-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Pina Vegas, Emilie Sbidian, Laetitia Penso, Pascal Claudepierre, Epidemiologic study of patients with psoriatic arthritis in a real-world analysis: a cohort study of the French health insurance database, Rheumatology, Volume 60, Issue 3, March 2021, Pages 1243–1251, https://doi.org/10.1093/rheumatology/keaa448
Close - Share Icon Share
Abstract
PsA is a chronic inflammatory arthritis with heterogeneous disease manifestations. Data on the prevalence of PsA in adults differ widely depending on the study and the country. This study aimed to estimate the prevalence and incidence of PsA in France, characterize comorbidities associated to PsA and identify prescribed treatments.
This nationwide cohort study involved the administrative healthcare database (Système National des Données de Santé) of the French health insurance scheme linked to the national hospital discharge database. All adults with PsA registered in the database and identified with a specific International Classification of Diseases, 10th revision code (M07) were included between 1 January 2015 and 31 December 2018.
A total of 63 598 patients were identified as having PsA [55.9 years (s.d. 14.4), 45.6% males]. The prevalence of PsA was estimated at 0.1% and the incidence at 8.4 per 100 000 person-years in the general population. The most common comorbidities were hypertension, diabetes, chronic obstructive pulmonary disease and dyslipidaemia. The prevalence of treatment with conventional synthetic DMARDs (csDMARDs), biological or biosimilar DMARDs (b/bsDMARDs) and apremilast for PsA was 25.9% (16 453), 30.4% (19 325) and 3.5% (2231), respectively. Overall, 8966 (14.1%) patients were new users of csDMARDs, 8311 (13.1%) were new users of b/bsDMARDs and 1529 (7.4%) were new users of apremilast. The most common first-line csDMARD was methotrexate (70.9%) and the most frequent first-line b/bsDMARD was adalimumab (30.8%).
Our results lead to a better understanding of PsA. Results were similar to those from other published studies using other data sources, which highlights the reliability of insurance databases for studies.
The prevalence of PsA was estimated at 0.1% and the incidence at 8.4 per 100 000 person-years.
The most common comorbidities were hypertension, diabetes, chronic obstructive pulmonary disease and dyslipidaemia.
Results were consistent with previous studies, which underscores the robustness of this database.
Introduction
PsA is a heterogeneous chronic inflammatory arthritis with a combination of articular and peri-articular but also extra-articular manifestations. This arthritis can lead to irreversible joint damage and progressive disability [1], thereby representing a real public health issue. Data on the prevalence of PsA in adults differ widely depending on the study and the country [2], from 0.01 to 0.19% of the general population [3] and from 6 to 41% of patients with psoriasis [4]. Similarly, the annual incidence varies from 0.1 to 23.1 cases per 100 000 inhabitants in the general population [5).
Moreover, PsA is not only associated with other diseases of the spectrum of SpA (psoriasis, AS, uveitis and IBD), but also appears to be linked to an increased prevalence of numerous comorbidities, such as cardiovascular disease, obesity and metabolic syndrome, diabetes and kidney disease [6–9].
Many therapeutic options are available. Several molecules, such as TNF inhibitors (etanercept, infliximab, adalimumab, certolizumab pegol, golimumab), an IL-12/23 inhibitor (ustekizumab), an IL-17 inhibitor (secukinumab) or targeted synthetic DMARDs (apremilast, tofacitinib) are recommended second-line therapies for moderate–severe disease when standard treatments [including conventional synthetic DMARDs (csDMARDs)] fail to control disease or are not tolerated [10]. Thus the clinician can propose the most appropriate therapeutic approach for the patient. Indeed, the choice of therapy for PsA, but also the response to treatment and adverse events, may depend on both arthritis activity and severity and patient characteristics, including comorbidities [11].
Thus, studying the PsA population is of major interest because it leads to a better understanding of the disease and allows for greater consideration of its burden for healthcare policies. The current study aimed to estimate the prevalence and incidence of PsA in France, characterize comorbidities associated with PsA and identify first-line treatments.
Methods
Data source and study design
This French nationwide cohort study was based on analyses of the French national health insurance database [Système National des Données de Santé (SNDS)] and the French national hospital discharge database [Programme de Médicalisation des Systèmes d’Information (PMSI)]. This is a nationwide database that contains individualized health data and covers 98.8% of the French population (>67 million persons) [12]. In France, all citizens have free, equal, universal access to healthcare for chronic diseases. The French social security number (a unique identifier assigned to each citizen at birth or upon immigration) enables the data in the SNDS database to be linked anonymously to those in the PMSI [13]. The SNDS contains exhaustive data on all reimbursements for health-related expenditures, outpatient medical care and nursing care prescribed or performed by healthcare professionals, together with demographic data such as age, sex, area of residence (postcode) and vital status. The data on all pharmacy-dispensed medications include the date of prescription fulfilment, the type of formulation prescribed and the quantity prescribed. Active compounds are coded in the SNDS according to the international Anatomical Therapeutic Chemical classification system. Although the SNDS does not specify the medical indication for all outpatient reimbursements, the health status of patients being treated for fully reimbursed care related to severe, costly, chronic diseases, called ‘Affection de Longue Durée’, is recorded and coded according to the International Classification of Diseases, 10th revision (ICD-10). The PMSI database provides detailed medical information on all admissions to French public- and private-sector hospitals, including the dates of hospital admission and discharge, the ICD-10 code on discharge, the medical procedures performed in hospital and costly drugs [such as biologic DMARDS (bDMARDs)] administered in hospital.
