-
PDF
- Split View
-
Views
-
Cite
Cite
Chanyuan Wu, Qian Wang, Dong Xu, Mengtao Li, Xiaofeng Zeng, Sirolimus for patients with connective tissue disease-related refractory thrombocytopenia: a single-arm, open-label clinical trial, Rheumatology, Volume 60, Issue 6, June 2021, Pages 2629–2634, https://doi.org/10.1093/rheumatology/keaa645
Close - Share Icon Share
Abstract
Connective tissue disease-related thrombocytopenia (CTD-TP) is a problematic disorder in clinical practice. Because the first-line therapy of glucocorticoid and/or immunosuppressants is not effective for refractory cases, alternative treatment approaches are urgently needed. The present study investigated the efficacy and safety of sirolimus in refractory CTD-TP patients.
This single-centre, single-arm, phase II study enrolled 20 refractory CTD-TP patients between September 2017 and September 2018 (registered on ClinicalTrials.gov: NCT03688191). Oral sirolimus administration was dose-adjusted to maintain a therapeutic range of 6–15 ng/ml for 6 months. The primary endpoints were partial and complete remission rates at 6 months.
Twelve (60%) patients achieved the primary end point with a 50% complete remission rate after 6 months. Among the 14 SLE patients, the overall response rate was 71.4%, with a complete remission rate of 64.3%. None of the primary Sjögren's syndrome cases responded to sirolimus. There was no significant difference in baseline clinical characteristics or lymphocyte subpopulations between responders and non-responders. No severe side effect was detected during the study.
Sirolimus is an effective and safe treatment option for refractory CTD-TP patients.
https://clinicaltrials.gov, NCT03688191.
Oral sirolimus showed promising efficacy to treat refractory CTD-related thrombocytopenia with an acceptable safety profile.
Oral sirolimus may be an alternative to CTD-TP, especially to SLE-TP.
The efficacy of sirolimus in the treatment of pSS awaits further evaluation.
Introduction
Connective tissue disease (CTD) refers to a group of autoimmune disorders, including SLE, primary Sjögren's syndrome (pSS), systemic sclerosis, etc., that are classified among the systemic rheumatic diseases [1]. Haematological disorder is commonly reported to be associated with CTD. One of the most severe forms of haematological involvement in CTD is thrombocytopenia (CTD-TP). CTD-TP can range from a slight decrease in platelet count to fatal bleeding, which occurs even when other systems are stable. Moreover, it does not respond well to glucocorticoids (GCs) or immunosuppressants (ISs), making it problematic in clinical practice.
Activation of mammalian target of rapamycin (mTOR) was reported to be associated to the T-cell dysfunction in SLE-related thrombocytopenia (SLE-TP) patients [2]. Sirolimus, as mTOR inhibitor, inhibits antigen-induced T-cell proliferation and has been reported to be effective in SLE with limited side effects [3]. A multi-institutional study indicated that sirolimus led to complete and durable responses in a majority of children with refractory multilineage autoimmune cytopenias [4]. Another study showed that sirolimus was potentially a safe and efficacious treatment in patients with active SLE who were unresponsive to conventional medications [5]. To date, some successful cases of sirolimus treatment of refractory SLE-TP have been reported [6]. The present clinical trial investigated the efficacy and safety of sirolimus treatment of refractory CTD-TP including SLE.
Methods
Study design and participants
This prospective, single-arm, open-label, phase II clinical trial was approved by the Institutional Review Board (approval no. HS-1349) of Peking Union Medical College Hospital, a tertiary referral hospital in Beijing, China. This trial is registered on ClinicalTrials.gov (no. NCT03688191). Refractory CTD-TP patients (aged ≥18 years) who visited our referral centre from 18 September 2017 to 20 September 2018 were enrolled. Patients with SLE fulfilled the 2012 SLE International Collaborating Clinics criteria for the classification of SLE [7]. Other CTDs, including pSS and undifferentiated CTD, were diagnosed according to the revised 2002 International European criteria for pSS [8] as well as preliminary classification criteria [9], respectively; newly-diagnosed CTD was excluded. Patients were excluded if they were pregnant or had life-threatening manifestations of CTD (e.g. rapidly progressive glomerulonephritis, neuropsychiatric lupus, alveolar haemorrhage or pulmonary hypertension), active infections or malignant diseases. All patients had thrombocytopenia and failed at least one course of methylprednisolone pulse therapy (1 g/d for 3 consecutive days) and/or intravenous immunoglobulin G (20 g/d for 3–5 consecutive days) and high-dose GCs in combination with ISs, including cyclophosphamide (1000 mg intravenous pulse every month or 100 mg/d orally), mycophenolate mofetil (2–3 g/d), FK506 (tacrolimus; 2–3 mg/d), or MMF A (3–5 mg/kg/d). All eligible participants provided informed consent using forms approved by the Institutional Review Board of Peking Union Medical College Hospital prior to study inclusion.
