-
PDF
- Split View
-
Views
-
Cite
Cite
Sara Monti, Paolo Delvino, Mattia Riboli, Chiara Rebuffi, Blerina Xoxi, Annalisa De Silvestri, Carlomaurizio Montecucco, The role of trimethoprim/sulfametoxazole in reducing relapses and risk of infections in ANCA-associated vasculitis: a meta-analysis, Rheumatology, Volume 60, Issue 8, August 2021, Pages 3553–3564, https://doi.org/10.1093/rheumatology/keab267
Close - Share Icon Share
Abstract
To assess available evidence from randomized controlled trials (RCTs) and observational studies including a control group regarding the role of trimethoprim/sulfametoxazole (TMP/SMX) in reducing the relapse rate in patients with granulomatosis with polyangiitis (GPA) and the risk of infections in patients with ANCA-associated vasculitis (AAV).
MEDLINE, EMBASE, The Cochrane Library databases, Scopus, Web of Science and ClinicalTrials.gov were searched from inception until 15 January 2020 to identify controlled studies assessing the role of TMP/SMX in reducing the rate of relapse in patients with GPA (primary outcome) and the number and/or severity of infections in patients with AAV (secondary outcome). Two reviewers independently selected eligible studies and extracted data. Cumulative risk ratios (RRs) with 95% CI were calculated using a random effect meta-analysis.
Eight studies were selected out of 2907 records. Seven studies (520 patients) (of which two were RCTs) assessed the role of TMP/SMX in the relapse rate in patients with GPA. TMP/SMX was not associated with a reduced risk of relapse (RR = 1.15, 95% CI: 0.51, 2.55; I2 = 78.5%; P < 0.001). Sensitivity analysis according to the dose of TMP/SMX (960 mg twice daily vs three times/week) confirmed the results. One retrospective cohort study (192 patients) was identified demonstrating a significant reduction of severe infections in patients with AAV receiving prophylaxis with TMP/SMX in association with rituximab.
TMP/SMX was not associated with a reduced risk of relapse in patients with GPA. TMP/SMX might be useful in the reduction of infectious complications.
CRD42019118983.
Trimethoprim/sulfametoxazole was not associated with a reduced risk of relapse in patients with granulomatosis with polyangiitis.
Trimethoprim/sulfametoxazole did not reduce relapses regardless of the dose (prophylactic vs full daily dose).
Trimethoprim/sulfametoxazole might be useful in the reduction of infectious complications in patients with ANCA-associated vasculitis.
Introduction
ANCA-associated vasculitis (AAV) encompasses three entities: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic GPA (EGPA). The treatment of AAV has gone through paradigmatic changes in recent years, along with a radical change in the prognosis of the disease. Increasing evidence from high-quality randomized controlled trials (RCTs) has led to the approval of new efficacious drugs and has significantly improved and standardized the management of these conditions. Nevertheless, some uncertainty in the optimal strategies to control disease activity, prevent relapses that still occur in up to 50% of patients and reduce permanent damage still exists [1]. Moreover, improvement of preventive strategies to control the significant mortality risk related to infections is pivotal. The role of trimethoprim/sulfametoxazole (TMP/SMX)—co-trimoxazole—in this context still remains controversial. Previous studies had suggested a relationship between Staphylococcus aureus (SA) nasal carriage and risk of relapses in GPA, supporting a rationale for the antimicrobial role of TMP/SMX. The hypothesized mechanisms through which SA could trigger disease activity include the pro-inflammatory signalling connected with the chronic infection or the activation of the innate and adaptive immune system. SA carriers among patients with GPA have been reported to experience more frequent relapses, but a causative role has never been proven [2–4]. A direct anti-inflammatory effect of TMP/SMX has also been postulated through the interference with the production of oxygen-derived radicals by primed neutrophils leading to local tissue destruction in GPA. Finally, TMP/SMX may exert an immunosuppressive effect contributing to the control of disease activity through the antagonism of folic acid metabolism [5]. The currently available EULAR recommendations for the management of AAV suggest that the addition of TMP/SMX to standard remission maintenance can reduce the risk of relapse in GPA, but there is no clear-cut indication for the routine use of TMP/SMX as a co-intervention in addition to standard immunosuppressive regimens to control disease activity, and no indication on the duration of such treatment. TMP/SMX has been routinely used as prophylaxis against Pneumocystis jirovecii (PCP) in all patients treated with CYC, but its use in patients treated with other immunosuppressants or during high-dose glucocorticoid (GC) or prolonged lower-dose GC therapy has not been specifically and routinely recommended in AAV.
To address these controversies, we performed a systematic literature review and meta-analysis to address the role of TMP/SMX in reducing the risk of relapse in GPA and its role in preventing infections in patients with AAV. We included RCTs and observational studies if they included a comparator group not exposed to TMP/SMX.
Methods
Definition of outcomes
A systematic literature review was performed to assess the role of TMP/SMX on the relapse rate of patients with GPA and on the risk of infections in patients with AAV. The primary outcome was the reduction in the rate of relapses in GPA, measured using time to first relapse and percentage of relapses or patients in remission at different time points. Secondary outcomes were reduction in the number and/or severity of infections (including PCP) in patients with GPA/AAV.
Data sources and search strategy
We searched the following electronic bibliographic databases: MEDLINE, EMBASE, The Cochrane Library databases, Scopus, Web of Science and ClinicalTrials.gov from inception until 15 January 2020 to identify eligible studies. The search strategy was developed using Thesaurus terms and free-text terms according to a population, intervention, comparison and outcomes (PICO) strategy. Details on the search strategy are presented in the Supplementary material, Appendix A, available at Rheumatology online.
Eligible studies were all full research articles with the following language restrictions: English, French, German, Portuguese, Spanish and Italian. All study designs were included if a comparator/control group was available. Abstracts from conferences were screened and included if relevant.
Patients with a diagnosis of GPA or AAV were included if exposed to TMP/SMX. Comparator/control groups were patients exposed to placebo (or no exposure to the experimental drug) and/or on remission maintenance immunosuppressive treatment prescribed to treat AAV.
Study selection
Selection of studies was based on title and/or abstract and was performed by two independent reviewers (P.D., M.R.). Controversies were solved by discussion after full-text review by a third investigator (S.M.). The preferred reporting items for systematic reviews and meta-analyses flowchart diagram displaying the number of records identified by the systematic literature review (SLR), the number of excluded articles with reasons and the final number of studies included is presented in Fig. 1.
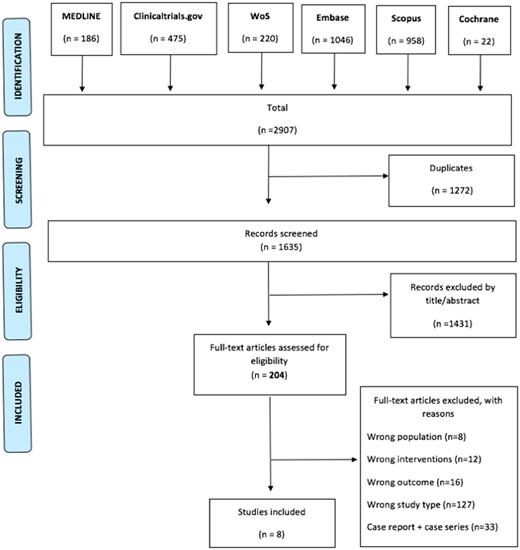
Preferred reporting items for systematic reviews and meta-analyses flow chart for the selected studies
WoS: Web of Science.
Data extraction and collection
Data extraction and creation of summary of evidence tables using a standardized data extraction form was performed by two independent reviewers (P.D., M.R.). We recorded for each trial the following data: population demographics and general characteristics, definitions of outcomes used by each study, description of the intervention and number of patients included in the intervention or comparator group, follow-up duration, concomitant immunosuppressive treatments, hazard ratios and odds ratios for the primary and secondary end point of the SLR crude or adjusted for confounders, and description of subpopulations influencing the outcome (e.g. SA carriage, limited GPA).
