-
PDF
- Split View
-
Views
-
Cite
Cite
Antao Xu, Yan Ye, Qiong Fu, Xinyue Lian, Sheng Chen, Qiang Guo, Liang-jing Lu, Min Dai, Xia Lv, Chunde Bao, Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease, Rheumatology, Volume 60, Issue 7, July 2021, Pages 3343–3351, https://doi.org/10.1093/rheumatology/keaa786
Close - Share Icon Share
Abstract
Anti-Ro52 antibody often co-occurs with anti-Jo1 antibody in antisynthetase syndrome and their co-occurrence correlates with a more aggressive clinical phenotype and poorer prognosis. The strong association of anti-Ro52 antibody with anti-melanoma differentiation-associated protein-5 (anti-MDA5) antibody has been indicated in juvenile myositis. The aim of this study was to assess the clinical significance of anti-Ro52 antibody in a cohort of adult patients with anti-MDA5-positive clinically amyopathic dermatomyositis with interstitial lung disease (CADM-ILD).
We assessed a cohort of 83 consecutive patients with anti-MDA5-positive CADM-ILD. Anti-MDA5 antibodies and anti-Ro52 antibodies were detected in immunoblotting and semi-quantitatively analysed by densitometry. Clinical features and the 24 month survival were compared between anti-MDA5-positive patients with and without anti-Ro52 antibodies.
Anti-Ro52 antibodies were found in 74.7% of anti-MDA5-positive CADM-ILD patients and were associated with an increased frequency of rapidly progressive interstitial lung disease (RP-ILD; 54.8% vs 23.8%; P = 0.014) and cutaneous ulcerations (27.4% vs 4.8%; P = 0.033). The cumulative 24 month survival rate tended to be lower in patients with anti-Ro52 antibodies than patients without (59.9% vs 85.7%; P = 0.051). The combination of anti-Ro52 antibody status and anti-MDA5 antibody levels further stratified patients’ survival rates, showing that the survival rate of patients who were dual positive for anti-MDA5 antibody and anti-Ro52 antibody was significantly lower than patients with mild positive anti-MDA5 antibody alone (59.9% vs 100%; P = 0.019).
Anti-Ro52 antibody is highly prevalent in anti-MDA5-positive CADM-ILD patients and their coexistence correlates with a subgroup of patients with more aggressive phenotypes. The combination of anti-MDA5 antibody levels and anti-Ro52 antibody status could help to predict patients’ prognosis and guide risk-based therapy.
74.7% of a Chinese cohort of adult patients with anti-MDA5-positive CADM-ILD has coexistent anti-Ro52 antibody.
Anti-MDA5-positive CADM-ILD patients with anti-Ro52 antibody are more likely to develop RP-ILD and cutaneous ulceration.
Anti-Ro52 antibodies have prognostic values for poor survival in patients with anti-MDA5-positive ADM-ILD.
Introduction
Clinically amyopathic dermatomyositis (CADM) is a unique subtype of idiopathic inflammatory myopathy (IIM) characterized by having the hallmark cutaneous lesions of DM but with no or mild muscle involvement. The prognosis of patients with CADM is largely determined by underlying complications, especially interstitial lung disease (ILD). A large proportion of patients with CADM, mainly from eastern Asia, develop rapidly progressive ILD (RP-ILD), which is resistant to high-dose glucocorticoids and immunosuppressive treatments and has a high mortality rate [1, 2]. Emerging evidence supports the correlation of myositis-specific antibodies (MSAs) with specific clinical phenotypes, disease progression and treatment response in IIMs [3]. In the last 2 decades, a major advance in this field has been the identification of anti-melanoma differentiation-associated protein-5 (anti-MDA5) antibody as one of the most important risk factors for developing RP-ILD and poor survival in CADM [4–6].
The management of anti-MDA5-positive CADM complicated with RP-ILD has been challenging in clinical practice [2, 7]. However, several recent studies have demonstrated that early and aggressive treatment significantly improves the 6 month survival rate of this group of patients from 33% to 89% [8–10]. In these studies, DM/CADM patients with anti-MDA5 antibodies receive combined immunosuppressive therapy before the development of RP-ILD or even prior to ILD onset. This regimen is obviously more effective than the conventional ‘step-up’ strategy, which is based on careful follow-up and switches to an intensive immunosuppressive therapy once patients show signs of RP-ILD. However, despite the great advance in clinical management, this intensive immunosuppressive therapy could be overtreatment for a large portion of patients who may only present with chronic or stable ILD [11]. Studies are required for further subclassification of anti-MDA5-positive CADM patients to recognize patients with a poor prognosis.
Although first identified as a serological marker for SS, anti-Ro52 antibodies often co-occur with myositis-specific antibodies (MSAs), such as anti-Jo1 antibodies, rather than anti-Ro60 antibodies in IIMs [12]. The clinical significance of isolated anti-Ro52 antibodies in myositis remains controversial. However, the coexistence of anti-Ro52 with its closely related antibodies may help to identify a specific clinical subtype of the disease. The frequency of anti-Ro52 antibodies could be 58–72% in anti-Jo1-positive IIM patients [12, 13]. Anti-Ro52 antibodies correlate with the development of more severe ILD [14], myositis, articular symptoms and poorer prognosis in anti-Jo1-positive IIM patients [15]. Importantly, coincidence of anti-Jo1 and anti-Ro52 antibodies also helps to predict patients’ resistance to conventional immunosuppressive drugs, recommending the early use of rituximab [14].
Recently Sabbagh et al. [16] reported that anti-Ro52 antibodies are significantly increased in anti-MDA5-positive juvenile myositis and associated with the presence of ILD as well as poorer prognosis. In the present study we assessed clinical features and long-term outcome associated with anti-Ro52 antibodies in adult patients with anti-MDA5-positive CADM-ILD.