Procedures
Oral sirolimus was administered at a dose of 2 mg/d for the first 3 d, with a sequential dose of 1 mg/d. Doses were adjusted according to tolerance and to maintain a therapeutic range of 6–15 ng/ml for 6 months. During the trial, previous doses of oral prednisone or prednisolone were continued and gradually tapered to minimal maintenance doses. All previous ISs were either continued or withdrawn; however, no new ISs were added during the study. Patient follow-up occurred 2, 4, 8, 16 and 24 weeks after the start of the first dose of sirolimus. The final follow-up date was 18 March 2019.
Outcomes and assessments
According to the ITP International Working Group Criteria [10], response criteria were defined as follows: partial response/remission (PR) was defined as a platelet count above 30 × 109 cells/l, with at least doubling of the baseline platelet count, and complete response/remission (CR) was defined as a platelet count ≥100 × 109 cells/l. No response was defined as those without a CR or PR. Overall response included patients with a CR or PR. The primary efficacy end point was CR and PR rates at 6 months. The secondary efficacy end point was CR and PR rates at 3 months. Hence, efficacy endpoints were assessed in all patients who completed at least 3 or more months of treatment. Safety outcomes included tolerance as assessed by the occurrence of common side effects, and all patients who received at least one dose of treatment were included in safety analyses.
Statistical analysis
Statistical analyses were performed with SPSS 22 software (SPSS, Inc., Chicago, IL, USA). The independent samples t test (for parameters with normal distribution) and Mann–Whitney test (for parameters which were not normally distributed) were used for comparison of baseline characteristics between responders and non-responders. The Wilcoxon signed-rank test was used to compare parameters before and after sirolimus treatment. For patients who were evaluated as ‘treatment failure’, the time of treatment failure was considered as the end of treatment. A P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 20 refractory CTD-TP patients participated in this phase II study and were followed for at least 24 weeks. Fourteen patients met the diagnostic criteria for SLE, three fulfilled the criteria for pSS, and three fulfilled the criteria for undifferentiated CTD. All 20 patients had a positive antinuclear antibodies test, and five were antiphospholipid antibody-positive. Baseline demographic and disease characteristics are summarized in Table 1. The median age was 37 years (range, 18–55 years), with a male:female ratio of 1:19. The majority of patients (60%) demonstrated thrombocytopenia as the first clinical manifestation. The median course of disease was 6.5 years (range, 0.5–17 years). The median platelet count was 34 × 109 cells/l (range, 2 × 109–72 × 109 cells/l) before trial admission. Median haemoglobin, white blood cell count, serum creatinine, CRP, ESR, serum cholesterol and immunoglobin G levels were 118 g/l (range, 68–160 g/l), 4.61 × 109 cells/l (range, 1.64 × 109–13.77 × 109 cells/l), 63 µmol/l (range, 44–151 µmol/l), 1.42 mg/l (range, 0.25–9.91 mg/l), 10 mm/h (range, 1–40 mm/h), 3.33 mmol/l (range, 2.59–4.79 mmol/l) and 12.81 g/l (range, 5.05–19.39 g/l), respectively.