Quality assessment
Risk of bias (RoB) was assessed through the Cochrane RoB assessment tool for RCTs [6] and with the Newcastle–Ottawa scale for observational studies [7]. RoB was assessed at outcome or at study level as appropriate.
Data synthesis and analysis
Incidence rates were calculated as the ratio of total number of events and persons year in each arm. Within each study, the risk ratio (RR) with 95% CI for each categorical outcome were calculated from the available data, if not provided by the articles. Finally, study RRs were pooled according to the Mantel–Haenszel/Cohen fixed effects method or DerSimonian and Laird random effects models, to account for differences among studies more effectively.
Statistical heterogeneity was evaluated by the Cochran Q test and measured by the I2 statistic. If the I2 statistic was >50%, we considered the random effect RR to be preferable. To evaluate publication bias funnel plots were drawn.
Pre-specified sub-analyses were planned, given a sufficient number of studies were retrieved by the SLR, on patients with GPA limited to the ENT and according to the nasal carriage state of SA. For the infection risk, we planned to access the influence of the type and duration of immunosuppressive regimens used in addition to TMP/SMX prophylaxis. The SLR protocol was registered on the Prospero database under the registration code CRD42019118983.
Results
Results of the systematic literature review and study selection
The SLR identified 2907 potentially relevant records by searching MEDLINE (n = 186), EMBASE (n = 1046), Cochrane (n = 22), Scopus (n = 958), Web of Science (n = 220) and ClinicalTrials.gov (n = 475). After deduplication, 1635 unique articles were retrieved and screened by title and abstract. A total of 204 articles were assessed for eligibility by full-text review. The number of records excluded, with reasons, is displayed in Fig. 1. Seven articles (n = 520 patients) assessing the efficacy of TMP/SMX in reducing the rate of flares in GPA were identified and included for meta-analysis: two RCTs [5, 8], four controlled prospective cohort studies [4, 9–11] and one retrospective cohort study [12]. The studies were published between 1996 and 2018. Only one article (n = 192), a retrospective cohort study [13], was selected on the role of TMP/SMX in the reduction of infections in AAV. The impact of TMP/SMX on the risk of infection was also a secondary outcome of the two RCTs included in the analysis [5, 8]. Inclusion/exclusion criteria and details on the outcomes assessed by the included studies are presented in Supplementary Tables S1 and S2 in Appendix B, available at Rheumatology online. The demographic data of patients included in the selected studies are displayed in Table 1.
Demographics of patients included in studies assessing the role of TMP/SMX to prevent relapses
| Reference . | Type of study . | Diagnosis . | TMP/SMX, no. of patients (%) . | Controls, no. of patients (%) . | TMP/SMX, median age (IQR)/ no. of females (%) . | Controls, median age (IQR)/ no. of females (%) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | RCT | Systemic/generalized GPA | 41 (50.6) | 40 (49.4) | 56 (21–82)/ 11 (26.8) | 57 (25–83)/ 12 (30.0) |
Zycinska et al. [8] | RCT | Systemic/generalized GPA | 16 (51.6) | 15 (48.4) | 46.1 (21.1–56.5)/ 8 (50.0) | 51.4 (28.4–76.0)/ 8 (53.3) |
Reinhold-Keller et al. [9] | Prospective cohort study | Systemic/generalized GPA | Group 1a: initial phase GPA: 19/72 (26.4) Group 2b: TMP/SMX: 24/53 (45.3) ; TMP/SMX + GC: 8/53 (15.1) | Group 1a: generalized GPA: 53/72 (73.6) Group 2b: no therapy: 21/53 (39.6) | Group 1a: initial phase GPA: 41 (23–67)/ 14 (73.7) Group 2b: TMP/SMX: 48 (17–73)/ 14 (59.3); TMP/SMX + GC: 46.5 (21–63)/5 (62.5) | Group 1a: generalized GPA: NS Group 2b: no therapy: 37 (12–70)/ 9 (42.9) |
de Groot et al. [10] | Prospective cohort study | Systemic/generalized GPA | Group B: TMP/SMX: 24 (36.9) Group D: TMP/SMX + GC: 8 (12.3) | Group A: MTX: 22 (33.8) Group C: MTX + GC: 11 (16.9) | Group B: TMP/SMX: 48 (17–73)/ 14 (58.3) Group D: TMP/SMX + GC: 46.5 (21–63)/ 5 (62.5) | Group A: MTX: 48 (12–60)/ 6 (27.3) Group C: MTX + GC: 45 (31–67)/ 7 (63.6) |
Holle et al. [11] | Prospective cohort study | Limited ENT GPA | 26 (52.0) | 24 (48.0) | NS | NS |
Salmela et al. [4] | Prospective analysis of two RCTs | Systemic/generalized GPA, MPA | 55 (27.5) | 145 (72.5) | NS (no differences) | NS (no differences) |
Yegin et al. [12] | Retrospective cohort study | Systemic/generalized GPA | 12 (75) | 4 (25) | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] |
| Reference . | Type of study . | Diagnosis . | TMP/SMX, no. of patients (%) . | Controls, no. of patients (%) . | TMP/SMX, median age (IQR)/ no. of females (%) . | Controls, median age (IQR)/ no. of females (%) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | RCT | Systemic/generalized GPA | 41 (50.6) | 40 (49.4) | 56 (21–82)/ 11 (26.8) | 57 (25–83)/ 12 (30.0) |
Zycinska et al. [8] | RCT | Systemic/generalized GPA | 16 (51.6) | 15 (48.4) | 46.1 (21.1–56.5)/ 8 (50.0) | 51.4 (28.4–76.0)/ 8 (53.3) |
Reinhold-Keller et al. [9] | Prospective cohort study | Systemic/generalized GPA | Group 1a: initial phase GPA: 19/72 (26.4) Group 2b: TMP/SMX: 24/53 (45.3) ; TMP/SMX + GC: 8/53 (15.1) | Group 1a: generalized GPA: 53/72 (73.6) Group 2b: no therapy: 21/53 (39.6) | Group 1a: initial phase GPA: 41 (23–67)/ 14 (73.7) Group 2b: TMP/SMX: 48 (17–73)/ 14 (59.3); TMP/SMX + GC: 46.5 (21–63)/5 (62.5) | Group 1a: generalized GPA: NS Group 2b: no therapy: 37 (12–70)/ 9 (42.9) |
de Groot et al. [10] | Prospective cohort study | Systemic/generalized GPA | Group B: TMP/SMX: 24 (36.9) Group D: TMP/SMX + GC: 8 (12.3) | Group A: MTX: 22 (33.8) Group C: MTX + GC: 11 (16.9) | Group B: TMP/SMX: 48 (17–73)/ 14 (58.3) Group D: TMP/SMX + GC: 46.5 (21–63)/ 5 (62.5) | Group A: MTX: 48 (12–60)/ 6 (27.3) Group C: MTX + GC: 45 (31–67)/ 7 (63.6) |
Holle et al. [11] | Prospective cohort study | Limited ENT GPA | 26 (52.0) | 24 (48.0) | NS | NS |
Salmela et al. [4] | Prospective analysis of two RCTs | Systemic/generalized GPA, MPA | 55 (27.5) | 145 (72.5) | NS (no differences) | NS (no differences) |
Yegin et al. [12] | Retrospective cohort study | Systemic/generalized GPA | 12 (75) | 4 (25) | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] |
Initial phase GPA: symptoms restricted to the upper and/or lower airways without constitutional symptoms or systemic vasculitis (limited GPA).
Group 1: induction therapy in initial phase/limited GPA.
Group 2: maintenance of remission in generalized GPA. GC: glucocorticoid; GPA: granulomatosis with polyangiitis; IQR: interquartile range; MPA: microscopic polyangiitis; NS: not specified; RCT: randomized controlled trial; TMP/SMX: trimethoprim/sulfametoxazole.