Methods
Patients
A total of 251 adult CADM patients were recruited in an inception cohort study from June 2012 to August 2019 in the Department of Rheumatology, Renji Hospital, Shanghai, China, once the diagnosis of ILD was confirmed [17]. In this cohort study, patients were screened for MSAs and myositis-associated antibodies (MAAs) using a commercial immunoblot assay with 16 autoantigens since December 2015. A total of 141 consecutive CADM-ILD patients were admitted to our centre from December 2015 to August 2019 and 83 were positive for anti-MDA5 antibodies and included in this study. The diagnosis of CADM was based on the criteria suggested by Sontheimer [18] and Concha et al. [19]. ILD was diagnosed according to respiratory symptoms (dry cough and dyspnoea on exertion), physical examinations (such as Velcro rales in the lung bases) and high-resolution CT (HRCT) findings (substantial ILD findings such as ground-glass attenuations, consolidations, reticulations and/or honeycombing), with the exclusion of infection and drug-induced interstitial changes [2]. RP-ILD was defined according to the criteria suggested by Akira et al. [20]: dyspnoea exacerbated within 1 month, new appearance of pulmonary opacities on chest HRCT, a decrease of >10 mmHg in the partial pressure of oxygen (PaO2) and no evidence of infection, pulmonary embolism, congestive heart failure or pneumothorax. HRCT scores were evaluated by two independent radiologists as indicated in our previous study, based on the method described by Ichikado et al. [17, 21], which assessed the percentage of abnormal lung parenchyma including ground-glass attenuation, consolidation, traction bronchiectasis or bronchiolectasis and honeycombing. The average of the HRCT scores for each patient calculated by the two radiologists was taken as the final score. Baseline characteristics of patients on admission, including demographic, clinical and laboratory data, were acquired from a standardized retrospective chart review. Reassessment of patients was conducted once a month during the first 3 months and every 3–6 months thereafter. Follow-up data were collected until December 2019. The cumulative survival rates at 24 months were evaluated, with exacerbated ILD being the major cause of death. Informed consent was obtained from each study participant. The study protocol was approved by the Ethics Committee of Renji Hospital (2013-126).
Detection of autoantibodies
A total of 16 autoantigens were detected in immunoblot testing (Euroimmun, Lübeck, Germany) based on the manufacturer’s instructions. Anti-MDA5 antibodies and anti-Ro52 antibodies were semi-quantitatively analysed by densitometry. The results were defined as negative with greyscale values of 0–10 U/l; weak positive, 11–25 U/l; moderate positive, 26–50 U/l; and strong positive, >50 U/l. ANA were determined by the Nova Lite Hep-2 ANA kit (Inova Diagnostics, San Diego, CA, USA).
Statistical analysis
The chi-squared or Fisher’s exact test was used for comparisons between groups of categorical variables. Based on data distribution, Student’s t-test or Mann–Whitney U-test was employed for continuous data. The Kaplan–Meier curve with logrank test was used to access differences in survival. Two-sided P-values <0.05 were considered statistically significant. All statistical analysis was performed using SPSS software (version 23; IBM, Armonk, NY, USA) and Prism (GraphPad Software, San Diego, CA, USA).
Results
General features of patients with anti-MDA5-positive CADM-ILD
Our consecutive cohort of anti-MDA5-positive CADM-ILD patients recruited 83 patients, 74.7% (62/83) of which were anti-Ro52 positive. The clinical features are summarized in Tables 1 and 2. The mean age of these patients at diagnosis was 51.5 years (s.d. 10.3) and 67.5% (56/83) were female. Seventeen patients also showed low to moderate levels of concurrent myositis-specific or associated antibodies other than anti-Ro52 and 70.6% (12/17) of them were strong positive for anti-MDA5 antibodies. More than one kind of concurrent myositis antibody other than anti-Ro52 was detected in six patients. All patients had Gottron papules/signs and/or heliotrope rash, which are typical DM skin manifestations. A total of 21.7% of patients had cutaneous ulcers, including ulceration of the Gottron papules/signs, the digital pulp and the periungual region, which are characteristic skin manifestations for anti-MDA5-positive DM/CADM patients [18, 22]. Patients also presented with periungual erythema [24.1% (20/83)], articular symptoms [33.7% (28/83)], hoarseness [7.2% (6/83)] and sore throat [3.6% (3/83)]. Thirty-nine patients (47%) developed RP-ILD during follow-up. Patients received glucocorticoids alone or in combination with immunosuppressive drugs such as cyclophosphamide, ciclosporin, tacrolimus, azathioprine or mycophenolate mofetil (Table 2).