| Patient no. . | Age (years) . | Sex . | CTD disease . | Prior therapies . | Time to sirolimus (years) . | Sirolimus duration (m) . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 36 | F | SLE, APS | GCs, MMF, AZA | 15 | 18 | CR |
| 2 | 44 | F | SLE | GCs, FK506 | 13 | 17 | CR |
| 3 | 48 | F | SLE | GCs, MMF | 7 | 17 | CR |
| 4 | 47 | F | pSS | GCs, FK506 | 18 | 4 | NR |
| 5 | 35 | F | SLE | GCs, MMF | 2 | 12 | CR |
| 6 | 52 | F | SLE | GCs, CsA | 3 | 12 | CR |
| 7 | 31 | F | SLE | GCs, FK506 | 7 | 12 | CR |
| 8 | 43 | F | pSS | GCs, FK506, CsA, IVIG | 8 | 6 | NR |
| 9 | 39 | F | SLE | GCs, CTX | 8 | 6 | NR |
| 10 | 30 | F | SLE | GCs, MMF, CsA, TII | 15 | 5 | PR |
| 11 | 29 | F | SLE | GCs, FK506 | 7 | 9 | NR |
| 12 | 27 | F | CTD | GCs, CsA | 5 | 9 | CR |
| 13 | 54 | F | CTD | GCs, CsA | 5 | 8 | PR |
| 14 | 55 | F | pSS | GCs, AZA | 14 | 6 | NR |
| 15 | 18 | M | SLE | GCs, MMF, FK506, CsA | 3 | 6 | NR |
| 16 | 39 | F | CTD | GCs, CTX, FK506, CsA | 6 | 6 | NR |
| 17 | 20 | F | SLE | GCs, CTX, FK506, CsA, MMF, IVIg | 14 | 6 | NR |
| 18 | 18 | F | SLE | GCs, AZA, FK506 | 1 | 6 | CR |
| 19 | 31 | F | SLE | GCs, CsA, CTX | 6 | 6 | CR |
| 20 | 38 | F | SLE | GCs, HCQ | 4 | 6 | CR |
| Patient no. . | Age (years) . | Sex . | CTD disease . | Prior therapies . | Time to sirolimus (years) . | Sirolimus duration (m) . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 36 | F | SLE, APS | GCs, MMF, AZA | 15 | 18 | CR |
| 2 | 44 | F | SLE | GCs, FK506 | 13 | 17 | CR |
| 3 | 48 | F | SLE | GCs, MMF | 7 | 17 | CR |
| 4 | 47 | F | pSS | GCs, FK506 | 18 | 4 | NR |
| 5 | 35 | F | SLE | GCs, MMF | 2 | 12 | CR |
| 6 | 52 | F | SLE | GCs, CsA | 3 | 12 | CR |
| 7 | 31 | F | SLE | GCs, FK506 | 7 | 12 | CR |
| 8 | 43 | F | pSS | GCs, FK506, CsA, IVIG | 8 | 6 | NR |
| 9 | 39 | F | SLE | GCs, CTX | 8 | 6 | NR |
| 10 | 30 | F | SLE | GCs, MMF, CsA, TII | 15 | 5 | PR |
| 11 | 29 | F | SLE | GCs, FK506 | 7 | 9 | NR |
| 12 | 27 | F | CTD | GCs, CsA | 5 | 9 | CR |
| 13 | 54 | F | CTD | GCs, CsA | 5 | 8 | PR |
| 14 | 55 | F | pSS | GCs, AZA | 14 | 6 | NR |
| 15 | 18 | M | SLE | GCs, MMF, FK506, CsA | 3 | 6 | NR |
| 16 | 39 | F | CTD | GCs, CTX, FK506, CsA | 6 | 6 | NR |
| 17 | 20 | F | SLE | GCs, CTX, FK506, CsA, MMF, IVIg | 14 | 6 | NR |
| 18 | 18 | F | SLE | GCs, AZA, FK506 | 1 | 6 | CR |
| 19 | 31 | F | SLE | GCs, CsA, CTX | 6 | 6 | CR |
| 20 | 38 | F | SLE | GCs, HCQ | 4 | 6 | CR |
AZA: azathioprine; CH: Chinese herbs; CR: complete response; CsA: ciclosporin A; CTD-TP: connective tissue disease-related thrombocytopenia; CTX: cyclophosphamide; FK506: tacrolimus; GCs: glucocorticoids; IVIG: intravenous immunoglobulin G; NA: not available; NR: no response; PR: partial response; pSS: primary Sjögren's syndrome; TII: tripterygium glycosides.