Demographics of patients included in studies assessing the role of TMP/SMX to prevent relapses
| Reference . | Type of study . | Diagnosis . | TMP/SMX, no. of patients (%) . | Controls, no. of patients (%) . | TMP/SMX, median age (IQR)/ no. of females (%) . | Controls, median age (IQR)/ no. of females (%) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | RCT | Systemic/generalized GPA | 41 (50.6) | 40 (49.4) | 56 (21–82)/ 11 (26.8) | 57 (25–83)/ 12 (30.0) |
Zycinska et al. [8] | RCT | Systemic/generalized GPA | 16 (51.6) | 15 (48.4) | 46.1 (21.1–56.5)/ 8 (50.0) | 51.4 (28.4–76.0)/ 8 (53.3) |
Reinhold-Keller et al. [9] | Prospective cohort study | Systemic/generalized GPA | Group 1a: initial phase GPA: 19/72 (26.4) Group 2b: TMP/SMX: 24/53 (45.3) ; TMP/SMX + GC: 8/53 (15.1) | Group 1a: generalized GPA: 53/72 (73.6) Group 2b: no therapy: 21/53 (39.6) | Group 1a: initial phase GPA: 41 (23–67)/ 14 (73.7) Group 2b: TMP/SMX: 48 (17–73)/ 14 (59.3); TMP/SMX + GC: 46.5 (21–63)/5 (62.5) | Group 1a: generalized GPA: NS Group 2b: no therapy: 37 (12–70)/ 9 (42.9) |
de Groot et al. [10] | Prospective cohort study | Systemic/generalized GPA | Group B: TMP/SMX: 24 (36.9) Group D: TMP/SMX + GC: 8 (12.3) | Group A: MTX: 22 (33.8) Group C: MTX + GC: 11 (16.9) | Group B: TMP/SMX: 48 (17–73)/ 14 (58.3) Group D: TMP/SMX + GC: 46.5 (21–63)/ 5 (62.5) | Group A: MTX: 48 (12–60)/ 6 (27.3) Group C: MTX + GC: 45 (31–67)/ 7 (63.6) |
Holle et al. [11] | Prospective cohort study | Limited ENT GPA | 26 (52.0) | 24 (48.0) | NS | NS |
Salmela et al. [4] | Prospective analysis of two RCTs | Systemic/generalized GPA, MPA | 55 (27.5) | 145 (72.5) | NS (no differences) | NS (no differences) |
Yegin et al. [12] | Retrospective cohort study | Systemic/generalized GPA | 12 (75) | 4 (25) | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] |
| Reference . | Type of study . | Diagnosis . | TMP/SMX, no. of patients (%) . | Controls, no. of patients (%) . | TMP/SMX, median age (IQR)/ no. of females (%) . | Controls, median age (IQR)/ no. of females (%) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | RCT | Systemic/generalized GPA | 41 (50.6) | 40 (49.4) | 56 (21–82)/ 11 (26.8) | 57 (25–83)/ 12 (30.0) |
Zycinska et al. [8] | RCT | Systemic/generalized GPA | 16 (51.6) | 15 (48.4) | 46.1 (21.1–56.5)/ 8 (50.0) | 51.4 (28.4–76.0)/ 8 (53.3) |
Reinhold-Keller et al. [9] | Prospective cohort study | Systemic/generalized GPA | Group 1a: initial phase GPA: 19/72 (26.4) Group 2b: TMP/SMX: 24/53 (45.3) ; TMP/SMX + GC: 8/53 (15.1) | Group 1a: generalized GPA: 53/72 (73.6) Group 2b: no therapy: 21/53 (39.6) | Group 1a: initial phase GPA: 41 (23–67)/ 14 (73.7) Group 2b: TMP/SMX: 48 (17–73)/ 14 (59.3); TMP/SMX + GC: 46.5 (21–63)/5 (62.5) | Group 1a: generalized GPA: NS Group 2b: no therapy: 37 (12–70)/ 9 (42.9) |
de Groot et al. [10] | Prospective cohort study | Systemic/generalized GPA | Group B: TMP/SMX: 24 (36.9) Group D: TMP/SMX + GC: 8 (12.3) | Group A: MTX: 22 (33.8) Group C: MTX + GC: 11 (16.9) | Group B: TMP/SMX: 48 (17–73)/ 14 (58.3) Group D: TMP/SMX + GC: 46.5 (21–63)/ 5 (62.5) | Group A: MTX: 48 (12–60)/ 6 (27.3) Group C: MTX + GC: 45 (31–67)/ 7 (63.6) |
Holle et al. [11] | Prospective cohort study | Limited ENT GPA | 26 (52.0) | 24 (48.0) | NS | NS |
Salmela et al. [4] | Prospective analysis of two RCTs | Systemic/generalized GPA, MPA | 55 (27.5) | 145 (72.5) | NS (no differences) | NS (no differences) |
Yegin et al. [12] | Retrospective cohort study | Systemic/generalized GPA | 12 (75) | 4 (25) | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] | NS [mean (s.d.) total population: 51.6 (11.9)]/NS [total population: 6 (28%)] |
Initial phase GPA: symptoms restricted to the upper and/or lower airways without constitutional symptoms or systemic vasculitis (limited GPA).
Group 1: induction therapy in initial phase/limited GPA.
Group 2: maintenance of remission in generalized GPA. GC: glucocorticoid; GPA: granulomatosis with polyangiitis; IQR: interquartile range; MPA: microscopic polyangiitis; NS: not specified; RCT: randomized controlled trial; TMP/SMX: trimethoprim/sulfametoxazole.
Experimental intervention
TMP/SMX was prescribed at full dose (960 mg twice daily) in one RCT [5], in one prospective cohort study assessing the role of TMP/SMX monotherapy as induction and maintenance of remission in GPA [9] and in a prospective study comparing the effectiveness of TMP/SMX with or without concomitant GC vs MTX in maintaining remission [10]. In the study by Holle et al. [11], in which TMP/SMX was used in a step-up fashion according to disease severity, the dose of TMP/SMX used was not specified, similarly to the retrospective cohort study from Yegin et al. [12] in which the use of TMP/SMX as adjuvant to maintenance of remission was reported to be an independent factor protecting against relapse. The second RCT [8] included in the analyses and the remaining observational studies used a reduced (prophylactic) dose (960 mg three times per week).
The study assessing the role of TMP/SMX as prophylaxis of PCP infection applied three different schemes (480 mg on alternate days, 960 mg on alternate days or 960 mg twice a day) [13]. Details on the interventions and controls are presented in Table 2.