This recently available large database has been used to conduct several large epidemiological studies [14, 15].
Specific approval was obtained from the French data protection agency (Comission nationale de l’informatique et des libertés: reference SLN/CBO/AR197671) to conduct this study.
Study population
All adults (≥18 years of age) with PsA registered in the SNDS between 1 January 2015 and 31 December 2018 were eligible for inclusion. Adults with PsA included all inpatients admitted with the ICD-10 diagnostic code for PsA (M07 except M07.4 and M07.5, which correspond to arthropathy in Crohn’s disease and ulcerative colitis, respectively) and all patients with fully reimbursed care procedures related to PsA. The hospital stay with an ICD-10 code for PsA could have taken place at any time between 1 January 2010 and 31 December 2018. The date of inclusion in the study cohort (index date) was defined as either the date of the fully reimbursed care related to PsA acceptance or the date of the first hospital stay. If the hospital stay was recorded between 1 January 2010 and 31 December 2014, then the index date was 1 January 2015. Participants were followed up until 31 December 2018 or the censorship dates (death or loss to follow-up, defined by the absence of prescription fulfilments for 12 consecutive months).
Treatments
The treatments studied included csDMARDs (methotrexate, leflunomide, sulfasalazine), biological originator and biosimilar DMARDs (b/bsDMARD; etanercept, infliximab, adalimumab, certolizumab pegol, golimumab, ustekizumab, secukinumab), a targeted synthetic DMARD (apremilast), NSAIDs and glucocorticoids. Ixekizumab, a bDMARD that received marketing authorization for PsA in April 2018, and tofacitinib, a targeted synthetic DMARDs marketed in December 2018, were not studied. Drugs were identified by Anatomical Therapeutic Chemical codes in outpatient databases and by Unité Commune de Dispensation codes in hospital discharge databases.
New users of csDMARDs were defined as patients who had filled a first prescription for one of these drugs after 1 year without any previous exposure to a csDMARD, b/bsDMARD or apremilast. New users of b/bsDMARDs and new users of apremilast were defined as those who had filled a first prescription for one of these drugs after 1 year without any previous exposure to a b/bsDMARD or apremilast. A patient could belong to both the csDMARD cohort and the b/bsDMARD or apremilast cohort. Patients without any DMARD were defined as those with no reimbursement data for cs/b/bsDMARD or apremilast in the year prior to the cohort entry date. However, they could use NSAIDs or prednisone during the study period.
Covariates
For each patient, we collected data on basic demographics, including age and sex, inflammatory diseases associated with PsA (psoriasis, IBD and uveitis) and comorbidities (diabetes, hypertension, dyslipidaemia, chronic ischaemic heart disease, major adverse cardiovascular events, atherosclerosis of arteries of extremities, chronic renal failure and chronic end-stage kidney failure, chronic liver disease (including cirrhosis and viral hepatitis), chronic obstructive pulmonary disease (COPD) and other hospital discharge diagnoses related to tobacco. These diseases were identified by using the disease definition algorithms developed by the French National Health Insurance Fund for Employees (Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés) when available or by the presence of ICD codes and the repeated reimbursement of specific treatments (supplementary Table S1, available at Rheumatology online).
Statistical analyses
Quantitative variables are reported as mean (s.d.) and categorical variables as frequency (percentage). The prevalence and annual incidence of adults with PsA were estimated: prevalence is reported with frequencies and annual incidence is expressed per 100 000 person-years for all inhabitants in France. The total number of patients with prescribed DMARDs and the treatments prescribed as first-line therapy are reported. Comorbidities and PsA-associated inflammatory diseases are described in total and for the three groups of new DMARD users, as well as patients without DMARDs. These characteristics were then compared between new b/bsDMARD and new apremilast users, new b/bsDMARD and new csDMARD users, and new csDMARD and non-DMARD users by t test or Mann–Whitney test for quantitative data and χ2 test for categorical data. Because of multiple comparisons, Bonferroni correction was applied and P < 0.001 was considered significant. All tests were bilateral. All analyses were performed with SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC, USA).
Results
Prevalence and incidence of PsA
A total of 63 598 patients (Fig. 1 and Table 1) had PsA [mean age 55.9 years (s.d. 14.4), 45.6% males]. Patients were followed up for a mean of 3.2 years (s.d. 1.2). The prevalence of PsA in our database was estimated at 0.1% and the incidence at 8.4 per 100 000 person-years.