| Patient no. . | Age (years) . | Sex . | CTD disease . | Prior therapies . | Time to sirolimus (years) . | Sirolimus duration (m) . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 36 | F | SLE, APS | GCs, MMF, AZA | 15 | 18 | CR |
| 2 | 44 | F | SLE | GCs, FK506 | 13 | 17 | CR |
| 3 | 48 | F | SLE | GCs, MMF | 7 | 17 | CR |
| 4 | 47 | F | pSS | GCs, FK506 | 18 | 4 | NR |
| 5 | 35 | F | SLE | GCs, MMF | 2 | 12 | CR |
| 6 | 52 | F | SLE | GCs, CsA | 3 | 12 | CR |
| 7 | 31 | F | SLE | GCs, FK506 | 7 | 12 | CR |
| 8 | 43 | F | pSS | GCs, FK506, CsA, IVIG | 8 | 6 | NR |
| 9 | 39 | F | SLE | GCs, CTX | 8 | 6 | NR |
| 10 | 30 | F | SLE | GCs, MMF, CsA, TII | 15 | 5 | PR |
| 11 | 29 | F | SLE | GCs, FK506 | 7 | 9 | NR |
| 12 | 27 | F | CTD | GCs, CsA | 5 | 9 | CR |
| 13 | 54 | F | CTD | GCs, CsA | 5 | 8 | PR |
| 14 | 55 | F | pSS | GCs, AZA | 14 | 6 | NR |
| 15 | 18 | M | SLE | GCs, MMF, FK506, CsA | 3 | 6 | NR |
| 16 | 39 | F | CTD | GCs, CTX, FK506, CsA | 6 | 6 | NR |
| 17 | 20 | F | SLE | GCs, CTX, FK506, CsA, MMF, IVIg | 14 | 6 | NR |
| 18 | 18 | F | SLE | GCs, AZA, FK506 | 1 | 6 | CR |
| 19 | 31 | F | SLE | GCs, CsA, CTX | 6 | 6 | CR |
| 20 | 38 | F | SLE | GCs, HCQ | 4 | 6 | CR |
| Patient no. . | Age (years) . | Sex . | CTD disease . | Prior therapies . | Time to sirolimus (years) . | Sirolimus duration (m) . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 36 | F | SLE, APS | GCs, MMF, AZA | 15 | 18 | CR |
| 2 | 44 | F | SLE | GCs, FK506 | 13 | 17 | CR |
| 3 | 48 | F | SLE | GCs, MMF | 7 | 17 | CR |
| 4 | 47 | F | pSS | GCs, FK506 | 18 | 4 | NR |
| 5 | 35 | F | SLE | GCs, MMF | 2 | 12 | CR |
| 6 | 52 | F | SLE | GCs, CsA | 3 | 12 | CR |
| 7 | 31 | F | SLE | GCs, FK506 | 7 | 12 | CR |
| 8 | 43 | F | pSS | GCs, FK506, CsA, IVIG | 8 | 6 | NR |
| 9 | 39 | F | SLE | GCs, CTX | 8 | 6 | NR |
| 10 | 30 | F | SLE | GCs, MMF, CsA, TII | 15 | 5 | PR |
| 11 | 29 | F | SLE | GCs, FK506 | 7 | 9 | NR |
| 12 | 27 | F | CTD | GCs, CsA | 5 | 9 | CR |
| 13 | 54 | F | CTD | GCs, CsA | 5 | 8 | PR |
| 14 | 55 | F | pSS | GCs, AZA | 14 | 6 | NR |
| 15 | 18 | M | SLE | GCs, MMF, FK506, CsA | 3 | 6 | NR |
| 16 | 39 | F | CTD | GCs, CTX, FK506, CsA | 6 | 6 | NR |
| 17 | 20 | F | SLE | GCs, CTX, FK506, CsA, MMF, IVIg | 14 | 6 | NR |
| 18 | 18 | F | SLE | GCs, AZA, FK506 | 1 | 6 | CR |
| 19 | 31 | F | SLE | GCs, CsA, CTX | 6 | 6 | CR |
| 20 | 38 | F | SLE | GCs, HCQ | 4 | 6 | CR |
AZA: azathioprine; CH: Chinese herbs; CR: complete response; CsA: ciclosporin A; CTD-TP: connective tissue disease-related thrombocytopenia; CTX: cyclophosphamide; FK506: tacrolimus; GCs: glucocorticoids; IVIG: intravenous immunoglobulin G; NA: not available; NR: no response; PR: partial response; pSS: primary Sjögren's syndrome; TII: tripterygium glycosides.