Immunosuppressive therapeutic approach and TMP/SMX dosage in studies assessing the role of TMP/SMX to prevent relapses
| References . | TMP/SMX indication . | Intervention . | Controls . | TMP/SMX dosage . | Remission induction treatment . |
|---|---|---|---|---|---|
Stegeman et al. [5] | Maintenance of remission | TMP/SMX ± CYC (21/41 patients) and GC (23/41) | Placebo ± CYC (20/40 patients) and GC (19/40) | 960 mg twice a day | CYC and GC |
Zycinska et al. [8] | Maintenance of remission | TMP/SMX monotherapy | Placebo | 960 mg three times a week | CYC and GC |
Reinhold-Keller et al. [9] | Group 1a: induction in limited GPA Group 2b: maintenance after remission induction | Group 1a: TMP/SMX monotherapy Group 2b: TMP/SMX monotherapy; TMP/SMX + GC | Group 1a: NS Group 2b: no treatment | Group 1a and group 2b: 960 mg twice a day | Group 1a: TMP/SMX monotherapy Group 2b: CYC and GC |
de Groot et al. [10] | Maintenance of remission | Group B: TMP/SMX monotherapy Group D: TMP/SMX + GC | Group A: MTX monotherapy Group C: MTX + GC | 960 mg twice a day | CYC and GC |
Holle et al. [11] | Induction and maintenance in limited disease with escalation of treatment if refractory/generalized | TMP/SMX monotherapy | Induction for refractory/generalized GPA: Cyc + GC/MTX, AZA, LEF, MMF + GC RTX, TNFi, IVIG Maintenance: TMP/SMX monotherapy (only for limited GPA) MTX, LEF, AZA, MMF | NS | CYC, MTX, AZA, MMF, LEF, RTX, TNF-I, IVIG |
Salmela et al. [4] | Prophylaxis for PCP | Study 1c: TMP/SMX + Cyc + GC vs MTX + GC Study 2c: TMP/SMX + CYC + GC vs AZA + GC | Study 1c: CYC + GC vs MTX + GC Study 2c: Cyc + GC vs AZA + GC | 960 mg three times a week | Study 1c: Cyc, MTX, GC Study 2c: CYC, AZA, GC |
Yegin et al. [12] | Maintenance of remission | TMP/SMX + AZA + GC | AZA | 960 mg on alternate days | CYC + GC ± PLEX or MTX + GC |
| References . | TMP/SMX indication . | Intervention . | Controls . | TMP/SMX dosage . | Remission induction treatment . |
|---|---|---|---|---|---|
Stegeman et al. [5] | Maintenance of remission | TMP/SMX ± CYC (21/41 patients) and GC (23/41) | Placebo ± CYC (20/40 patients) and GC (19/40) | 960 mg twice a day | CYC and GC |
Zycinska et al. [8] | Maintenance of remission | TMP/SMX monotherapy | Placebo | 960 mg three times a week | CYC and GC |
Reinhold-Keller et al. [9] | Group 1a: induction in limited GPA Group 2b: maintenance after remission induction | Group 1a: TMP/SMX monotherapy Group 2b: TMP/SMX monotherapy; TMP/SMX + GC | Group 1a: NS Group 2b: no treatment | Group 1a and group 2b: 960 mg twice a day | Group 1a: TMP/SMX monotherapy Group 2b: CYC and GC |
de Groot et al. [10] | Maintenance of remission | Group B: TMP/SMX monotherapy Group D: TMP/SMX + GC | Group A: MTX monotherapy Group C: MTX + GC | 960 mg twice a day | CYC and GC |
Holle et al. [11] | Induction and maintenance in limited disease with escalation of treatment if refractory/generalized | TMP/SMX monotherapy | Induction for refractory/generalized GPA: Cyc + GC/MTX, AZA, LEF, MMF + GC RTX, TNFi, IVIG Maintenance: TMP/SMX monotherapy (only for limited GPA) MTX, LEF, AZA, MMF | NS | CYC, MTX, AZA, MMF, LEF, RTX, TNF-I, IVIG |
Salmela et al. [4] | Prophylaxis for PCP | Study 1c: TMP/SMX + Cyc + GC vs MTX + GC Study 2c: TMP/SMX + CYC + GC vs AZA + GC | Study 1c: CYC + GC vs MTX + GC Study 2c: Cyc + GC vs AZA + GC | 960 mg three times a week | Study 1c: Cyc, MTX, GC Study 2c: CYC, AZA, GC |
Yegin et al. [12] | Maintenance of remission | TMP/SMX + AZA + GC | AZA | 960 mg on alternate days | CYC + GC ± PLEX or MTX + GC |
Group 1: induction therapy in initial-phase/limited GPA.
Group 2: maintenance of remission in generalized GPA.
Studies 1 and 2: data collected from two different randomized controlled trials (NORAM [14] and CYCAZAREM [15]) to assess the association between chronic nasal carriage of Staphylococcus aureus and relapses. GC: glucocorticoid; GPA: granulomatosis with polyangiitis; NS: not specified; PCP: Pneumocystis jirovecii; PLEX: plasma-exchange; RTX: rituximab; TMP/SMX: trimethoprim/sulfametoxazole.
Immunosuppressive therapeutic approach and TMP/SMX dosage in studies assessing the role of TMP/SMX to prevent relapses
| References . | TMP/SMX indication . | Intervention . | Controls . | TMP/SMX dosage . | Remission induction treatment . |
|---|---|---|---|---|---|
Stegeman et al. [5] | Maintenance of remission | TMP/SMX ± CYC (21/41 patients) and GC (23/41) | Placebo ± CYC (20/40 patients) and GC (19/40) | 960 mg twice a day | CYC and GC |
Zycinska et al. [8] | Maintenance of remission | TMP/SMX monotherapy | Placebo | 960 mg three times a week | CYC and GC |
Reinhold-Keller et al. [9] | Group 1a: induction in limited GPA Group 2b: maintenance after remission induction | Group 1a: TMP/SMX monotherapy Group 2b: TMP/SMX monotherapy; TMP/SMX + GC | Group 1a: NS Group 2b: no treatment | Group 1a and group 2b: 960 mg twice a day | Group 1a: TMP/SMX monotherapy Group 2b: CYC and GC |
de Groot et al. [10] | Maintenance of remission | Group B: TMP/SMX monotherapy Group D: TMP/SMX + GC | Group A: MTX monotherapy Group C: MTX + GC | 960 mg twice a day | CYC and GC |
Holle et al. [11] | Induction and maintenance in limited disease with escalation of treatment if refractory/generalized | TMP/SMX monotherapy | Induction for refractory/generalized GPA: Cyc + GC/MTX, AZA, LEF, MMF + GC RTX, TNFi, IVIG Maintenance: TMP/SMX monotherapy (only for limited GPA) MTX, LEF, AZA, MMF | NS | CYC, MTX, AZA, MMF, LEF, RTX, TNF-I, IVIG |
Salmela et al. [4] | Prophylaxis for PCP | Study 1c: TMP/SMX + Cyc + GC vs MTX + GC Study 2c: TMP/SMX + CYC + GC vs AZA + GC | Study 1c: CYC + GC vs MTX + GC Study 2c: Cyc + GC vs AZA + GC | 960 mg three times a week | Study 1c: Cyc, MTX, GC Study 2c: CYC, AZA, GC |
Yegin et al. [12] | Maintenance of remission | TMP/SMX + AZA + GC | AZA | 960 mg on alternate days | CYC + GC ± PLEX or MTX + GC |
| References . | TMP/SMX indication . | Intervention . | Controls . | TMP/SMX dosage . | Remission induction treatment . |
|---|---|---|---|---|---|
Stegeman et al. [5] | Maintenance of remission | TMP/SMX ± CYC (21/41 patients) and GC (23/41) | Placebo ± CYC (20/40 patients) and GC (19/40) | 960 mg twice a day | CYC and GC |
Zycinska et al. [8] | Maintenance of remission | TMP/SMX monotherapy | Placebo | 960 mg three times a week | CYC and GC |
Reinhold-Keller et al. [9] | Group 1a: induction in limited GPA Group 2b: maintenance after remission induction | Group 1a: TMP/SMX monotherapy Group 2b: TMP/SMX monotherapy; TMP/SMX + GC | Group 1a: NS Group 2b: no treatment | Group 1a and group 2b: 960 mg twice a day | Group 1a: TMP/SMX monotherapy Group 2b: CYC and GC |
de Groot et al. [10] | Maintenance of remission | Group B: TMP/SMX monotherapy Group D: TMP/SMX + GC | Group A: MTX monotherapy Group C: MTX + GC | 960 mg twice a day | CYC and GC |
Holle et al. [11] | Induction and maintenance in limited disease with escalation of treatment if refractory/generalized | TMP/SMX monotherapy | Induction for refractory/generalized GPA: Cyc + GC/MTX, AZA, LEF, MMF + GC RTX, TNFi, IVIG Maintenance: TMP/SMX monotherapy (only for limited GPA) MTX, LEF, AZA, MMF | NS | CYC, MTX, AZA, MMF, LEF, RTX, TNF-I, IVIG |
Salmela et al. [4] | Prophylaxis for PCP | Study 1c: TMP/SMX + Cyc + GC vs MTX + GC Study 2c: TMP/SMX + CYC + GC vs AZA + GC | Study 1c: CYC + GC vs MTX + GC Study 2c: Cyc + GC vs AZA + GC | 960 mg three times a week | Study 1c: Cyc, MTX, GC Study 2c: CYC, AZA, GC |
Yegin et al. [12] | Maintenance of remission | TMP/SMX + AZA + GC | AZA | 960 mg on alternate days | CYC + GC ± PLEX or MTX + GC |
Group 1: induction therapy in initial-phase/limited GPA.