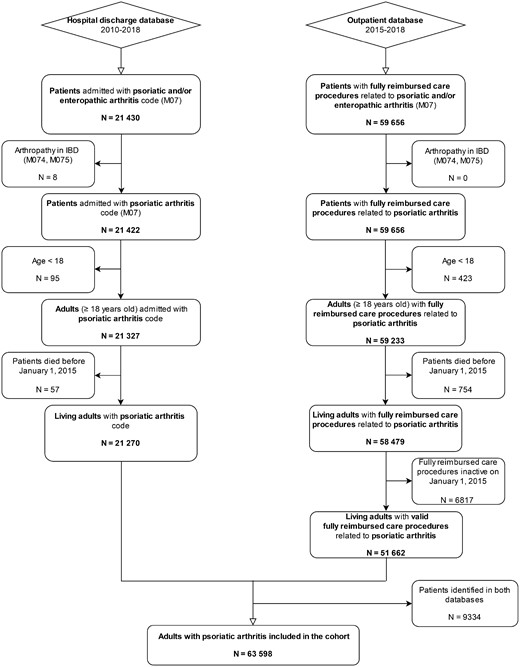
Flow chart of the selection of patients for inclusion in the study
Characteristics of patients identified as having PsA in the database (n = 63 598)
| Age at the index date, mean (s.d.), years | 55.9 (14.4) |
| Males, n (%) | 29 010 (45.6) |
| Associated inflammatory diseases, n (%) | |
| Psoriasis treated with topical therapies | 26 572 (41.8) |
| IBD | 2754 (4.3) |
| Uveitis | 81 (0.1) |
| Comorbidities, n (%) | |
| Essential hypertension | 13 809 (21.7) |
| Diabetes | 7707 (12.1) |
| COPD | 5771 (9.1) |
| Other hospital discharge diagnosis related to tobacco | 1971 (3.1) |
| Dyslipidaemia without associated pathology | 5658 (8.9) |
| Chronic ischaemic heart disease | 3503 (5.5) |
| Sequelae of stroke | 788 (1.2) |
| Major adverse cardiovascular events, n (%) | |
| Acute myocardial infarction | 142 (0.2) |
| Acute stroke | 160 (0.2) |
| Atherosclerosis of arteries of extremities, n (%) | 1146 (1.8) |
| End-stage kidney failure, n (%) | 82 (0.1) |
| Transplanted, n (%) | 37 (0.1) |
| Dialyzed, n (%) | 48 (0.1) |
| Liver disease, n (%) | 1557 (2.4) |
| Age at the index date, mean (s.d.), years | 55.9 (14.4) |
| Males, n (%) | 29 010 (45.6) |
| Associated inflammatory diseases, n (%) | |
| Psoriasis treated with topical therapies | 26 572 (41.8) |
| IBD | 2754 (4.3) |
| Uveitis | 81 (0.1) |
| Comorbidities, n (%) | |
| Essential hypertension | 13 809 (21.7) |
| Diabetes | 7707 (12.1) |
| COPD | 5771 (9.1) |
| Other hospital discharge diagnosis related to tobacco | 1971 (3.1) |
| Dyslipidaemia without associated pathology | 5658 (8.9) |
| Chronic ischaemic heart disease | 3503 (5.5) |
| Sequelae of stroke | 788 (1.2) |
| Major adverse cardiovascular events, n (%) | |
| Acute myocardial infarction | 142 (0.2) |
| Acute stroke | 160 (0.2) |
| Atherosclerosis of arteries of extremities, n (%) | 1146 (1.8) |
| End-stage kidney failure, n (%) | 82 (0.1) |
| Transplanted, n (%) | 37 (0.1) |
| Dialyzed, n (%) | 48 (0.1) |
| Liver disease, n (%) | 1557 (2.4) |
Characteristics of patients identified as having PsA in the database (n = 63 598)
| Age at the index date, mean (s.d.), years | 55.9 (14.4) |
| Males, n (%) | 29 010 (45.6) |
| Associated inflammatory diseases, n (%) | |
| Psoriasis treated with topical therapies | 26 572 (41.8) |
| IBD | 2754 (4.3) |
| Uveitis | 81 (0.1) |
| Comorbidities, n (%) | |
| Essential hypertension | 13 809 (21.7) |
| Diabetes | 7707 (12.1) |
| COPD | 5771 (9.1) |
| Other hospital discharge diagnosis related to tobacco | 1971 (3.1) |
| Dyslipidaemia without associated pathology | 5658 (8.9) |
| Chronic ischaemic heart disease | 3503 (5.5) |
| Sequelae of stroke | 788 (1.2) |
| Major adverse cardiovascular events, n (%) | |
| Acute myocardial infarction | 142 (0.2) |
| Acute stroke | 160 (0.2) |
| Atherosclerosis of arteries of extremities, n (%) | 1146 (1.8) |
| End-stage kidney failure, n (%) | 82 (0.1) |
| Transplanted, n (%) | 37 (0.1) |
| Dialyzed, n (%) | 48 (0.1) |
| Liver disease, n (%) | 1557 (2.4) |
| Age at the index date, mean (s.d.), years | 55.9 (14.4) |
| Males, n (%) | 29 010 (45.6) |
| Associated inflammatory diseases, n (%) | |
| Psoriasis treated with topical therapies | 26 572 (41.8) |
| IBD | 2754 (4.3) |
| Uveitis | 81 (0.1) |
| Comorbidities, n (%) | |
| Essential hypertension | 13 809 (21.7) |
| Diabetes | 7707 (12.1) |
| COPD | 5771 (9.1) |
| Other hospital discharge diagnosis related to tobacco | 1971 (3.1) |
| Dyslipidaemia without associated pathology | 5658 (8.9) |
| Chronic ischaemic heart disease | 3503 (5.5) |
| Sequelae of stroke | 788 (1.2) |
| Major adverse cardiovascular events, n (%) | |
| Acute myocardial infarction | 142 (0.2) |
| Acute stroke | 160 (0.2) |
| Atherosclerosis of arteries of extremities, n (%) | 1146 (1.8) |
| End-stage kidney failure, n (%) | 82 (0.1) |
| Transplanted, n (%) | 37 (0.1) |
| Dialyzed, n (%) | 48 (0.1) |
| Liver disease, n (%) | 1557 (2.4) |
Treatments
A total of 16 453 (25.9%) patients had a prescription for csDMARDs during the study period; 8966 (14.1%) were new csDMARDs users [mean age 54.2 years (s.d. 13.8), 42.2% males] (Table 2). The most common first-line csDMARD was methotrexate (70.9%), followed by leflunomide (15.4%) and sulfasalazine (13.5%).