Response
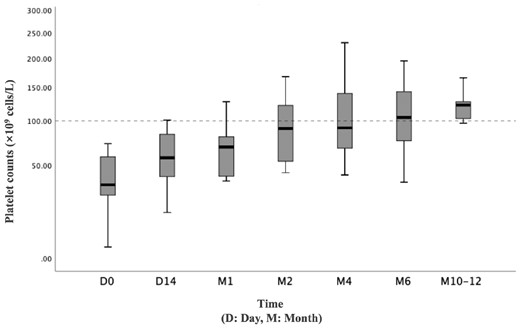
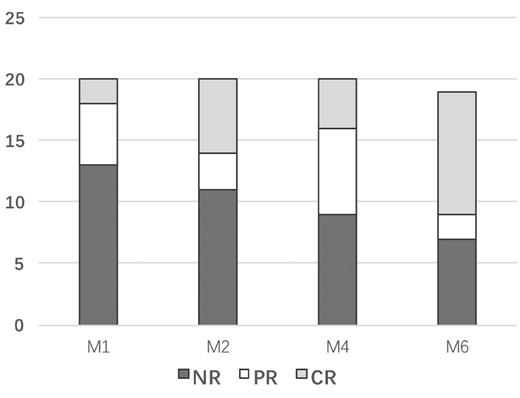
Nineteen patients completed 6 months of oral sirolimus; one patient withdrew in the fourth month because of poor effect. Eight patients continued sirolimus therapy after 6 months, and the longest follow-up period was 14 months. After administration of sirolimus, 12 (60%) patients (including 10 SLE patients) achieved TP response (CR+PR) at the 6-month follow-up visit, and the median platelet count was 82 × 109 cells/l (range, 11 × 109–195 × 109 cells/l) (Fig. 1A). At the 1-month visit, two (10%) patients achieved CR, and the CR rate increased to 30% (6 patients) at the 3-month visit. At the 6-month visit, nine (45%) patients achieved durable CR, and one achieved CR at 10 months (Fig. 1B). The median CR time was 2 months (range, 1–10 months). Among the 14 SLE patients, the overall response rate was 71.4%, with a CR rate of 64.3%. However, all three cases of pSS showed no response to sirolimus. Comparison of baseline parameters between the 12 responders and eight non-responders demonstrated no significant difference in clinical characteristics (Table 2). Comparison of the 4- and 6-month plasma concentration of rapamycin between responders and non-responders was not significantly different. Moreover, lymphocyte subpopulations were not significantly different between responders and non-responders (Fig. 2).
Comparison of variables before initiation of sirolimus treatment in responders and non-responders
| Characteristics . | Overall response (n = 12) . | No response (n = 8) . | P . |
|---|---|---|---|
| Age (years) | 37 (10.7) | 36 ( 12.9) | 0.91 |
| Duration of CTD (years) | 6.9 ( 4.8) | 10 ( 5.0) | 0.22 |
| SLE | 10 | 4 | NA |
| pSS | 0 | 3 | NA |
| Undifferentiated CTD | 2 | 1 | NA |
| Baseline platelet count (×109 cells/l) | 36.8 ( 21.7) | 39.1 ( 21.8) | 0.82 |
| aNA (n) | 12 | 8 | NA |
| anti-dsDNA (n) | 4 | 4 | 0.46 |
| anti-Sm (n) | 0 | 3 | 0.09 |
| anti-RNP (n) | 4 | 3 | 0.77 |
| anti-SSA (n) | 5 | 6 | 0.31 |
| anti-SSB (n) | 0 | 3 | 0.09 |
| anti-rRNP (n) | 3 | 2 | 0.59 |
| Coombs positive (n) | 4 | 0 | 0.21 |
| aCL (n) | 1 | 2 | 0.70 |
| anti-B2GP1 (n) | 2 | 2 | 0.91 |
| Characteristics . | Overall response (n = 12) . | No response (n = 8) . | P . |
|---|---|---|---|
| Age (years) | 37 (10.7) | 36 ( 12.9) | 0.91 |
| Duration of CTD (years) | 6.9 ( 4.8) | 10 ( 5.0) | 0.22 |
| SLE | 10 | 4 | NA |
| pSS | 0 | 3 | NA |
| Undifferentiated CTD | 2 | 1 | NA |
| Baseline platelet count (×109 cells/l) | 36.8 ( 21.7) | 39.1 ( 21.8) | 0.82 |
| aNA (n) | 12 | 8 | NA |
| anti-dsDNA (n) | 4 | 4 | 0.46 |
| anti-Sm (n) | 0 | 3 | 0.09 |
| anti-RNP (n) | 4 | 3 | 0.77 |
| anti-SSA (n) | 5 | 6 | 0.31 |
| anti-SSB (n) | 0 | 3 | 0.09 |
| anti-rRNP (n) | 3 | 2 | 0.59 |
| Coombs positive (n) | 4 | 0 | 0.21 |
| aCL (n) | 1 | 2 | 0.70 |
| anti-B2GP1 (n) | 2 | 2 | 0.91 |
ACL: anticardiolipin antibody; B2GP1: β2-glycoprotein 1; CTD: connective tissue disease; dsDNA: double-stranded DNA; NA: not available; pSS: primary Sjögren's syndrome; RNP: ribonucleoprotein; rRNP: recombinant RNP; SSA/SSB: Sjögren's syndrome-related antigen A/B.