Group 2: maintenance of remission in generalized GPA.
Studies 1 and 2: data collected from two different randomized controlled trials (NORAM [14] and CYCAZAREM [15]) to assess the association between chronic nasal carriage of Staphylococcus aureus and relapses. GC: glucocorticoid; GPA: granulomatosis with polyangiitis; NS: not specified; PCP: Pneumocystis jirovecii; PLEX: plasma-exchange; RTX: rituximab; TMP/SMX: trimethoprim/sulfametoxazole.
Control intervention
In the two RCTs included in the analysis, the control group received placebo. In the remaining observational cohort studies, the control group received no intervention [9] or standard immunosuppressive co-intervention for GPA [4, 10–12].
RoB assessment and publication bias
The RoB of the two RCTs was generally high, with unclear RoB in most items assessed by the Cochrane tool (randomization, blinding, completeness of data and selective reporting). Supplementary Table S3 in Appendix B, available at Rheumatology online. The Newcastle–Ottawa scale for observational studies ranged from 5 to 6 points (Supplementary Table S4 in Appendix B, available at Rheumatology online). A funnel plot was symmetric (Supplementary Fig. S1 in Appendix C, available at Rheumatology online).
Subgroup analyses
Given the low number of studies and the heterogeneity of the study designs, intervention therapeutic schemes and outcomes, the pre-specified subgroup analyses, including the influence of TMP/SMX in the prevention of relapses in specific subgroups of patients (e.g. limited GPA or nasal carriers of SA), could not be performed.
Similarly, evidence was too scarce to stratify the risk and type of infections according to immunosuppressive treatment prescribed in combination with TMP/SMX for the secondary outcome.
Meta-analysis
Primary outcome: relapse rate
Details on the relapse rates reported by each study are presented in Table 3. Overall comparison of TMP/SMX with placebo, no treatment or other immunosuppressants was not significant (RR = 1.15, 95% CI: 0.51, 2.55) as reported in Fig. 2.
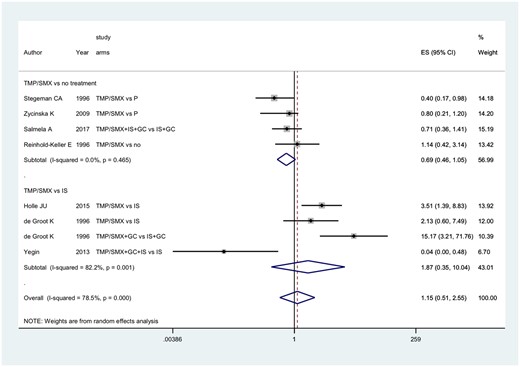
Primary outcome: role of TMP/SMX on the risk of relapses in patients with granulomatosis with polyangiitis
B: TMP/SMX; ES: effect size; GC: glucocorticoids; IS: immunosuppressant drug; P: placebo; TMP/SMX: trimethoprim/sulfametoxazole.
Risk of relapse in patients treated and not treated with TMP/SMX in the different studies
| References . | Median follow-up duration, months (IQR) . | TMP/SMX, no. of patients in remission (%) . | Controls, no. of patients in remission (%) . | TMP/SMX, no. of patients with relapse (%) . | Controls, no. of patients with relapse (%) . | RR for relapse (95% CI) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | 24 | 31 (82) | 23 (60) | NS | NS | 0.4 (0.17–0.98) |
Zycinska et al. [8] | 18 | 12 (75) | 8 (55) | NS | NS | 0.8 (0.21–1.20) |
Reinhold-Keller et al. [9] | Group 1a: TMP/SMX monotherapy: 33 (3–88) Group 2b: TMP/SMX monotherapy: 23 (4–73); no treatment: 18 (range 6–84); TMP/SMX + GC: 14.5 (2–24) | Group 1a: 11 (57.9) Group 2b: TMP/SMX monotherapy: 14 (58.3); TMP/SMX + GC: 0 (0) | Group 2b: no treatment: 15 (71.4) | Group 1a: 8 (42.1) Group 2b: TMP/SMX monotherapy: 10 (41.7); TMP/SMX + GC: 8 (100) | — Group 2b: No treatment: 6 (28.6) | — NS |
de Groot et al. [10] | Group A: MTX: 16 (5–30) Group B: TMP/SMX: 23 (4–73) Group C: MTX + GC: 20 (4–34) Group D: TMP/SMX + GC: 14.5 (2–24) | Group B: TMP/SMX: 14 (58.3) Group D: TMP/SMX + GC: 0 (0) | Group A: MTX: 19 (86.3) Group C: MTX + GC: 10 (90.9) | Group B: TMP/SMX: 10 (41.7) Group D: TMP/SMX + GC: 8 (100) | Group A: MTX: 3 (13.6) Group C: MTX + GC: 10 (9.1) | Group A vs B: P < 0.05 Group C vs D: P < 0.005 |
Holle et al. [11] | 4 years (1–14.5) | 7 (27): 4 (8) remained on maintenance with TMP/SMX monotherapy + 3 (6) with TMP/SMX + GC | 10 (41) | 19 (73) | NS | NS |
Salmela et al. [4] | Study 1c: 12 Study 2c: 18 | NS | NS | 15 (27.3) | 50 (34.5) | 0.71 (0.36–1.41); P = 0.33 |
Yegin et al. [12] | Mean (s.d.): 38.3 (37.4) | NS | NS | No relapse: 8/8 (100) on TMP/SMX ; relapse: 4/8 (50) on TMP/SMX | NS | 23.2 (2.07–250); P = 0.011 for reduced risk of relapse |
| References . | Median follow-up duration, months (IQR) . | TMP/SMX, no. of patients in remission (%) . | Controls, no. of patients in remission (%) . | TMP/SMX, no. of patients with relapse (%) . | Controls, no. of patients with relapse (%) . | RR for relapse (95% CI) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | 24 | 31 (82) | 23 (60) | NS | NS | 0.4 (0.17–0.98) |
Zycinska et al. [8] | 18 | 12 (75) | 8 (55) | NS | NS | 0.8 (0.21–1.20) |
Reinhold-Keller et al. [9] | Group 1a: TMP/SMX monotherapy: 33 (3–88) Group 2b: TMP/SMX monotherapy: 23 (4–73); no treatment: 18 (range 6–84); TMP/SMX + GC: 14.5 (2–24) | Group 1a: 11 (57.9) Group 2b: TMP/SMX monotherapy: 14 (58.3); TMP/SMX + GC: 0 (0) | Group 2b: no treatment: 15 (71.4) | Group 1a: 8 (42.1) Group 2b: TMP/SMX monotherapy: 10 (41.7); TMP/SMX + GC: 8 (100) | — Group 2b: No treatment: 6 (28.6) | — NS |
de Groot et al. [10] | Group A: MTX: 16 (5–30) Group B: TMP/SMX: 23 (4–73) Group C: MTX + GC: 20 (4–34) Group D: TMP/SMX + GC: 14.5 (2–24) | Group B: TMP/SMX: 14 (58.3) Group D: TMP/SMX + GC: 0 (0) | Group A: MTX: 19 (86.3) Group C: MTX + GC: 10 (90.9) | Group B: TMP/SMX: 10 (41.7) Group D: TMP/SMX + GC: 8 (100) | Group A: MTX: 3 (13.6) Group C: MTX + GC: 10 (9.1) | Group A vs B: P < 0.05 Group C vs D: P < 0.005 |
Holle et al. [11] | 4 years (1–14.5) | 7 (27): 4 (8) remained on maintenance with TMP/SMX monotherapy + 3 (6) with TMP/SMX + GC | 10 (41) | 19 (73) | NS | NS |
Salmela et al. [4] | Study 1c: 12 Study 2c: 18 | NS | NS | 15 (27.3) | 50 (34.5) | 0.71 (0.36–1.41); P = 0.33 |
Yegin et al. [12] | Mean (s.d.): 38.3 (37.4) | NS | NS | No relapse: 8/8 (100) on TMP/SMX ; relapse: 4/8 (50) on TMP/SMX | NS | 23.2 (2.07–250); P = 0.011 for reduced risk of relapse |
Group 1: induction therapy in initial-phase/limited GPA.