| Characteristics . | New b/ bsDMARD users (N = 8311) . | New apremilast users (N = 1529) . | New csDMARD users (N = 8966) . | Non- DMARD users (N = 29 467) . | P-valuea, comparison of new b/bsDMARD and new apremilast users . | P-valuea, comparison of new b/bsDMARD and new csDMARD users . | P-valuea, comparison of new csDMARD users and non-DMARD users . |
|---|---|---|---|---|---|---|---|
| Age at the index date, mean (s.d.), years | 49.1 (12.9) | 55.1 (12.5) | 54.2 (13.8) | 59.0 (14.8) | <0.0001 | <0.0001 | <0.0001 |
| Males, n (%) | 3603 (43.4) | 710 (46.4) | 3781 (42.2) | 13 431 (45.6) | 0.0267 | 0.1167 | <0.0001 |
| Associated inflammatory diseases, n (%) | |||||||
| Psoriasis treated with topical therapies | 4001 (48.1) | 1011 (66.1) | 3791 (42.3) | 11 472 (38.9) | <0.0001 | <0.0001 | <0.0001 |
| IBD | 409 (4.9) | 9 (0.6) | 229 (2.5) | 2754 (4.7) | <0.0001 | <0.0001 | <0.0001 |
| Uveitis | 26 (0.3) | 1 (0.1) | 7 (0.1) | 81 (0.3) | 0.1097 | 0.0004 | 0.0006 |
| Comorbidities, n (%) | |||||||
| Essential hypertension | 1457 (17.5) | 366 (23.9) | 1919 (21.4) | 6704 (22.7) | <0.0001 | <0.0001 | 0.0077 |
| Diabetes | 802 (9.6) | 210 (13.7) | 1004 (11.2) | 4030 (13.7) | <0.0001 | 0.0009 | <0.0001 |
| COPD | 737 (7.5) | 183 (12.0) | 911 (10.2) | 2823 (9.6) | 0.0001 | 0.0038 | 0.1000 |
| Other hospital discharge diagnosis related to tobacco | 497 (6.0) | 70 (4.6) | 365 (4.1) | 819 (2.8) | 0.0306 | <0.0001 | <0.0001 |
| Dyslipidaemia without associated pathology | 514 (6.2) | 127 (8.3) | 766 (8.5) | 2838 (9.6) | 0.0020 | <0.0001 | 0.0021 |
| Acute myocardial infarction | 19 (0.2) | 3 (0.2) | 26 (0.3) | 71 (0.2) | 0.0608 | 0.4291 | 0.4901 |
| Chronic ischaemic heart disease | 292 (3.5) | 102 (6.7) | 462 (5.2) | 1945 (6.6) | <0.0001 | <0.0001 | <0.0001 |
| Acute stroke | 9 (0.1) | 2 (0.1) | 25 (0.3) | 78 (0.3) | 0.0586 | 0.0115 | 0.9125 |
| Sequelae of stroke | 66 (0.8) | 13 (0.8) | 124 (1.4) | 460 (1.6) | 0.8213 | 0.0002 | 0.2470 |
| Atherosclerosis of arteries of extremities | 85 (1.0) | 29 (1.9) | 146 (1.6) | 707 (2.4) | 0.0033 | 0.0005 | <0.0001 |
| End-stage kidney failure | 12 (0.1) | 0 (0) | 8 (0.1) | 50 (0.2) | 0.1371 | 0.2867 | 0.1180 |
| Transplanted | 6 (0.1) | 0 (0) | 4 (0.0) | 20 (0.1) | |||
| Dialyzed | 8 (0.1) | 0 (0) | 5 (0.1) | 32 (0.1) | |||
| Liver disease | 314 (3.8) | 70 (4.6) | 184 (2.1) | 703 (2.4) | 0.1376 | <0.0001 | 0.0655 |
| Characteristics . | New b/ bsDMARD users (N = 8311) . | New apremilast users (N = 1529) . | New csDMARD users (N = 8966) . | Non- DMARD users (N = 29 467) . | P-valuea, comparison of new b/bsDMARD and new apremilast users . | P-valuea, comparison of new b/bsDMARD and new csDMARD users . | P-valuea, comparison of new csDMARD users and non-DMARD users . |
|---|---|---|---|---|---|---|---|
| Age at the index date, mean (s.d.), years | 49.1 (12.9) | 55.1 (12.5) | 54.2 (13.8) | 59.0 (14.8) | <0.0001 | <0.0001 | <0.0001 |
| Males, n (%) | 3603 (43.4) | 710 (46.4) | 3781 (42.2) | 13 431 (45.6) | 0.0267 | 0.1167 | <0.0001 |
| Associated inflammatory diseases, n (%) | |||||||
| Psoriasis treated with topical therapies | 4001 (48.1) | 1011 (66.1) | 3791 (42.3) | 11 472 (38.9) | <0.0001 | <0.0001 | <0.0001 |
| IBD | 409 (4.9) | 9 (0.6) | 229 (2.5) | 2754 (4.7) | <0.0001 | <0.0001 | <0.0001 |