General features of patients with anti-MDA5-positive CADM with and without anti-Ro52 antibodies
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Age, years, mean (s.d.) | 51.5 (10.3) | 51.8 (10.4) | 50.8 (10.3) | 0.700 |
| Female, % (n/N) | 67.5 (56/83) | 64.5 (40/62) | 76.2 (16/21) | 0.324 |
| Ferritin, μg/ml, mean (s.d.) | 868.7 (531.9) | 913.9 (511.3) | 742.0 (580.3) | 0.213 |
| CK level, μ/ml, mean (s.d.) | 75.5 (70.3) | 79.9 (73.8) | 62.3 (58.5) | 0.176 |
| Albumin, g/l, mean (s.d.) | 31.5 (5.4) | 31.06 (5.3) | 32.9 (5.6) | 0.190 |
| LDH, μ/l, mean (s.d.) | 342.9 (148.4) | 351.5 (152.8) | 318.3 (135.4) | 0.174 |
| CRP, mg/l, mean (s.d.) | 10.4 (21.4) | 11.2 (23.3) | 8.2 (14.2) | 0.741 |
| ESR, mm/h, mean (s.d.) | 36.7 (22.2) | 38.8 (23.2) | 30.3 (17.9) | 0.142 |
| ANA (≥1:80), % (n/N) | 52 (39/75) | 53.6 (30/56)a | 47.4 (9/19)b | 0.640 |
| Myositis-specific and associated antibodies, % (n/N) | ||||
| Anti-OJ | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-Mi-2β | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-Ku | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| Anti-PM-SCL75 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
| Anti-Jo-1 | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-SRP | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-PL7 | 1.2 (1/83) | 1.6 (1/62) | 0 | 1.000 |
| Anti-PL12 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Age, years, mean (s.d.) | 51.5 (10.3) | 51.8 (10.4) | 50.8 (10.3) | 0.700 |
| Female, % (n/N) | 67.5 (56/83) | 64.5 (40/62) | 76.2 (16/21) | 0.324 |
| Ferritin, μg/ml, mean (s.d.) | 868.7 (531.9) | 913.9 (511.3) | 742.0 (580.3) | 0.213 |
| CK level, μ/ml, mean (s.d.) | 75.5 (70.3) | 79.9 (73.8) | 62.3 (58.5) | 0.176 |
| Albumin, g/l, mean (s.d.) | 31.5 (5.4) | 31.06 (5.3) | 32.9 (5.6) | 0.190 |
| LDH, μ/l, mean (s.d.) | 342.9 (148.4) | 351.5 (152.8) | 318.3 (135.4) | 0.174 |
| CRP, mg/l, mean (s.d.) | 10.4 (21.4) | 11.2 (23.3) | 8.2 (14.2) | 0.741 |
| ESR, mm/h, mean (s.d.) | 36.7 (22.2) | 38.8 (23.2) | 30.3 (17.9) | 0.142 |
| ANA (≥1:80), % (n/N) | 52 (39/75) | 53.6 (30/56)a | 47.4 (9/19)b | 0.640 |
| Myositis-specific and associated antibodies, % (n/N) | ||||
| Anti-OJ | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-Mi-2β | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-Ku | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| Anti-PM-SCL75 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
| Anti-Jo-1 | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-SRP | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-PL7 | 1.2 (1/83) | 1.6 (1/62) | 0 | 1.000 |
| Anti-PL12 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
The chi-squared or Fisher’s exact test was used to compare categorical variables, while continuous data were compared with the Student’s t-test or Mann–Whitney U-test.
N ≠ 62 due to missing data.
N ≠ 21 due to missing data.
CK: creatine kinase; LDH: lactate dehydrogenase.
General features of patients with anti-MDA5-positive CADM with and without anti-Ro52 antibodies
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Age, years, mean (s.d.) | 51.5 (10.3) | 51.8 (10.4) | 50.8 (10.3) | 0.700 |
| Female, % (n/N) | 67.5 (56/83) | 64.5 (40/62) | 76.2 (16/21) | 0.324 |
| Ferritin, μg/ml, mean (s.d.) | 868.7 (531.9) | 913.9 (511.3) | 742.0 (580.3) | 0.213 |
| CK level, μ/ml, mean (s.d.) | 75.5 (70.3) | 79.9 (73.8) | 62.3 (58.5) | 0.176 |
| Albumin, g/l, mean (s.d.) | 31.5 (5.4) | 31.06 (5.3) | 32.9 (5.6) | 0.190 |
| LDH, μ/l, mean (s.d.) | 342.9 (148.4) | 351.5 (152.8) | 318.3 (135.4) | 0.174 |
| CRP, mg/l, mean (s.d.) | 10.4 (21.4) | 11.2 (23.3) | 8.2 (14.2) | 0.741 |
| ESR, mm/h, mean (s.d.) | 36.7 (22.2) | 38.8 (23.2) | 30.3 (17.9) | 0.142 |
| ANA (≥1:80), % (n/N) | 52 (39/75) | 53.6 (30/56)a | 47.4 (9/19)b | 0.640 |
| Myositis-specific and associated antibodies, % (n/N) | ||||
| Anti-OJ | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-Mi-2β | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-Ku | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| Anti-PM-SCL75 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
| Anti-Jo-1 | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-SRP | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-PL7 | 1.2 (1/83) | 1.6 (1/62) | 0 | 1.000 |
| Anti-PL12 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Age, years, mean (s.d.) | 51.5 (10.3) | 51.8 (10.4) | 50.8 (10.3) | 0.700 |
| Female, % (n/N) | 67.5 (56/83) | 64.5 (40/62) | 76.2 (16/21) | 0.324 |
| Ferritin, μg/ml, mean (s.d.) | 868.7 (531.9) | 913.9 (511.3) | 742.0 (580.3) | 0.213 |
| CK level, μ/ml, mean (s.d.) | 75.5 (70.3) | 79.9 (73.8) | 62.3 (58.5) | 0.176 |
| Albumin, g/l, mean (s.d.) | 31.5 (5.4) | 31.06 (5.3) | 32.9 (5.6) | 0.190 |
| LDH, μ/l, mean (s.d.) | 342.9 (148.4) | 351.5 (152.8) | 318.3 (135.4) | 0.174 |
| CRP, mg/l, mean (s.d.) | 10.4 (21.4) | 11.2 (23.3) | 8.2 (14.2) | 0.741 |
| ESR, mm/h, mean (s.d.) | 36.7 (22.2) | 38.8 (23.2) | 30.3 (17.9) | 0.142 |
| ANA (≥1:80), % (n/N) | 52 (39/75) | 53.6 (30/56)a | 47.4 (9/19)b | 0.640 |
| Myositis-specific and associated antibodies, % (n/N) | ||||
| Anti-OJ | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-Mi-2β | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-Ku | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| Anti-PM-SCL75 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
| Anti-Jo-1 | 2.4 (2/83) | 1.6 (1/62) | 4.8 (1/21) | 0.444 |
| Anti-SRP | 6.0 (5/83) | 4.8 (3/62) | 9.5 (2/21) | 0.597 |
| Anti-PL7 | 1.2 (1/83) | 1.6 (1/62) | 0 | 1.000 |
| Anti-PL12 | 3.6 (3/83) | 3.2 (2/62) | 4.8 (1/21) | 1.000 |
The chi-squared or Fisher’s exact test was used to compare categorical variables, while continuous data were compared with the Student’s t-test or Mann–Whitney U-test.