Comparison of variables before initiation of sirolimus treatment in responders and non-responders
| Characteristics . | Overall response (n = 12) . | No response (n = 8) . | P . |
|---|---|---|---|
| Age (years) | 37 (10.7) | 36 ( 12.9) | 0.91 |
| Duration of CTD (years) | 6.9 ( 4.8) | 10 ( 5.0) | 0.22 |
| SLE | 10 | 4 | NA |
| pSS | 0 | 3 | NA |
| Undifferentiated CTD | 2 | 1 | NA |
| Baseline platelet count (×109 cells/l) | 36.8 ( 21.7) | 39.1 ( 21.8) | 0.82 |
| aNA (n) | 12 | 8 | NA |
| anti-dsDNA (n) | 4 | 4 | 0.46 |
| anti-Sm (n) | 0 | 3 | 0.09 |
| anti-RNP (n) | 4 | 3 | 0.77 |
| anti-SSA (n) | 5 | 6 | 0.31 |
| anti-SSB (n) | 0 | 3 | 0.09 |
| anti-rRNP (n) | 3 | 2 | 0.59 |
| Coombs positive (n) | 4 | 0 | 0.21 |
| aCL (n) | 1 | 2 | 0.70 |
| anti-B2GP1 (n) | 2 | 2 | 0.91 |
| Characteristics . | Overall response (n = 12) . | No response (n = 8) . | P . |
|---|---|---|---|
| Age (years) | 37 (10.7) | 36 ( 12.9) | 0.91 |
| Duration of CTD (years) | 6.9 ( 4.8) | 10 ( 5.0) | 0.22 |
| SLE | 10 | 4 | NA |
| pSS | 0 | 3 | NA |
| Undifferentiated CTD | 2 | 1 | NA |
| Baseline platelet count (×109 cells/l) | 36.8 ( 21.7) | 39.1 ( 21.8) | 0.82 |
| aNA (n) | 12 | 8 | NA |
| anti-dsDNA (n) | 4 | 4 | 0.46 |
| anti-Sm (n) | 0 | 3 | 0.09 |
| anti-RNP (n) | 4 | 3 | 0.77 |
| anti-SSA (n) | 5 | 6 | 0.31 |
| anti-SSB (n) | 0 | 3 | 0.09 |
| anti-rRNP (n) | 3 | 2 | 0.59 |
| Coombs positive (n) | 4 | 0 | 0.21 |
| aCL (n) | 1 | 2 | 0.70 |
| anti-B2GP1 (n) | 2 | 2 | 0.91 |
ACL: anticardiolipin antibody; B2GP1: β2-glycoprotein 1; CTD: connective tissue disease; dsDNA: double-stranded DNA; NA: not available; pSS: primary Sjögren's syndrome; RNP: ribonucleoprotein; rRNP: recombinant RNP; SSA/SSB: Sjögren's syndrome-related antigen A/B.