| Uveitis | 26 (0.3) | 1 (0.1) | 7 (0.1) | 81 (0.3) | 0.1097 | 0.0004 | 0.0006 |
| Comorbidities, n (%) | |||||||
| Essential hypertension | 1457 (17.5) | 366 (23.9) | 1919 (21.4) | 6704 (22.7) | <0.0001 | <0.0001 | 0.0077 |
| Diabetes | 802 (9.6) | 210 (13.7) | 1004 (11.2) | 4030 (13.7) | <0.0001 | 0.0009 | <0.0001 |
| COPD | 737 (7.5) | 183 (12.0) | 911 (10.2) | 2823 (9.6) | 0.0001 | 0.0038 | 0.1000 |
| Other hospital discharge diagnosis related to tobacco | 497 (6.0) | 70 (4.6) | 365 (4.1) | 819 (2.8) | 0.0306 | <0.0001 | <0.0001 |
| Dyslipidaemia without associated pathology | 514 (6.2) | 127 (8.3) | 766 (8.5) | 2838 (9.6) | 0.0020 | <0.0001 | 0.0021 |
| Acute myocardial infarction | 19 (0.2) | 3 (0.2) | 26 (0.3) | 71 (0.2) | 0.0608 | 0.4291 | 0.4901 |
| Chronic ischaemic heart disease | 292 (3.5) | 102 (6.7) | 462 (5.2) | 1945 (6.6) | <0.0001 | <0.0001 | <0.0001 |
| Acute stroke | 9 (0.1) | 2 (0.1) | 25 (0.3) | 78 (0.3) | 0.0586 | 0.0115 | 0.9125 |
| Sequelae of stroke | 66 (0.8) | 13 (0.8) | 124 (1.4) | 460 (1.6) | 0.8213 | 0.0002 | 0.2470 |
| Atherosclerosis of arteries of extremities | 85 (1.0) | 29 (1.9) | 146 (1.6) | 707 (2.4) | 0.0033 | 0.0005 | <0.0001 |
| End-stage kidney failure | 12 (0.1) | 0 (0) | 8 (0.1) | 50 (0.2) | 0.1371 | 0.2867 | 0.1180 |
| Transplanted | 6 (0.1) | 0 (0) | 4 (0.0) | 20 (0.1) | |||
| Dialyzed | 8 (0.1) | 0 (0) | 5 (0.1) | 32 (0.1) | |||
| Liver disease | 314 (3.8) | 70 (4.6) | 184 (2.1) | 703 (2.4) | 0.1376 | <0.0001 | 0.0655 |
P-value based on t test or Mann–Whitney test for quantitative data and χ2 or Fisher’s exact test for categorical data. P < 0.001 was considered significant.
| Characteristics . | New b/ bsDMARD users (N = 8311) . | New apremilast users (N = 1529) . | New csDMARD users (N = 8966) . | Non- DMARD users (N = 29 467) . | P-valuea, comparison of new b/bsDMARD and new apremilast users . | P-valuea, comparison of new b/bsDMARD and new csDMARD users . | P-valuea, comparison of new csDMARD users and non-DMARD users . |
|---|---|---|---|---|---|---|---|
| Age at the index date, mean (s.d.), years | 49.1 (12.9) | 55.1 (12.5) | 54.2 (13.8) | 59.0 (14.8) | <0.0001 | <0.0001 | <0.0001 |
| Males, n (%) | 3603 (43.4) | 710 (46.4) | 3781 (42.2) | 13 431 (45.6) | 0.0267 | 0.1167 | <0.0001 |
| Associated inflammatory diseases, n (%) | |||||||
| Psoriasis treated with topical therapies | 4001 (48.1) | 1011 (66.1) | 3791 (42.3) | 11 472 (38.9) | <0.0001 | <0.0001 | <0.0001 |
| IBD | 409 (4.9) | 9 (0.6) | 229 (2.5) | 2754 (4.7) | <0.0001 | <0.0001 | <0.0001 |
| Uveitis | 26 (0.3) | 1 (0.1) | 7 (0.1) | 81 (0.3) | 0.1097 | 0.0004 | 0.0006 |
| Comorbidities, n (%) | |||||||
| Essential hypertension | 1457 (17.5) | 366 (23.9) | 1919 (21.4) | 6704 (22.7) | <0.0001 | <0.0001 | 0.0077 |
| Diabetes | 802 (9.6) | 210 (13.7) | 1004 (11.2) | 4030 (13.7) | <0.0001 | 0.0009 | <0.0001 |
| COPD | 737 (7.5) | 183 (12.0) | 911 (10.2) | 2823 (9.6) | 0.0001 | 0.0038 | 0.1000 |
| Other hospital discharge diagnosis related to tobacco | 497 (6.0) | 70 (4.6) | 365 (4.1) | 819 (2.8) | 0.0306 | <0.0001 | <0.0001 |
| Dyslipidaemia without associated pathology | 514 (6.2) | 127 (8.3) | 766 (8.5) | 2838 (9.6) | 0.0020 | <0.0001 | 0.0021 |
| Acute myocardial infarction | 19 (0.2) | 3 (0.2) | 26 (0.3) | 71 (0.2) | 0.0608 | 0.4291 | 0.4901 |