N ≠ 62 due to missing data.
N ≠ 21 due to missing data.
CK: creatine kinase; LDH: lactate dehydrogenase.
General features of patients with anti-MDA5-positive CADM with and without anti-Ro52 antibodies
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Gottron papule/sign, % (n/N) | 92.8 (77/83) | 93.5 (58/62) | 90.5 (19/21) | 0.640 |
| Heliotrope, % (n/N) | 57.8 (48/83) | 56.5 (35/62) | 61.9 (13/21) | 0.662 |
| Cutaneous ulceration, % (n/N) | 21.7 (18/83) | 27.4 (17/62) | 4.8 (1/21) | 0.033 |
| Periungual erythema, % (n/N) | 24.1 (20/83) | 24.2 (15/62) | 23.8 (5/21) | 0.972 |
| Articular symptom, % (n/N) | 33.7 (28/83) | 32.3 (20/62) | 38.1% (8/21) | 0.625 |
| Hoarseness, % (n/N) | 7.2 (6/83) | 9.7 (6/62) | 0 | 0.330 |
| Sore throat, % (n/N) | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| HRCT score, % (n/N) | 138.4 (32.7) | 141.2 (34.6) | 130.1 (25.1) | 0.179 |
| RP-ILD, % (n/N) | 47.0 (39/83) | 54.8 (34/62) | 23.8 (5/21) | 0.014 |
| Exposure to high-dose glucocorticoid (>80 mg), % (n/N) | 30.1 (25/83) | 35.5 (22/62) | 14.3 (3/21) | 0.067 |
| Immunosuppressants, % (n/N) | ||||
| Ciclosporin | 25.3 (21/83) | 25.8 (16/62) | 23.8 (5/21) | 0.856 |
| Tacrolimus | 31.3 (26/83) | 33.8 (21/62) | 23.8 (5/21) | 0.390 |
| Cyclophosphamide | 10.8 (9/83) | 11.3 (7/62) | 9.5 (2/21) | 1.000 |
| Mycophenolate mofetil | 8.4 (7/83) | 6.5 (4/62) | 14.2 (3/21) | 0.362 |
| Azathioprine | 6.0 (5/83) | 8.1 (5/62) | 0 | 0.323 |
| Exposure to pirfenidone, % (n/N) | 14.5 (12/83) | 12.9 (8/62) | 19.0 (4/21) | 0.488 |
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Gottron papule/sign, % (n/N) | 92.8 (77/83) | 93.5 (58/62) | 90.5 (19/21) | 0.640 |
| Heliotrope, % (n/N) | 57.8 (48/83) | 56.5 (35/62) | 61.9 (13/21) | 0.662 |
| Cutaneous ulceration, % (n/N) | 21.7 (18/83) | 27.4 (17/62) | 4.8 (1/21) | 0.033 |
| Periungual erythema, % (n/N) | 24.1 (20/83) | 24.2 (15/62) | 23.8 (5/21) | 0.972 |
| Articular symptom, % (n/N) | 33.7 (28/83) | 32.3 (20/62) | 38.1% (8/21) | 0.625 |
| Hoarseness, % (n/N) | 7.2 (6/83) | 9.7 (6/62) | 0 | 0.330 |
| Sore throat, % (n/N) | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| HRCT score, % (n/N) | 138.4 (32.7) | 141.2 (34.6) | 130.1 (25.1) | 0.179 |
| RP-ILD, % (n/N) | 47.0 (39/83) | 54.8 (34/62) | 23.8 (5/21) | 0.014 |
| Exposure to high-dose glucocorticoid (>80 mg), % (n/N) | 30.1 (25/83) | 35.5 (22/62) | 14.3 (3/21) | 0.067 |
| Immunosuppressants, % (n/N) | ||||
| Ciclosporin | 25.3 (21/83) | 25.8 (16/62) | 23.8 (5/21) | 0.856 |
| Tacrolimus | 31.3 (26/83) | 33.8 (21/62) | 23.8 (5/21) | 0.390 |
| Cyclophosphamide | 10.8 (9/83) | 11.3 (7/62) | 9.5 (2/21) | 1.000 |
| Mycophenolate mofetil | 8.4 (7/83) | 6.5 (4/62) | 14.2 (3/21) | 0.362 |
| Azathioprine | 6.0 (5/83) | 8.1 (5/62) | 0 | 0.323 |
| Exposure to pirfenidone, % (n/N) | 14.5 (12/83) | 12.9 (8/62) | 19.0 (4/21) | 0.488 |
The chi-squared or Fisher’s exact test was used to compare categorical variables, while continuous data were compared employing the Student’s t-test or Mann–Whitney U-test.