Safety
With regard to the safety of sirolimus treatment, no patient experienced Grade 2 or higher adverse events. Leukopoenia (15%), prolonged menstrual cycle (15%), elevated serum triglycerides (10%) and elevated cholesterol (10%) were the most common adverse events. Hypertriglyceridaemia occurred in patient 10 and patient 16, and the serum triglycerides elevated from 0.96–1.91 mmol/l, and from 1.21–2.91 mmol/l, respectively. The serum Low Density Lipoprotein (LDL) of patient 13 and patient 20 increased from 2.49–4.48 mmol/l and from 4.1–5.34 mmol/l, respectively. The summary of adverse events following initiation of sirolimus are listed in Table 3. No thrombotic events were observed during follow-up, and no bleeding events were documented.
| Adverse effects . | Cases . | Occurrence rate . |
|---|---|---|
| Leukopoenia | 3 | 15% |
| Prolonged menstrual cycle | 3 | 15% |
| Elevated serum triglyceride | 2 | 10% |
| Elevated serum cholesterol | 2 | 10% |
| Cheilitis | 1 | 5% |
| Slow recovery from muscle strain | 1 | 5% |
| Rash | 1 | 5% |
| Adverse effects . | Cases . | Occurrence rate . |
|---|---|---|
| Leukopoenia | 3 | 15% |
| Prolonged menstrual cycle | 3 | 15% |
| Elevated serum triglyceride | 2 | 10% |
| Elevated serum cholesterol | 2 | 10% |
| Cheilitis | 1 | 5% |
| Slow recovery from muscle strain | 1 | 5% |
| Rash | 1 | 5% |
CTD-TP: connective tissue disease-related thrombocytopenia.
| Adverse effects . | Cases . | Occurrence rate . |
|---|---|---|
| Leukopoenia | 3 | 15% |
| Prolonged menstrual cycle | 3 | 15% |
| Elevated serum triglyceride | 2 | 10% |
| Elevated serum cholesterol | 2 | 10% |
| Cheilitis | 1 | 5% |
| Slow recovery from muscle strain | 1 | 5% |
| Rash | 1 | 5% |
| Adverse effects . | Cases . | Occurrence rate . |
|---|---|---|
| Leukopoenia | 3 | 15% |
| Prolonged menstrual cycle | 3 | 15% |
| Elevated serum triglyceride | 2 | 10% |
| Elevated serum cholesterol | 2 | 10% |
| Cheilitis | 1 | 5% |
| Slow recovery from muscle strain | 1 | 5% |
| Rash | 1 | 5% |
CTD-TP: connective tissue disease-related thrombocytopenia.
Discussion
Herein are reported the results of the first phase II trial of oral sirolimus for the treatment of refractory CTD-TP patients. This is also the first CTD-TP clinical trial of an agent directed against mTOR. According to the results, sirolimus induced a durable symptomatic response in 60% (12/20 patients) of CTD patients and gained a CR rate of 50% (10/20 patients) for at least 24 weeks. Of the 14 SLE patients, the overall response rate was 71.4%, with a CR of 64.3%. Unfortunately, none of the three pSS cases responded to sirolimus treatment.
Because the mechanism of CTD-TP is still unclear, no therapeutic regimen guideline has yet been approved worldwide [11]. Considering B cells are involved in the pathogenesis of SLE and pSS, their depletion by targeting CD20 using rituximab has emerged as a promising treatment option for CTD-TP [11–13]. T-cell defection is also mentioned in the pathogenesis of SLE, while previous studies indicate the mTOR pathway is activated before disease onset in lupus-prone mice [14] and before flares in patients with SLE [3]. One study reported use of an mTOR inhibitor to treat SLE with a positive result [15]. Current knowledge on the performance of sirolimus in rheumatology is mainly focused on musculoskeletal manifestation, skin involvement and mild lupus nephritis [5, 6, 16]. A trial using sirolimus for relapsed/refractory autoimmune cytopenia children indicated that six out of seven children with thrombocytopenia achieved a CR within 3 months of starting sirolimus [4]. However, observational data are still missing for refractory thrombocytopenia. To our knowledge, the present study is the largest prospective trial of sirolimus for CTD-TP to date. The current results not only confirm its effectiveness, but also suggest involvement of mTOR in the pathogenesis of CTD-TP.