| Chronic ischaemic heart disease | 292 (3.5) | 102 (6.7) | 462 (5.2) | 1945 (6.6) | <0.0001 | <0.0001 | <0.0001 |
| Acute stroke | 9 (0.1) | 2 (0.1) | 25 (0.3) | 78 (0.3) | 0.0586 | 0.0115 | 0.9125 |
| Sequelae of stroke | 66 (0.8) | 13 (0.8) | 124 (1.4) | 460 (1.6) | 0.8213 | 0.0002 | 0.2470 |
| Atherosclerosis of arteries of extremities | 85 (1.0) | 29 (1.9) | 146 (1.6) | 707 (2.4) | 0.0033 | 0.0005 | <0.0001 |
| End-stage kidney failure | 12 (0.1) | 0 (0) | 8 (0.1) | 50 (0.2) | 0.1371 | 0.2867 | 0.1180 |
| Transplanted | 6 (0.1) | 0 (0) | 4 (0.0) | 20 (0.1) | |||
| Dialyzed | 8 (0.1) | 0 (0) | 5 (0.1) | 32 (0.1) | |||
| Liver disease | 314 (3.8) | 70 (4.6) | 184 (2.1) | 703 (2.4) | 0.1376 | <0.0001 | 0.0655 |
| Characteristics . | New b/ bsDMARD users (N = 8311) . | New apremilast users (N = 1529) . | New csDMARD users (N = 8966) . | Non- DMARD users (N = 29 467) . | P-valuea, comparison of new b/bsDMARD and new apremilast users . | P-valuea, comparison of new b/bsDMARD and new csDMARD users . | P-valuea, comparison of new csDMARD users and non-DMARD users . |
|---|---|---|---|---|---|---|---|
| Age at the index date, mean (s.d.), years | 49.1 (12.9) | 55.1 (12.5) | 54.2 (13.8) | 59.0 (14.8) | <0.0001 | <0.0001 | <0.0001 |
| Males, n (%) | 3603 (43.4) | 710 (46.4) | 3781 (42.2) | 13 431 (45.6) | 0.0267 | 0.1167 | <0.0001 |
| Associated inflammatory diseases, n (%) | |||||||
| Psoriasis treated with topical therapies | 4001 (48.1) | 1011 (66.1) | 3791 (42.3) | 11 472 (38.9) | <0.0001 | <0.0001 | <0.0001 |
| IBD | 409 (4.9) | 9 (0.6) | 229 (2.5) | 2754 (4.7) | <0.0001 | <0.0001 | <0.0001 |
| Uveitis | 26 (0.3) | 1 (0.1) | 7 (0.1) | 81 (0.3) | 0.1097 | 0.0004 | 0.0006 |
| Comorbidities, n (%) | |||||||
| Essential hypertension | 1457 (17.5) | 366 (23.9) | 1919 (21.4) | 6704 (22.7) | <0.0001 | <0.0001 | 0.0077 |
| Diabetes | 802 (9.6) | 210 (13.7) | 1004 (11.2) | 4030 (13.7) | <0.0001 | 0.0009 | <0.0001 |
| COPD | 737 (7.5) | 183 (12.0) | 911 (10.2) | 2823 (9.6) | 0.0001 | 0.0038 | 0.1000 |
| Other hospital discharge diagnosis related to tobacco | 497 (6.0) | 70 (4.6) | 365 (4.1) | 819 (2.8) | 0.0306 | <0.0001 | <0.0001 |
| Dyslipidaemia without associated pathology | 514 (6.2) | 127 (8.3) | 766 (8.5) | 2838 (9.6) | 0.0020 | <0.0001 | 0.0021 |
| Acute myocardial infarction | 19 (0.2) | 3 (0.2) | 26 (0.3) | 71 (0.2) | 0.0608 | 0.4291 | 0.4901 |
| Chronic ischaemic heart disease | 292 (3.5) | 102 (6.7) | 462 (5.2) | 1945 (6.6) | <0.0001 | <0.0001 | <0.0001 |
| Acute stroke | 9 (0.1) | 2 (0.1) | 25 (0.3) | 78 (0.3) | 0.0586 | 0.0115 | 0.9125 |
| Sequelae of stroke | 66 (0.8) | 13 (0.8) | 124 (1.4) | 460 (1.6) | 0.8213 | 0.0002 | 0.2470 |
| Atherosclerosis of arteries of extremities | 85 (1.0) | 29 (1.9) | 146 (1.6) | 707 (2.4) | 0.0033 | 0.0005 | <0.0001 |
| End-stage kidney failure | 12 (0.1) | 0 (0) | 8 (0.1) | 50 (0.2) | 0.1371 | 0.2867 | 0.1180 |
| Transplanted | 6 (0.1) | 0 (0) | 4 (0.0) | 20 (0.1) | |||
| Dialyzed | 8 (0.1) | 0 (0) | 5 (0.1) | 32 (0.1) | |||
| Liver disease | 314 (3.8) | 70 (4.6) | 184 (2.1) | 703 (2.4) | 0.1376 | <0.0001 | 0.0655 |
P-value based on t test or Mann–Whitney test for quantitative data and χ2 or Fisher’s exact test for categorical data. P < 0.001 was considered significant.