General features of patients with anti-MDA5-positive CADM with and without anti-Ro52 antibodies
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Gottron papule/sign, % (n/N) | 92.8 (77/83) | 93.5 (58/62) | 90.5 (19/21) | 0.640 |
| Heliotrope, % (n/N) | 57.8 (48/83) | 56.5 (35/62) | 61.9 (13/21) | 0.662 |
| Cutaneous ulceration, % (n/N) | 21.7 (18/83) | 27.4 (17/62) | 4.8 (1/21) | 0.033 |
| Periungual erythema, % (n/N) | 24.1 (20/83) | 24.2 (15/62) | 23.8 (5/21) | 0.972 |
| Articular symptom, % (n/N) | 33.7 (28/83) | 32.3 (20/62) | 38.1% (8/21) | 0.625 |
| Hoarseness, % (n/N) | 7.2 (6/83) | 9.7 (6/62) | 0 | 0.330 |
| Sore throat, % (n/N) | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| HRCT score, % (n/N) | 138.4 (32.7) | 141.2 (34.6) | 130.1 (25.1) | 0.179 |
| RP-ILD, % (n/N) | 47.0 (39/83) | 54.8 (34/62) | 23.8 (5/21) | 0.014 |
| Exposure to high-dose glucocorticoid (>80 mg), % (n/N) | 30.1 (25/83) | 35.5 (22/62) | 14.3 (3/21) | 0.067 |
| Immunosuppressants, % (n/N) | ||||
| Ciclosporin | 25.3 (21/83) | 25.8 (16/62) | 23.8 (5/21) | 0.856 |
| Tacrolimus | 31.3 (26/83) | 33.8 (21/62) | 23.8 (5/21) | 0.390 |
| Cyclophosphamide | 10.8 (9/83) | 11.3 (7/62) | 9.5 (2/21) | 1.000 |
| Mycophenolate mofetil | 8.4 (7/83) | 6.5 (4/62) | 14.2 (3/21) | 0.362 |
| Azathioprine | 6.0 (5/83) | 8.1 (5/62) | 0 | 0.323 |
| Exposure to pirfenidone, % (n/N) | 14.5 (12/83) | 12.9 (8/62) | 19.0 (4/21) | 0.488 |
| Characteristics . | Total (N = 83) . | Anti-Ro52 antibody positive (N = 62) . | Anti-Ro52 antibody negative (N = 21) . | P-value . |
|---|---|---|---|---|
| Gottron papule/sign, % (n/N) | 92.8 (77/83) | 93.5 (58/62) | 90.5 (19/21) | 0.640 |
| Heliotrope, % (n/N) | 57.8 (48/83) | 56.5 (35/62) | 61.9 (13/21) | 0.662 |
| Cutaneous ulceration, % (n/N) | 21.7 (18/83) | 27.4 (17/62) | 4.8 (1/21) | 0.033 |
| Periungual erythema, % (n/N) | 24.1 (20/83) | 24.2 (15/62) | 23.8 (5/21) | 0.972 |
| Articular symptom, % (n/N) | 33.7 (28/83) | 32.3 (20/62) | 38.1% (8/21) | 0.625 |
| Hoarseness, % (n/N) | 7.2 (6/83) | 9.7 (6/62) | 0 | 0.330 |
| Sore throat, % (n/N) | 3.6 (3/83) | 4.8 (3/62) | 0 | 0.568 |
| HRCT score, % (n/N) | 138.4 (32.7) | 141.2 (34.6) | 130.1 (25.1) | 0.179 |
| RP-ILD, % (n/N) | 47.0 (39/83) | 54.8 (34/62) | 23.8 (5/21) | 0.014 |
| Exposure to high-dose glucocorticoid (>80 mg), % (n/N) | 30.1 (25/83) | 35.5 (22/62) | 14.3 (3/21) | 0.067 |
| Immunosuppressants, % (n/N) | ||||
| Ciclosporin | 25.3 (21/83) | 25.8 (16/62) | 23.8 (5/21) | 0.856 |
| Tacrolimus | 31.3 (26/83) | 33.8 (21/62) | 23.8 (5/21) | 0.390 |
| Cyclophosphamide | 10.8 (9/83) | 11.3 (7/62) | 9.5 (2/21) | 1.000 |
| Mycophenolate mofetil | 8.4 (7/83) | 6.5 (4/62) | 14.2 (3/21) | 0.362 |
| Azathioprine | 6.0 (5/83) | 8.1 (5/62) | 0 | 0.323 |
| Exposure to pirfenidone, % (n/N) | 14.5 (12/83) | 12.9 (8/62) | 19.0 (4/21) | 0.488 |
The chi-squared or Fisher’s exact test was used to compare categorical variables, while continuous data were compared employing the Student’s t-test or Mann–Whitney U-test.
Characteristics of anti-MDA5-positive CADM-ILD patients with anti-Ro52 antibodies
There was a trend towards the coexistence of anti-Ro52 antibodies with high levels of anti-MDA5 antibody, which occurred in 57.1% of patients with weak positive anti-MDA5 antibodies, 68% of patients with moderate positive and 80.4% of patients with strong positive (Table 3). No significant differences were found in age, gender and laboratory examinations such as levels of creatine kinase, albumin, lactate dehydrogenase, CRP and ESR between anti-MDA5-positive CADM patients with and without anti-Ro52 antibodies. There were also no associations between anti-Ro52 antibodies and the presence of ANA or other myositis antibodies. Among the clinical manifestations of CADM, cutaneous ulceration was significantly increased in anti-MDA5-positive patients with anti-Ro52 antibodies (27.4% vs 4.8%; P = 0.033; Table 2). However, there was no association of the presence of cutaneous ulcerations with RP-ILD and patient’s prognosis with respect to the cumulative survival in the anti-MDA5-positive CADM-ILD cohort (Supplementary Table S1, available at Rheumatology online). Hoarseness and sore throat presented in only patients with anti-Ro52 antibodies. There were significantly more patients with the combined presence of anti-Ro52 and anti-MDA5 antibodies who developed RP-ILD than patients with anti-MDA5 antibody alone (54.8% vs 23.8%; P = 0.014). With regard to different anti-MDA5 antibody levels, the presence of anti-Ro52 antibody significantly correlated with the development of RP-ILD in patients with moderate positive anti-MDA5 (64.7% vs 12.5%; P = 0.030; Table 4). The maximum dosage of glucocorticoids in treatment also had a positively higher trend in the group of patients with anti-Ro52 antibodies (P = 0.067). There was no significant difference in therapeutic regimens between the two groups.
