1 nausea | 1 abdominal pain | 1 appendicitis |
| 7 infections | 8 infections | 1 gastroenteritis | |
| 1 loss of consciousness | 1 abdominal pain | ||
| 1 urticaria | 2 burning/pain at the injection site | ||
| 11 infections | |||
| Biosimilar | 7/33 | 14/29 | 9/23 |
| ADEs description | 1 nausea | 1 gastroenteritis | 7 infections |
| 1 burning/pain at the injection site | 2 burning/pain at the injection site | 1 gastroenteritis | |
| 5 infections | 9 infections | 1 headache | |
| 1 headache | 1 impetigo | ||
| χ 1.83 P =0.170 | χ 0.82 P =0.360 | χ 0.11 P =0.730 | |
| n of serious ADEs | — | 1/29 | — |
| Etanercept | |||
| Originator | 4/17 | 6/17 | 6/17 |
| ADEs description | 4 infections | 1 burning/pain at the injection site | 1 anaemia |
| 1 headache | 4 infections | 1 burning/pain at the injection site | |
| 1 psoriasis | 3 infections | ||
| 1 headache | |||
| 1 erythema | |||
| Biosimilar | 1/20 | 4/14 | 3/13 |
| ADEs description | 1 burning at the injection site | 1 burning/pain at the injection site | 2 burning/pain at the injection site |
| 3 infections | 2 infections | ||
| 1 cutaneous rash | |||
| 1 chondromatosis of the knee | |||
| χ 2.69 P =0.100 | χ 0.15 P =0.690 | χ 0.52 P =0.460 | |
| n of serious ADEs | — | — | — |
ADE: adverse drug event.
No statistically significant difference was observed between originator and BIOs of ADA and ETA (Fig. 1A and B), nor between originator and BIOs (Fig. 1C), and between ADA and ETA (Fig. 1D) in time to inactive disease achievement. For all these comparisons, no difference was observed for the outcome time to relapse (Supplementary Fig. S1, available at Rheumatology online).
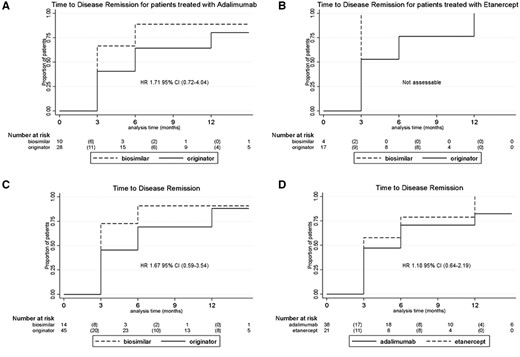
Time to disease remission
Difference in time of disease remission between originator and BIOs of ADA and ETA (A and B), between originator and BIOs (C) and between ADA and ETA (D). Numbers below each figure part represent the number or patients still at risk at a considered timepoint, whereas numbers in brackets represent the number of events occurring in the interval between the two follow-up timepoints.
Discussion
Despite national and international recommendations to use BIOs in clinical practice for the treatment of JIA patients, there are no randomized controlled trials or cohort studies to inform clinicians on the efficacy and safety of BIOs in paediatric JIA patients. The European Medicines Agency has encouraged a Bayesian approach to the development of BIOs in order to shorten the approval process, reduce costs and thereby increase access to biologic therapy [26, 29]. The European Medicines Agency, providing a strict similarity process control, extrapolated data from originators and claimed for BIOs the same rate of efficacy and safety due to randomized controlled trials in adults [26, 30, 31]. Only a few conference abstracts are available dealing with this relevant topic. Experience regarding real-world use of BIOs is needed to supplement RCTs that evaluate highly selected populations and adult patients. To our knowledge, this is the first and largest cohort reporting data on the efficacy and safety of BIOs in paediatric patients with juvenile idiopathic arthritis.
Our multicentre Italian cohort found no difference in a real-world setting in terms of efficacy and safety of switching from the reference drug to the BIOs of ADA and ETA in 59 JIA paediatric patients. No increase in the rate of relapse was observed, and the median levels of inflammatory index and JADAS10 score were always in the normal ranges during the follow-up period. Finally, no statistically significant difference was observed between originator and BIOs of ADA and ETA in time to disease remission achievement and in time to relapse.
The only two studies available in the literature reporting data about the use of BIOs in JIA are about a few adult patients treated with adalimumab and etanercept [37, 38]. The results of efficacy and safety of these drugs in this peculiar class of patients were presented aggregated with other adult patients with rheumatoid arthritis, psoriatic arthritis and axial spondylarthritis. The first study evaluated similar profiles of disease activity and safety profile after switching from reference ADA to its BIOs in a real-life setting with mild features of disease flare not leading to BIOs treatment interruption according to our data [38]. While the second study, which investigated the safety and treatment persistence of patients switching from the originator of ETA to its BIOs, showed a high persistence on treatment at 6, 12 and 18 months, loss of efficacy represented the most frequent reason for discontinuation and the 22.3% of patients experienced AEs [37]. The proportion of patients who discontinued the drug for loss of efficacy is higher in the study by Bruni et al. compared with our results, where the most frequent reason for discontinuation is the persistent remission.