A total of 19 325 (30.4%) patients had a prescription for b/bsDMARDs and 2231 (3.5%) for apremilast. Because of the recent marketing authorization of ustekinumab (October 2014), apremilast (December 2015) and secukinumab (June 2016), the distribution of prescribed treatments changed during the study period (Fig. 2). Overall, 8311 (13.1%) patients were new b/bsDMARDs users [mean age 49.1 years (s.d. 12.9), 43.4% males, monotherapy in 89.1% and combined with methotrexate in 6.5% at the time of the first prescription]. The most common first-line b/bsDMARD was adalimumab (36.4%), followed by etanercept (27.0%). Ustekinumab was prescribed for 11.5% of patients, secukinumab for 9.9%, golimumab for 9.0%, certolizumab for 5.7% and infliximab for 0.5%. There were 1529 (7.4%) new apremilast users [mean age 55.1 years (s.d. 12.5), 46.4% males, monotherapy in 86.5% and combined with methotrexate in 8.1% at the time of the first prescription] (Table 2). This treatment was prescribed as second-line therapy for 15.5% of patients. New b/bsDMARDs users were younger than new users of csDMARDs (P < 0.0001) or apremilast (P < 0.0001) (Table 2).
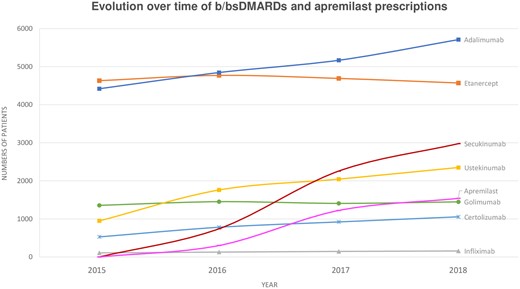
Evolution between 2015 and 2018 of prescriptions for b/bsDMARDs and apremilast
Among patients with PsA in the database, 44 972 (70.7%) had at least one NSAID reimbursement and 20 327 (32.0%) had at least one prednisone reimbursement during the study period. The most commonly prescribed NSAID class was arylpropionic acids, for 58.4% (37 157) of patients with PsA. Among new csDMARD, b/bsDMARD and apremilast users, 66.9% (5616 patients), 66.1% (5493 patients) and 56.2% (859 patients), respectively, had at least one NSAID reimbursement and 34.1% (3019 patients), 30.73% (2552 patients) and 21.6% (330 patients), respectively, at least one prednisone reimbursement during the study period. Among patients without any DMARD, 64.7% (19 057 patients) had at least one NSAID or prednisone reimbursement during the study period.
Associated inflammatory diseases and comorbidities with and without DMARDs
PsA was associated with IBDs and uveitis in 4.3% and 0.1% of cases, respectively (Table 1). In this population, 41.8% of patients had psoriasis treated with topical therapies. The most common comorbidity associated with PsA was essential hypertension, in 21.7% of patients. Other comorbidities frequently identified were diabetes (12.1%), COPD (9.1%), other hospital discharge diagnoses related to tobacco (3.1%) and dyslipidaemia without associated pathology (8.9%).
The most frequent comorbidities recorded in the three new-user groups were similar to those reported in the overall PsA patient group (Table 2). Among new csDMARD, b/bsDMARD and apremilast users, psoriasis treated with topical therapy was associated with PsA in 42.3%, 48.1% and 66.1% of cases, respectively, and IBDs in 2.5%, 4.9% and 0.6% of cases, respectively. Uveitis was recorded in 0.1%, 0.3% and 0.1%, respectively. Thus psoriasis treated with topical therapy and IBDs were more frequent among new b/bsDMARD than new csDMARD users (P < 0.0001) and uveitis was more frequent among new b/bsDMARD than csDMARD users (P < 0.0001). The difference between new b/bsDMARD and apremilast users was notable but did not reach significance (P = 0.11).
The previous results, in particular for new csDMARD users, were compared with the 29 467 patients without DMARDs (Table 2). PsA patients without DMARDs were older [mean age 59.0 years (s.d. 14.8)] than new csDMARD users (P < 0.0001), with a sex ratio of 0.8 (45.9% male). IBDs and uveitis were associated with PsA in 4.7% and 0.3% of cases, respectively. Thus the proportion of associated IBDs and uveitis was higher for non-DMARD than new csDMARD users (P < 0.0001 for both tests). The proportion of psoriasis treated with topical therapy was significantly lower for non-DMARD (38.9%) than new csDMARD users (P < 0.0001). The most frequent comorbidities associated with PsA for non-DMARD users were similar to those for the new csDMARD and apremilast users: essential hypertension (22.7%), diabetes (13.7%) and COPD (9.6%).