Prevalence of anti-Ro52 antibodies among patients with anti-MDA5-positive CADM
| Anti-MDA5 antibody level . | Anti-Ro52 antibody positive, % (n) . | Anti-Ro52 antibody negative, % (n) . | P-value . |
|---|---|---|---|
| Weak (n = 7) | 57.1 (4) | 42.9 (3) | 0.362 |
| Moderate (n = 25) | 68 (17) | 32 (8) | 0.357 |
| Strong (n = 51) | 80.4 (41) | 19.6 (10) | 0.132 |
| Anti-MDA5 antibody level . | Anti-Ro52 antibody positive, % (n) . | Anti-Ro52 antibody negative, % (n) . | P-value . |
|---|---|---|---|
| Weak (n = 7) | 57.1 (4) | 42.9 (3) | 0.362 |
| Moderate (n = 25) | 68 (17) | 32 (8) | 0.357 |
| Strong (n = 51) | 80.4 (41) | 19.6 (10) | 0.132 |
The chi-squared or Fisher’s exact test was used to compare categorical variables.
Prevalence of anti-Ro52 antibodies among patients with anti-MDA5-positive CADM
| Anti-MDA5 antibody level . | Anti-Ro52 antibody positive, % (n) . | Anti-Ro52 antibody negative, % (n) . | P-value . |
|---|---|---|---|
| Weak (n = 7) | 57.1 (4) | 42.9 (3) | 0.362 |
| Moderate (n = 25) | 68 (17) | 32 (8) | 0.357 |
| Strong (n = 51) | 80.4 (41) | 19.6 (10) | 0.132 |
| Anti-MDA5 antibody level . | Anti-Ro52 antibody positive, % (n) . | Anti-Ro52 antibody negative, % (n) . | P-value . |
|---|---|---|---|
| Weak (n = 7) | 57.1 (4) | 42.9 (3) | 0.362 |
| Moderate (n = 25) | 68 (17) | 32 (8) | 0.357 |
| Strong (n = 51) | 80.4 (41) | 19.6 (10) | 0.132 |
The chi-squared or Fisher’s exact test was used to compare categorical variables.
The association of RP-ILD with anti-MDA5 antibody levels and anti-Ro52 antibody status in CADM patients
| Anti-MDA5 antibody levels . | Anti-Ro52 antibody status . | RP-ILD, % (n) . | P-value . |
|---|---|---|---|
| Weak (+) | Positive (n = 4) | 25.0 (1) | 1.000 |
| Negative (n = 3) | 33.3 (1) | ||
| Moderate (++) | Positive (n = 17) | 64.7 (11) | 0.030 |
| Negative (n = 8) | 12.5 (1) | ||
| Strong (+++) | Positive (n = 41) | 53.7 (22) | 0.291 |
| Negative (n = 10) | 30 (3) |
| Anti-MDA5 antibody levels . | Anti-Ro52 antibody status . | RP-ILD, % (n) . | P-value . |
|---|---|---|---|
| Weak (+) | Positive (n = 4) | 25.0 (1) | 1.000 |
| Negative (n = 3) | 33.3 (1) | ||
| Moderate (++) | Positive (n = 17) | 64.7 (11) | 0.030 |
| Negative (n = 8) | 12.5 (1) | ||
| Strong (+++) | Positive (n = 41) | 53.7 (22) | 0.291 |
| Negative (n = 10) | 30 (3) |
The chi-squared or Fisher’s exact test was used to compare categorical variables.
The association of RP-ILD with anti-MDA5 antibody levels and anti-Ro52 antibody status in CADM patients
| Anti-MDA5 antibody levels . | Anti-Ro52 antibody status . | RP-ILD, % (n) . | P-value . |
|---|---|---|---|
| Weak (+) | Positive (n = 4) | 25.0 (1) | 1.000 |
| Negative (n = 3) | 33.3 (1) | ||
| Moderate (++) | Positive (n = 17) | 64.7 (11) | 0.030 |
| Negative (n = 8) | 12.5 (1) | ||
| Strong (+++) | Positive (n = 41) | 53.7 (22) | 0.291 |
| Negative (n = 10) | 30 (3) |
| Anti-MDA5 antibody levels . | Anti-Ro52 antibody status . | RP-ILD, % (n) . | P-value . |
|---|---|---|---|
| Weak (+) | Positive (n = 4) | 25.0 (1) | 1.000 |
| Negative (n = 3) | 33.3 (1) | ||
| Moderate (++) | Positive (n = 17) | 64.7 (11) | 0.030 |
| Negative (n = 8) | 12.5 (1) | ||
| Strong (+++) | Positive (n = 41) | 53.7 (22) | 0.291 |
| Negative (n = 10) | 30 (3) |
The chi-squared or Fisher’s exact test was used to compare categorical variables.
Long-term outcome among anti-MDA5-positive CADM-ILD patients with anti-Ro52 antibodies
The 24 month survival rate of anti-MDA5-positive CADM-ILD patients was 66.6%. The major cause of mortality was ILD-induced respiratory failure and most of these deaths happened within the first 6 months after diagnosis. Consistent with the higher frequency of RP-ILD, the cumulative survival rate at 24 months tended to be lower in patients with anti-Ro52 antibodies than in patients without the antibody (59.9% vs 85.7%; P = 0.051; Fig. 1A).