Moreover, our results are in line with the other studies conducted in adult patients with inflammatory arthritis treated with BIOs of ADA and ETA [40–48, 50]. The demonstration of similar efficacy of BIOs comes from different study settings, primarily RCTs for both drugs, while observational studies are available mainly for etanercept because for adalimumab they are still lacking.
In particular, we need to mention the DANBIO registry, one of the largest observational studies for etanercept, where 1621 of 2061 patients switched to the biosimilar of ETA and showed an unchanged disease activity 3 months pre- and post-switch [40]. Furthermore, they showed an 82% of persistence on treatment at 1 year of follow-up compared with the 88% of historic cohort; however, they evaluated an increased withdrawal in patients not in remission. These data might suggest that these differences are not influenced by drug effects but by patient-related factors [40]. This peculiar situation is called the nocebo effect, and it is related to the patients’ perceived lack of efficacy or subjective complaints rather than the objective worsening of the disease. This peculiar effect was observed in numerous studies conducted in adult patients, but in our study, we apparently did not observe such an effect, probably because the information about biosimilars was presented in a positive context and was supported by studies already performed, even if in adults or childhood inflammatory bowel diseases. Moreover, in the treatment of children, a fundamental role is played by parents, who help in the management and care of these patients.
A recent systematic review about the biosimilars of adalimumab showed comparable efficacy, safety and immunogenicity among the different types of BIOs compared with the reference drug [48]. Moreover, another review that highlights the real-world data of biosimilars in adult patients with arthritis showed no significant inferiority in treatment outcomes, resulting in considerable cost savings [49].
As in inflammatory arthritis, TNF-inhibitors have drastically changed the prognosis of inflammatory bowel diseases (IBDs), and BIOs have also become a reality in this peculiar category of patients. The only studies regarding the use of BIOs in paediatric age are focussed on IBDs and evaluated the efficacy, safety and immunogenicity of infliximab [32–36, 47]. Alongside our data, they showed that switching from the reference drug to the BIO did not affect the efficacy of the drug and did not increase the rate of ADEs in a real-world setting [32–36, 50]. Even though we did not directly evaluate the efficacy and safety of infliximab in our study, and IBDs and JIA are different diseases, these results are consistent, supporting that biosimilars also show comparable properties to originators in paediatric age.
Moreover, the safety profile of originator and BIO were similar for ADA and ETA in terms not only of proportion of ADEs but also of type of ADEs. In fact, in our sample, the most frequently reported ADEs were infections, both for originator and BIOs. Something that might be interesting is that there is a slightly increased reporting of site injection reaction with biosimilars. This ADE was also reported in adult cohorts and seems to be correlated to the presence of citrate in biosimilars [40–45]. Furthermore, in our cohort there is not an increased rate of serious ADEs.
Before drawing our conclusions, we need to discuss some specific caveats of our study. Our real-life study has some key strengths; first of all, it is the first study in JIA patients, it includes a medium-large sample size and shows a considerable follow-up duration. However, the sample of naïve patients to biologic limits the analysis only to patients who switched from the reference drug to its BIO. Moreover, the observational nature of the study leads to some missing data and different duration of follow-up.
Nonetheless, while this cohort represents the largest data collection so far on this topic, the number of enrolled patients might be insufficient to identify a real difference and the power of the sample is not reliable enough to detect a real difference if it is true. This caveat impacts even greater on the subgroup analysis for etanercept and adalimumab. However, this is a retro-prospective study aiming to mirror real life in clinical practice, thus we could not calculate a priori analysis.
As an additional caveat, oligo-arthritis represents the second most frequent category in our enrolled cohort, and, because this category tends to have better outcomes, our results regarding stable remission might have been affected.
Nonetheless, while our data do not show significant concerns, a longer follow-up and a larger cohort result are needed before we can clearly conclude and state the safety profile regarding the biosimilars use in JIA.
Additionally, we did not record data about the immunogenicity of these drugs and a cost analysis to quantify the money saving for the sanitary system was not carried out.
Conclusion
In the last two decades, treatment with biologics – and biosimilars in the last few years – has dramatically improved clinical outcomes of JIA patients resulting in considerable cost savings in the real world. Results of our observational real-life study would seem to confirm the efficacy and safety profile of switching from the reference drugs of ADA and ETA to their respective BIOs in paediatric patients with JIA, even though these results came from a limited number of patients and with short follow-up. These data can reassure families and will help paediatric rheumatologists in their clinical practice. There is a need for further investigation in larger cohorts. Moreover, investigations are needed in patients who are naïve to biologics and start treatment with biosimilars.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data will be available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
European Medicines Agency. Biosimilars in the EU - Information guide for healthcare professionals. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf (30 March 2021, date last accessed).
Biosimilars. FDA Mon, 03/23/2020 – 14:43. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
Author notes
Alfredo Vannacci and Gabriele Simonini contributed equally to this study.




Comments