Group 2: maintenance of remission in generalized GPA.
Studies 1 and 2: data collected from two different randomized controlled trials (NORAM [14] and CYCAZAREM [15]) to assess the association between chronic nasal carriage of Staphylococcus aureus and relapses. GC: glucocorticoid; GPA: granulomatosis with polyangiitis; IQR: interquartile range; NS: not specified; RR: risk ratio; TMP/SMX: trimethoprim/sulfametoxazole.
Risk of relapse in patients treated and not treated with TMP/SMX in the different studies
| References . | Median follow-up duration, months (IQR) . | TMP/SMX, no. of patients in remission (%) . | Controls, no. of patients in remission (%) . | TMP/SMX, no. of patients with relapse (%) . | Controls, no. of patients with relapse (%) . | RR for relapse (95% CI) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | 24 | 31 (82) | 23 (60) | NS | NS | 0.4 (0.17–0.98) |
Zycinska et al. [8] | 18 | 12 (75) | 8 (55) | NS | NS | 0.8 (0.21–1.20) |
Reinhold-Keller et al. [9] | Group 1a: TMP/SMX monotherapy: 33 (3–88) Group 2b: TMP/SMX monotherapy: 23 (4–73); no treatment: 18 (range 6–84); TMP/SMX + GC: 14.5 (2–24) | Group 1a: 11 (57.9) Group 2b: TMP/SMX monotherapy: 14 (58.3); TMP/SMX + GC: 0 (0) | Group 2b: no treatment: 15 (71.4) | Group 1a: 8 (42.1) Group 2b: TMP/SMX monotherapy: 10 (41.7); TMP/SMX + GC: 8 (100) | — Group 2b: No treatment: 6 (28.6) | — NS |
de Groot et al. [10] | Group A: MTX: 16 (5–30) Group B: TMP/SMX: 23 (4–73) Group C: MTX + GC: 20 (4–34) Group D: TMP/SMX + GC: 14.5 (2–24) | Group B: TMP/SMX: 14 (58.3) Group D: TMP/SMX + GC: 0 (0) | Group A: MTX: 19 (86.3) Group C: MTX + GC: 10 (90.9) | Group B: TMP/SMX: 10 (41.7) Group D: TMP/SMX + GC: 8 (100) | Group A: MTX: 3 (13.6) Group C: MTX + GC: 10 (9.1) | Group A vs B: P < 0.05 Group C vs D: P < 0.005 |
Holle et al. [11] | 4 years (1–14.5) | 7 (27): 4 (8) remained on maintenance with TMP/SMX monotherapy + 3 (6) with TMP/SMX + GC | 10 (41) | 19 (73) | NS | NS |
Salmela et al. [4] | Study 1c: 12 Study 2c: 18 | NS | NS | 15 (27.3) | 50 (34.5) | 0.71 (0.36–1.41); P = 0.33 |
Yegin et al. [12] | Mean (s.d.): 38.3 (37.4) | NS | NS | No relapse: 8/8 (100) on TMP/SMX ; relapse: 4/8 (50) on TMP/SMX | NS | 23.2 (2.07–250); P = 0.011 for reduced risk of relapse |
| References . | Median follow-up duration, months (IQR) . | TMP/SMX, no. of patients in remission (%) . | Controls, no. of patients in remission (%) . | TMP/SMX, no. of patients with relapse (%) . | Controls, no. of patients with relapse (%) . | RR for relapse (95% CI) . |
|---|---|---|---|---|---|---|
Stegeman et al. [5] | 24 | 31 (82) | 23 (60) | NS | NS | 0.4 (0.17–0.98) |
Zycinska et al. [8] | 18 | 12 (75) | 8 (55) | NS | NS | 0.8 (0.21–1.20) |
Reinhold-Keller et al. [9] | Group 1a: TMP/SMX monotherapy: 33 (3–88) Group 2b: TMP/SMX monotherapy: 23 (4–73); no treatment: 18 (range 6–84); TMP/SMX + GC: 14.5 (2–24) | Group 1a: 11 (57.9) Group 2b: TMP/SMX monotherapy: 14 (58.3); TMP/SMX + GC: 0 (0) | Group 2b: no treatment: 15 (71.4) | Group 1a: 8 (42.1) Group 2b: TMP/SMX monotherapy: 10 (41.7); TMP/SMX + GC: 8 (100) | — Group 2b: No treatment: 6 (28.6) | — NS |
de Groot et al. [10] | Group A: MTX: 16 (5–30) Group B: TMP/SMX: 23 (4–73) Group C: MTX + GC: 20 (4–34) Group D: TMP/SMX + GC: 14.5 (2–24) | Group B: TMP/SMX: 14 (58.3) Group D: TMP/SMX + GC: 0 (0) | Group A: MTX: 19 (86.3) Group C: MTX + GC: 10 (90.9) | Group B: TMP/SMX: 10 (41.7) Group D: TMP/SMX + GC: 8 (100) | Group A: MTX: 3 (13.6) Group C: MTX + GC: 10 (9.1) | Group A vs B: P < 0.05 Group C vs D: P < 0.005 |
Holle et al. [11] | 4 years (1–14.5) | 7 (27): 4 (8) remained on maintenance with TMP/SMX monotherapy + 3 (6) with TMP/SMX + GC | 10 (41) | 19 (73) | NS | NS |
Salmela et al. [4] | Study 1c: 12 Study 2c: 18 | NS | NS | 15 (27.3) | 50 (34.5) | 0.71 (0.36–1.41); P = 0.33 |
Yegin et al. [12] | Mean (s.d.): 38.3 (37.4) | NS | NS | No relapse: 8/8 (100) on TMP/SMX ; relapse: 4/8 (50) on TMP/SMX | NS | 23.2 (2.07–250); P = 0.011 for reduced risk of relapse |
Group 1: induction therapy in initial-phase/limited GPA.
Group 2: maintenance of remission in generalized GPA.
Studies 1 and 2: data collected from two different randomized controlled trials (NORAM [14] and CYCAZAREM [15]) to assess the association between chronic nasal carriage of Staphylococcus aureus and relapses. GC: glucocorticoid; GPA: granulomatosis with polyangiitis; IQR: interquartile range; NS: not specified; RR: risk ratio; TMP/SMX: trimethoprim/sulfametoxazole.
Treatment with TMP/SMX had similar efficacy in the prevention of relapses compared with placebo or no treatment (RR = 0.69, 95% CI: 0.46, 1.05). TMP/SMX tended to be less effective (although not significantly) in controlling the disease when compared with active treatments (MTX, AZA): RR = 1.87, 95% CI: 0.35, 10.04 (Fig. 2).
The sensitivity analysis performed based on the dosage of TMP/SMX (960 mg twice daily vs prophylactic dose) confirmed the same results (TMP/SMX full dose vs placebo/no treatment/MTX: RR = 2.12, 95% CI: 0.66, 6.74; TMP/SMX prophylactic dose vs placebo/MTX or AZA: RR = 0.50, 95% CI: 0.18, 1.40; Supplementary Figs S2 and S3 in Appendix C, available at Rheumatology online).
The analysis based on the type of publication (RCTs vs observational studies) confirmed the same results [TMP/SMX in RCTs: RR = 0.57 (95% CI: 0.29, 1.12) vs TMP/SMX in observational studies compared with no treatment: RR = 1.47 (95% CI: 0.50, 4.33) vs TMP/SMX in observational studies compared with immunosuppressive drug: RR = 1.87 (95% CI 0.35, 10.04); Supplementary Fig. S4 in Appendix D, available at Rheumatology online].
Secondary outcome: infection rate
A meta-analysis on the role of TMP/SMX on the infection risk in GPA/AAV could not be performed since the SLR retrieved only one study specifically assessing this research question. Details on the risk of infections reported by the included study that had infection risk as primary outcome are presented in Table 4.