Because rituximab has a 40% CR rate but with a high risk of infection in refractory SLE-TP [17], the 64.3% CR rate observed with sirolimus in the present study is a meaningful clinical benefit. After first-line GCs and ISs treatment, refractory thrombocytopenia patients are very difficult to treat in clinic practice as most other organs’ damages shift from active to stable. However, employment of another GCs or ISs therapy might increase side effects, leading to decreased treatment compliance. The present results show the effective rate of sirolimus treatment in refractory patients is >50%, suggesting that dramatic changes can be obtained with refractory disease without additional or exacerbation of potential side effects. The current trial confirmed the benefit of sirolimus with a 64.3% CR rate in refractory SLE-TP patients. Importantly, sirolimus is a less expensive, oral alternative that is potentially more accessible than rituximab or other ISs in certain areas of the world.
The mechanism of sirolimus action is directed towards mTOR, which is functional in several types of immune cells, including T cells, B cells and macrophages. The mTOR pathway is known to drive proinflammatory expansion of T helper type 1 and inhibit the development of CD4+CD25+FoxP3+ T regulatory cells [2, 18–20]. A recent study indicated the mTor pathway was also associated with B-cell immunity in SLE [21]. Activation of mTOR in B cells is associated with several rheumatic diseases. Herein, preliminarily analysis of immune cells before and after sirolimus treatment was conducted, but no significant changes in T or B cells were observed. Furthermore, though comparison of clinical characteristics or autoantibodies has no clue, none of the three pSS patients responded to sirolimus therapy. There is little literature about mTOR in pSS. Only one study [22] reported that mTOR reduced lymphocyte infiltration in lacrimal gland of mice. The clinical research about sirolimus for pSS is lacking. We found that refractory pSS-TP patients in our study were characterized by a long course of disease and splenomegaly, so we speculated whether there was a non-immune mechanism involved.
Another key finding of the present study is that the response time varied. Some patients can achieve a better curative effect by increasing the dose under a certain blood concentration, but the risk of side effects, such as infection, should be averted. In one patient, no response (platelet elevation did not reach PR) was evaluated during treatment, but the platelet count decreased to 10% after sirolimus withdrawal. Other patients did not suffer from recurrence of the disease with gradual reduction of sirolimus concentration after reaching CR or PR. In consideration of the adjustment of blood concentration, the curative effect is generally highest after 3–6 months of treatment. The lack of significant difference in blood concentration between CR and no response patients at 4 months and 6 months also suggests the efficacy was not completely concentration dependent. However, more clinical data are needed to find other biomarkers.
The sirolimus usage was also well-tolerated. Several anticipated side effects, such as leukopoenia, prolonged menstrual cycle, and elevated serum lipids, were observed at low grades. Our patients with hyperlipidaemia both had high LDL levels at basement, and received a slight elevation in the trial. Considering the damage of high LDL levels to cardiovascular diseases, although no more lipid-lowering drugs were added, we are still continuously observing the trend of blood lipid levels. Importantly, no severe infection, thrombotic events, or bleeding events were reported. Due to the small sample size, more attention to side effects is needed in future clinical practice and study of sirolimus treatment.
The present study has several limitations. Firstly, this was a single-centre trial with a small number of patients from a single ethnic group. Secondly, the trial focused on refractory CTD-TP, excluding newly diagnosed patients. Therefore, the results might underestimate the efficacy of sirolimus. Thirdly, no control arm was included. Lastly, the observation period was relatively short, and there is still no data on drug withdrawal programs. Whether bias from the investigators and/or subjects may exist with the non-blind peculiarity and long-term sequelae of exposure are unknown. Due to the heterogeneity of effects, more challenges were raised for further studies. Future studies will include an extended follow-up time to determine the long-term benefits and risks of this regimen.
In conclusion, the results of the present clinical trial indicate that sirolimus is an inexpensive oral therapy that is effective and safe for refractory CTD-TP.
Funding: This article is funded by Chinese National Key Technology R&D Program of Ministry of Science and Technology (2017YFC0907604), National Science and Technology Major Project of the Ministry of Science and Technology of China (2019ZX09734001-002–004), Medical and health science and technology innovation project of Chinese Academy of Medical Sciences (2019-I2M-2–008) and National Natural Science Foundation of China (81601430, 81471615).
Disclosure statement: The authors have declared no conflicts of interest.




Comments