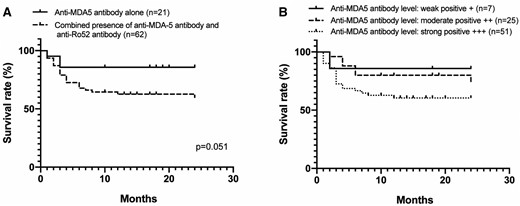
Overall survival in anti-MDA5-positive CADM-ILD patients
(A) The cumulative survival rate at 24 months in anti-MDA5-positive CADM-ILD patients with and without anti-Ro52 antibody. (B) Survival rates stratified according to anti-MDA5 antibody levels. Overall survival rates were determined by Kaplan–Meier test and plus signs represents censored patients.
Anti-MDA5 antibody levels have been reported to be associated with disease severity and long-term survival in patients with CADM/DM [23–25]. Patients were then subdivided into three groups according to anti-MDA5 antibody levels. Survival analysis showed that patients who were strong positive for anti-MDA5 antibodies tended to have poorer long-term outcomes than patients with weak or moderate positive anti-MDA5, although this difference did not reach statistical significance (Fig. 1B).
Since survival rates were similar between patients with weak and moderate positive anti-MDA5, these patients were reclassified into the same group and designated as ‘mild positive’. In order to better identify patients with the highest and lowest survival rates, patients were further subclassified based on the combination of anti-MDA5 antibody level and anti-Ro52 antibody status. Analysis showed that the survival rates increased in the following order (from lowest to highest): strong positive anti-MDA5 with anti-Ro52 (57.9%), mild positive anti-MDA5 with anti-Ro52 (65.5%), strong positive anti-MDA5 without anti-Ro52 (70.0%) and mild positive anti-MDA5 without anti-Ro52 (100.0%) (Fig. 2A). The prognosis of patients with mild positive anti-MDA5 alone was significantly better than that of patients with the combined presence of anti-MDA5 antibody and anti-Ro52 antibody (100.0% vs 59.9%; P = 0.019; Fig. 2B). Consistent with the results of survival analysis, significantly fewer patients with mild positive anti-MDA5 antibody alone developed RP-ILD than patients who were dual-positive for anti-MDA5 antibody and anti-Ro52 antibody (18.2% vs 54.8%; P = 0.025).
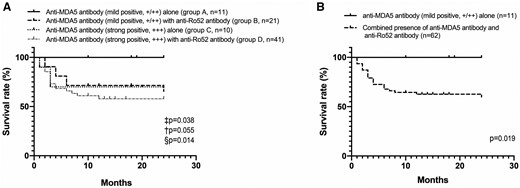
Survival rates stratified according to the combination of anti-MDA5 antibody levels and the anti-Ro52 antibody status
Patients with weak and moderate positive anti-MDA5 antibody were reclassified into one group and designated as ‘mild positive’. ‡, group A vs group B; †, group A vs group C; §, group A vs group D. +, weak positive; ++, moderate positive; +++, strong positive; +/++, weak or moderate positive. Overall survival rates were determined by Kaplan–Meier test and plus signs represents censored patients.
Discussion
Our current study underlines the high prevalence of anti-Ro52 antibody in patients with anti-MDA5-positive CADM-ILD. The presence of anti-Ro52 may help to define a specific subtype of anti-MDA5-positive CADM-ILD, which correlates with an increased frequency of RP-ILD and cutaneous ulceration. Anti-MDA5-positive CADM-ILD patients with coexistent anti-Ro52 antibodies tend to have poorer prognosis than patients without. The combination of anti-MDA5 antibody levels and anti-Ro52 antibody status further stratifies long-term survival rates of CADM-ILD patients with the identification of patients with the highest and lowest survival rates, which may help to improve patients’ clinical management.
The prognosis of anti-MDA5-positive CADM patients complicated with RP-ILD was very poor. Most mortalities occurred in the first 6 months, but for patients who survived this stage, long-term survival could be achieved. This indicated that the initial therapeutic strategy for this group of patients is critical. Recent studies have demonstrated that early interventions in anti-MDA5-positive CADM/DM patients who presented with ILD or even before signs of ILD could significantly improve patient survival [9]. Two effective modalities of early interventions have been proposed. Japanese investigators proposed a combined immunosuppressive regimen that included high-dose glucocorticoids, tacrolimus and intravenous cyclophosphamide [8, 9], while a clinical trial conducted at our hospital showed that initiation of the Janus kinase inhibitor tofacitinib in the early stage of disease was also effective [26]. In our present cohort, 47.0% (39/83) of the anti-MDA5-positive CADM-ILD patients developed RP-ILD, while about half of the patients remained chronic or stable ILD, which was consistent with previously reported cohort studies [5, 11]. This indicated that early intensive immunosuppressive therapy based on anti-MDA5 status alone or in combination with ILD would be overtreatment to a large proportion of patients for whom the disease activity could be well controlled under conventional therapies or the ‘step-up’ strategy [8, 11]. Of particular significance is the identification of anti-Ro52 antibody as an important prognostic factor in our anti-MDA5-positive CADM cohort. The administration of combined immunosuppressive therapy according to the coexistence of anti-Ro52 and anti-MDA5 antibody levels may help to avoid overtreatment in CADM-ILD patients, especially in patients with mild positive anti-MDA5 antibody alone.