Characteristics of the study assessing the role of TMP/SMX in preventing infections
| Characteristic . | Kronbichler et al. [13] . |
|---|---|
| Type of study | Retrospective cohort study |
Diagnosis | AAV |
Total no. of patients | 192 |
Patients with GPA, MPA, EGPA, n (%) | 134 (69.8), 28 (14.5), 30 (15.6) |
Immunosuppressive agent | GC + RTX (both remission induction and maintenance) |
TMP/SMX, n (%) | 73 (38.0) |
TMP/SMX indication | Prophylaxis for PCP |
TMP/SMX dosage | 480 mg on alternate days (38.4%) 960 mg on alternate days (21.9%) 960 mg twice a day (12.3%) |
| Patients with severe infections, n (%) | 49 (25.5) |
| Patients without severe infections, n (%) | 143 (74.4) |
Female patients with severe infection/female patients without severe infection, n (%) | 28 (59)/78 (55) |
Age of patients with severe infections/age of patients without severe infections, mean (range) | 60 (22–82)/56 (16–85) |
Patients on TMP/SMX with severe infections, n (%) | 11 (22) |
Patients on TMP/SMX without severe infections, n (%) | 62 (43) |
Duration of prophylaxis with TMP/SMX, mean, months | 14.67 |
RR for severe infections in patients on TMP/SMX (95% CI) | 0.30 (0.13–0.69) |
RR for PCP in patients on TMP/SMX (95% CI) | 0.45 (0.23–0.88) |
| Characteristic . | Kronbichler et al. [13] . |
|---|---|
| Type of study | Retrospective cohort study |
Diagnosis | AAV |
Total no. of patients | 192 |
Patients with GPA, MPA, EGPA, n (%) | 134 (69.8), 28 (14.5), 30 (15.6) |
Immunosuppressive agent | GC + RTX (both remission induction and maintenance) |
TMP/SMX, n (%) | 73 (38.0) |
TMP/SMX indication | Prophylaxis for PCP |
TMP/SMX dosage | 480 mg on alternate days (38.4%) 960 mg on alternate days (21.9%) 960 mg twice a day (12.3%) |
| Patients with severe infections, n (%) | 49 (25.5) |
| Patients without severe infections, n (%) | 143 (74.4) |
Female patients with severe infection/female patients without severe infection, n (%) | 28 (59)/78 (55) |
Age of patients with severe infections/age of patients without severe infections, mean (range) | 60 (22–82)/56 (16–85) |
Patients on TMP/SMX with severe infections, n (%) | 11 (22) |
Patients on TMP/SMX without severe infections, n (%) | 62 (43) |
Duration of prophylaxis with TMP/SMX, mean, months | 14.67 |
RR for severe infections in patients on TMP/SMX (95% CI) | 0.30 (0.13–0.69) |
RR for PCP in patients on TMP/SMX (95% CI) | 0.45 (0.23–0.88) |
EGPA: eosinophilic GPA; GC: glucocorticoid; GPA: granulomatosis with polyangiitis; MPA: mycroscopic polyangiitis; PCP: Pneumocystis jirovecii; RR: risk ratio; RTX: rituximab; TMP/SMX: trimethoprim/sulfametoxazole.
Characteristics of the study assessing the role of TMP/SMX in preventing infections
| Characteristic . | Kronbichler et al. [13] . |
|---|---|
| Type of study | Retrospective cohort study |
Diagnosis | AAV |
Total no. of patients | 192 |
Patients with GPA, MPA, EGPA, n (%) | 134 (69.8), 28 (14.5), 30 (15.6) |
Immunosuppressive agent | GC + RTX (both remission induction and maintenance) |
TMP/SMX, n (%) | 73 (38.0) |
TMP/SMX indication | Prophylaxis for PCP |
TMP/SMX dosage | 480 mg on alternate days (38.4%) 960 mg on alternate days (21.9%) 960 mg twice a day (12.3%) |
| Patients with severe infections, n (%) | 49 (25.5) |
| Patients without severe infections, n (%) | 143 (74.4) |
Female patients with severe infection/female patients without severe infection, n (%) | 28 (59)/78 (55) |
Age of patients with severe infections/age of patients without severe infections, mean (range) | 60 (22–82)/56 (16–85) |
Patients on TMP/SMX with severe infections, n (%) | 11 (22) |
Patients on TMP/SMX without severe infections, n (%) | 62 (43) |
Duration of prophylaxis with TMP/SMX, mean, months | 14.67 |
RR for severe infections in patients on TMP/SMX (95% CI) | 0.30 (0.13–0.69) |
RR for PCP in patients on TMP/SMX (95% CI) | 0.45 (0.23–0.88) |
| Characteristic . | Kronbichler et al. [13] . |
|---|---|
| Type of study | Retrospective cohort study |
Diagnosis | AAV |
Total no. of patients | 192 |
Patients with GPA, MPA, EGPA, n (%) | 134 (69.8), 28 (14.5), 30 (15.6) |
Immunosuppressive agent | GC + RTX (both remission induction and maintenance) |
TMP/SMX, n (%) | 73 (38.0) |
TMP/SMX indication | Prophylaxis for PCP |
TMP/SMX dosage | 480 mg on alternate days (38.4%) 960 mg on alternate days (21.9%) 960 mg twice a day (12.3%) |
| Patients with severe infections, n (%) | 49 (25.5) |
| Patients without severe infections, n (%) | 143 (74.4) |
Female patients with severe infection/female patients without severe infection, n (%) | 28 (59)/78 (55) |
Age of patients with severe infections/age of patients without severe infections, mean (range) | 60 (22–82)/56 (16–85) |
Patients on TMP/SMX with severe infections, n (%) | 11 (22) |
Patients on TMP/SMX without severe infections, n (%) | 62 (43) |
Duration of prophylaxis with TMP/SMX, mean, months | 14.67 |
RR for severe infections in patients on TMP/SMX (95% CI) | 0.30 (0.13–0.69) |
RR for PCP in patients on TMP/SMX (95% CI) | 0.45 (0.23–0.88) |
EGPA: eosinophilic GPA; GC: glucocorticoid; GPA: granulomatosis with polyangiitis; MPA: mycroscopic polyangiitis; PCP: Pneumocystis jirovecii; RR: risk ratio; RTX: rituximab; TMP/SMX: trimethoprim/sulfametoxazole.
Data regarding infections reported in the two RCTs [5, 8] included in the analysis were incompletely reported and could not be used to assess the research question. Stegeman et al. reported a significantly lower number of infections in the TMP/SMX group compared with placebo (median 0.0 vs 1.0; P < 0.001). Respiratory infections were the most frequent cause of infection in both groups. The second trial from Zycinska et al. reported a lower annual number of infections in TMP/SMX group compared with placebo (median 0.0 vs 4.0; P < 0.01). The two RCTs by Stegeman et al. and Zycinska et al. [5, 8] reported very similar results on cases of herpes zoster and cytomegalovirus infections (defined as opportunistic infections), which were not influenced by treatment with TMP/SMX on meta-analysed data (RR = 0.53, 95% CI: 0.18, 1.55).
Discussion
The SLR retrieved a number of studies describing the role of TMP/SMX on the relapse risk of patients with GPA. However, the majority of these studies were anecdotal case reports or small case series. To assess the adjunctive role of TMP/SMX we selected studies with a comparator group for a total of 520 participants. Only two RCTs assessed the role of TMP/SMX compared with placebo and only one of these was double-blinded, even though details on allocation concealment and blinding were not specified. Despite different intervention drug doses (TMP/SMX 960 mg twice daily in one study, and 960 mg three times a week in the other), both trials concluded for a significant reduction in the risk of relapse in patients with GPA having achieved remission with an induction treatment with CYC and GC. In the trial by Zycinska et al. [8] the use of co-trimoxazole was associated with longer disease-free interval, but the comparison of frequency of relapse between intervention and placebo group did not reach statistical significance (hazard ratio = 0.8, 95% CI: 0.21, 1.20). The pooled analysis of the results of the two trials concluded for the lack of efficacy of TMP/SMX in reducing the relapse rate of patient with GPA in remission (RR = 0.57, 95% CI: 0.29, 1.12; I2 = 17.3%; P = 0.272). The RoB of the two RCTs was generally unclear.