Cutaneous ulceration is one of the heterogeneous skin manifestations in DM/CADM, which is mainly in the Gottron papules/signs, the digital pulp or the periungual region and can be extremely painful with poor response to treatment [18, 27]. Anti-MDA5 antibody is significantly associated with the presentation of cutaneous ulcers, especially those located in the digital pulp [22]. Histopathological analysis has confirmed the severe cutaneous vasculopathy in anti-MDA5-positive DM/CADM patients [28]. However, the clinical significance of cutaneous ulcerations has been controversial. Cao et al. [29] reported that ulcerative Gottron papules/signs correlated with a significantly increased frequency of RP-ILD and decreased survival rates in DM/CADM patients, while Nagashima et al. [30] found no correlation in their cohort. Since the association between cutaneous ulcerations and ILD depends on the appearance of anti-MDA5 antibody [22], one possible explanation was the high frequency of anti-MDA5 antibody positivity in patients with ulcerative Gottron papules/signs in the cohort study of Cao et al. [29]. In the present study, we demonstrated that cutaneous ulcerations did not correlate with RP-ILD and patient survival in the anti-MDA5-positive CADM-ILD cohort, which indicated that the combined presence of cutaneous ulceration and anti-MDA5 antibody did not further dampen a patient’s prognosis. Thus, in clinical practice, cutaneous ulcerations may mainly serve as a characteristic clinical feature of anti-MDA5 antibody in DM/CADM.
Anti-Ro52 antibodies are prevalent in various systemic autoimmune conditions and may serve as a common element in the pathogenesis of these disease. In our anti-MDA5-positive CADM-ILD cohort, anti-Ro52 antibodies significantly correlated with the development of RP-ILD and cutaneous ulceration and this subgroup of patients is more likely to be refractory to the conventional immunosuppressive regimen, which leads to a high mortality rate. High risk of RP-ILD and ulcerative skin changes are generally taken as clinical features specific for anti-MDA5 antibody [18]. That is to say, once coupled with anti-Ro52 antibodies, anti-MDA5-positive CADM patients tend to present a more aggressive, anti-MDA5-specific clinical phenotype. Interestingly, when anti-Ro52 antibody co-occurred with anti-Jo1 antibody, DM patients showed more severe myositis, articular impairment and ILD, as well as more common presentation of mechanic’s hand, all of which lead to decreased survival rate [15]. As we know, the combination of myositis, arthritis, ILD and mechanic’s hands is a characteristic clinical feature of anti-Jo1-positive antisynthetase syndrome [31]. Thus the most important clinical significance of anti-Ro52 antibody in IIMs seems to serve as a risk factor that aggravates clinical phenotypes specific for its co-occurring MSAs. Thus DM patients with isolated anti-Ro52 antibody, even when complicated with RP-ILD, respond well to therapy and have a good prognosis [32].
Our study was based on the semi-quantitative analysis of anti-MDA5 levels employing a commercial line immunoassay (LIA). The advantages of an LIA are it is easy to use and inexpensive, which makes it favourable to be applied on a large scale in routine clinical practice [33]. As the significance of MSAs in the classification and further stratification of IIMs has been globally recognized, the measurement of MSAs has been increasingly introduced in clinical practices. The LIA is now becoming more and more widely adopted in the diagnostic workups of IIMs, which may greatly promote early diagnosis of aggressive conditions like anti-MDA5-positive CADM-ILD. On the other hand, because of a lack of internal controls and proper calibration, LIAs may suffer from low specificity. A current hot topic of debate is how to further improve the accuracy of LIAs and harmonize MSA-detecting methodologies [34–36]. An automated scanning system has been introduced to objectively interpret LIA results. The scanning system also provides a semi-quantitative approach for the analysis of antibody levels. Previously we developed and validated a ‘FLAIR’ score model to predict the mortality of patients with ADM-ILD, which included ferritin levels, lactate dehydrogenase levels, semi-quantitative anti-MDA5 antibody levels, HRCT scores and RP-ILD status [17]. The weak, moderate and strong positivity of anti-MDA5 received increasing weighted values in this model, indicating a significant prognostic value of semi-quantitative measurement of anti-MDA5 levels. In the present study, the prognostic value of the combination of semi-quantitative anti-MDA5 levels and anti-Ro52 status was shown. We also observed a semi-quantitative decrease of anti-MDA5 levels during patients’ follow-up after treatment (data not shown), which was consistent with previous studies that monitored anti-MDA5 titres using ELISA [37, 38]. Our study seems to expand the clinical significance of the LIA in the detection of MSAs. However, statistics-based method comparisons are needed to further confirm this.
Although the current study includes a large cohort of anti-MDA5-positive CADM-ILD patients that provides unique strength to the results, as a study conducted in a single tertiary academic medical centre in China, there are several limitations. Not only patients in Shanghai, but also patients from other areas of China are referred to our centre [2]. Many patients are severely ill and unable to complete pulmonary function tests on admission. Thus the results could possibly skew towards more severe conditions with an increased likelihood of RP-ILD and poor prognosis. The lack of pulmonary function tests, bronchoalveolar lavage fluid analysis, lung biopsy and monitoring changes of anti-MDA5 antibody levels was also a great limitation. Given the possible influences of environmental factors and genetic factors, the clinical significance of anti-Ro52 antibody in anti-MDA5-positive CADM-ILD patients should be further explored in other ethnicities.
In conclusion, anti-Ro52 antibody is highly prevalent in anti-MDA5-positive CADM-ILD and their coexistence could help to identify a more aggressive disease phenotype with an increased likelihood of RP-ILD and cutaneous ulceration. Further studies are required to confirm the predictive value of anti-Ro52 antibody status combined with anti-MDA5 antibody levels for RP-ILD and prognosis in CADM patients. Precise risk stratification and early intensive immunosuppressive therapy in patients at high risk may significantly improve the long-term outcome of anti-MDA5-positive CADM patients, while avoiding overtreatment at the same time.
Acknowledgements
The authors thank Ping Ye and Shuiying Li for clinical lab technique and follow-up support (Renji Hospital, School of Medicine, Shanghai Jiao Tong University, China).
Funding: This work was supported by the National Key Research and Development Program of China (2017YFC0909002) and National Natural Science Foundation of China (No. 81771733, No. 81771752 and No. 81600435).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
References
Author notes
Antao Xu and Yan Ye contributed equally to the article.




Comments