The SLR retrieved five additional comparative cohort studies confirming the lack of effectiveness of TMP/SMX in reducing the relapse rate in patient with GPA compared with lack of treatment and a trend towards reduced efficacy compared with standard immunosuppressive remission maintenance treatment (MTX). TMP/SMX was prescribed as co-treatment to maintain remission together with standard immunosuppressive therapies in two studies.
The overall results of the meta-analysis including all the types of evidence retrieved from randomized and non-randomized studies did not confirm a role for TMP/SMX in reducing the relapse risk in patients with GPA (RR = 1.15, 95% CI: 0.51, 2.55; I2 = 78.5%; P < 0.001). The same result concluding for lack of efficacy of TMP/SMX in reducing relapse rates was obtained even when separating the analysis based on the dose of TMP/SMX prescribed (full dose vs prophylactic dose).
The current EULAR recommendations for the treatment of AAV suggested a potential role for TMP/SMX as adjunctive treatment in association with standard immunosuppressive agents, mainly based on the results of the RCT published by Stegeman et al. in 1996 [5]. The pooled evidence summarized by the current SLR and meta-analysis might form the basis for a practice-changing future recommendation, minimizing the role of TMP/SMX in the modern setting of a more standardized sequential treatment of AAV based on intensive remission induction and long-term maintenance with standardized and efficacious immunosuppressive drugs, probably overcoming the previously reported beneficial effect of TMP/SMX. Moreover, it has been previously demonstrated that TMP/SMX does not completely eliminate SA carriage in GPA and the antibiotic drug might not be sufficient to restore the dysregulated epithelial nasal barrier, the unbalanced nasal flora and the aberrant inflammatory response that has been found in GPA [16]. These findings were confirmed by a recent study from the French Vasculitis Study Group that found no differences in SA carriage amongst patients with AAV, but reported higher frequency of the chronic carriage compared with controls without vasculitis. Although TMP/SMX prophylaxis reduced the frequency of nasal carriage, this did not influence the relapse rate [17]. Recent evidence has explored the changes in the nasal microbiota (with both standard cultures and modern deep sequencing methods) of patients with GPA and its association with disease activity demonstrating a specific dysbiosis compared with healthy controls and influenced by disease activity [18, 19]. The initial cause of this unbalanced local environment is still unknown, but it is unlikely that TMP/SMX alone could restore the physiological microbial composition. Moreover, it has been recently demonstrated that antibiotic resistance to TMP/SMX (up to 41%) is increasingly being recognized in GPA [18, 20]. Interestingly, the current or recent (prior 6 months) use of non-GC immunosuppressive therapies has been associated with a microbial environment similar to that of healthy controls compared with patients off-therapy or with active disease [19]. These findings still support the hypothesis that local infections might influence disease activity in GPA, but highlight a more complex interaction between resident pathogens, dysbiosis and inflammation that goes beyond the reach of antimicrobial therapy. An ongoing prospective study, the Trimethoprim–Sulfamethoxazole in Granulomatosis with Polyangiitis (TEMPO) study, is currently enrolling patients to evaluate changes in the nasal microbiome, mycobiome and host immunity in patients with GPA after a course of 4 weeks’ TMP/SMX and will further clarify this aspect [21].
On the other hand, prophylactic treatment to prevent serious infections in the delicate balance between intensive immunosuppressive treatments and risk of adverse events is still a real challenge in the management of AAV. The majority of deaths occur early in the disease, with infections rather than disease activity being the leading cause of mortality in the first year from diagnosis [22, 23]. Severe infections have been reported to occur in 20–60% of patients with AAV [24, 25]. Opportunistic infections are another significant threat, particularly during the remission induction phase. PCP pneumonia, a common complication in immunocompromised patients, is associated with significant morbidity and mortality. Lymphopenia is a well-known risk factor associated with this type of infection. TMP/SMX, pentamidine or atovaquone is used as prophylaxis against PCP [26, 27]. Ongoing efforts are being made to reduce the treatment-related adverse event frequency. Intravenous pulse CYC has been shown to be associated with lower cumulative doses and fewer infections compared with oral administration [28, 29]. Nevertheless, more recently introduced treatment with rituximab, despite its being a more targeted agent, has shown a comparable safety profile and a similar rate of infections compared with CYC [30, 31]. Future directions of RCT to minimize treatment-related adverse events have been focusing on testing treatment options that would allow a reduction in the exposure to high-dose GC [32, 33]. Current EULAR recommendations encourage the prescription of prophylaxis against PCP with TMP/SMX in all patients treated with CYC [34]; however, precise recommendations during other treatment regimens or according to the dose and duration of GC are still based on expert opinion and decided on an individual basis [26]. A conference abstract was identified by the SLR describing the use of TMP/SMX prophylaxis in a real-life retrospective cohort including 56 patients with AAV. Serious infections occurred in ∼40% of patients, with TMP/SMX being prescribed to only 70% of patients treated with CYC and/or RTX [35]. TMP/SMX 800/160 mg three times a week has been demonstrated to be a cost-effective option in patients with GPA treated with immunosuppressive drugs (mainly CYC, but also MTX). Whether this practice should be extended to all patients with AAV and for how long is still uncertain. Formal recommendations for prophylaxis have been developed for other diseases, but consensus is still generally lacking in rheumatology [24, 36, 37]. Our SLR failed to identify specific studies addressing this topic in patients with AAV except for one study demonstrating the advantages of PCP prophylaxis with TMP/SMX in patients with AAV treated with rituximab in reducing the occurrence of severe infections in general and the time to first significant infection [13]. The two RCTs included in the analysis assessed the risk of infection as secondary outcome and reported limited data suggesting lower rates of infections in patients treated with TMP/SMX compared with placebo. According to the available evidence, TMP/SMX prophylaxis should also be recommended to AAV patients receiving rituximab.
This study has some limitations. The quality of the studies retrieved was generally low. The only two RCTs identified on the topic were characterized by a generally high RoB, different doses of TMP/SMX used and different outcome measure definitions applied. The remaining studies were observational cohort studies often applying different concomitant treatments in association with TMP/SMX and different treatment protocols reducing the possibilities to generalize and compare the results. Moreover, previous induction of remission immunosuppressive regimens might have influenced the rate of relapses when TMP/SMX has been used as a maintenance treatment and this represents a potential bias to be considered when interpreting the results. Finally, the evidence supporting the secondary outcome of the study (reduction of infections risk) was only supported by one single study, preventing the possibility of performing a meta-analysis on this important topic in the management of AAV. Nevertheless, this study offers an overview of published and ongoing available evidence supporting the role of TMP/SMX for GPA that might lead to a reconsideration of its use in future clinical practice.
Conclusions
This SLR provides a comprehensive and contemporary appraisal of the available evidence supporting the adjunctive role of TMP/SMX in the treatment of patients with AAV. The results of our meta-analysis failed to support a role of TMP/SMX on the reduction of relapses in GPA; however, in view of the limitations of the existing evidence, the lack of effect for TMP/SMX in modulating disease activity in GPA should be interpreted cautiously. TMP/SMX has a role as prophylaxis against PCP and serious infections, but evidence specifically obtained from patients with AAV was scarce. Further high-quality evidence is warranted to clarify the role of TMP/SMX in the management of AAV.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: None to declare.
Data availability statement
Data extracted from the studies identified by the systematic literature review are displayed in the current manuscript. Complete data are available in the single publications of studies included in the meta-analysis.
Supplementary data
Supplementary data are available at Rheumatology online.
References
14.
TEMPO Study: Trimethoprim-sulfamethoxazole in granulomatosis with polyangiitis.
Author notes
Sara Monti and Paolo Delvino contributed equally to this study.




Comments