Discussion
In this French nationwide study based on the SNDS, the prevalence of PsA was estimated at 0.1% and the incidence at 8.4 per 100 000 person-years in the general population. Our prevalence and incidence data are similar to that from other studies. Indeed, in their systematic review, Alamanos et al. [5] found a prevalence ranging from 1 case per 100 000 population in a Japanese study to 420 cases per 100 000 population in an Italian study (median 180) and the incidence was estimated at 6.4 per 100 000 inhabitants (range 0.1–23.1 per 100 000 inhabitants). In the systematic review and meta-regression analysis by Stolwijk et al. [3], the prevalence estimates varied from 0.01% (95% CI 0.00, 0.17) in the Middle East to 0.19% (95% CI 0.16, 0.32) in Europe. Scotti et al. [2] found a random-effects pooled PsA prevalence of 133 per 100 000 individuals (95% CI 107, 164 per 100 000 individuals) and a random-effects pooled PsA incidence of 8.3 per 100 000 patient-years (95% CI 4.1, 16.7 per 100 000 person-years), close to our results.
These data differ widely by country, and few studies have focused specifically on the epidemiology of PsA in France. A nationwide sample survey conducted in 20 French counties [16] estimated the prevalence of PsA at 0.19% (95% CI 0.08, 0.35) in 2001. A second study of a sample of 6556 individuals from the French GAZEL cohort estimated the prevalence of PsA (n = 5) at 0.08% (95% CI 0.03, 0.18) in 2010 [17]. In our study, the prevalence was probably slightly underestimated because of the identification of patients by ICD-10 codes.
Grodner et al. [18] previously assessed the proportion of patients treated for psoriasis in France. In their study, the prevalence was estimated at 1.3–2.1% using sensitivity analyses and the incidence was estimated at 150–240 per 100 000 person-years. PsA was identified in 0.8% of patients (n = 5950) without systemic treatment, 7.4% of patients (n = 5403) with first csDMARDs and 33.6% of patients (n = 5560) with first b/bsDMARDs.
During the study period, the most frequently prescribed first-line csDMARD was methotrexate and the most frequently prescribed b/bsDMARD was adalimumab. With the recent market entry of new molecules such as apremilast, ustekinumab, secukinumab and ixekizumab and in view of the evolution of the prescriptions during our study period, this distribution may change. An NSAID or prednisone was prescribed in 70.7% and 32.0% of patients, respectively. According to the European League Against Rheumatism and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis recommendations [10, 19], when not contraindicated, NSAIDs may be used as first-line therapy to relieve musculoskeletal signs and symptoms. Oral glucocorticoid therapy should be used with caution and only when absolutely necessary, with the minimal effective dosage to limit the risk of adverse effects. In patients with peripheral arthritis, particularly those with severe disease at presentation, csDMARDs should be considered at an early stage. Finally, in patients with peripheral arthritis and an inadequate response to at least one csDMARD, therapy with a b/bsDMARD, usually a TNF inhibitor, is recommended. However, the position of apremilast and other more recently marketed b/bsDMARDs is less well defined [20], and other studies are needed.
Our results concerning comorbidities associated with PsA were similar to those from other studies using other data sources, especially well-recorded PsA cohorts [7, 8, 21, 22] (Table 3). Notably, many PsA patients, including non-DMARD users, had hypertension, diabetes and dyslipidaemia. In our study, the typical profile of PsA patients receiving biologic drugs was younger with fewer comorbidities as compared with those receiving csDMARDs or apremilast. Similarly, non-DMARD users were older and had more comorbidities than new csDMARD users.
| Comorbidities . | Husted et al. [7], University of Toronto Psoriatic Arthritis Clinic . | Jafri et al. [22], Health Improvement Network . | Shah et al. [8], Truven Health Analytics MarketScan Database . | Sinnathurai et al. [21], ARAD . | Pina Vegas et al., SNDS (this study) . |
|---|---|---|---|---|---|
| Study design | Prospective, observational cohort | Longitudinal database | Health insurance claims | National observational cohort | National administrative database |
| N | 611 | 12 548 | 47 438 | 490 | 63 598 |
| Period | 2006–10 | 1994–2014 | 2008–15 | 2003–15 | 2015–18 |
| Country | Canada | UK | USA | Australia | France |
| Comorbidities, n (%) | |||||
| Hypertension | 226 (37.1) | 2807 (22.4) | 23 811 (47.3) | 187 (38.2) | 24 785 (39.0) |
| Essential hypertension | — | — | — | — | 13 809 (21.7) |
| Diabetes | 73 (12.0) | 973 (7.8) | 10 036 (21.2) | 63 (12.9) | 7707 (12.1) |
| COPD | 68 (11.2) | — | — | — | 5771 (9.1) |
| Dyslipidaemia | 126 (20.7) | 1240 (9.9) | 23 811 (50.2) | 124 (25.3) | 12 153 (19.1) |
| Dyslipidaemia without associated pathology | — | — | — | — | 5658 (8.9) |
| Cardiovascular disease | 50 (8.2) | ||||
| Chronic ischaemic heart disease | — | — | 6005 (11.6) | 37 (7.6) | 3503 (5.5) |
| Acute myocardial infarction | — | — | 292 (0.6) | — | 142 (0.2) |
| Sequelae of stroke | — | — | — | 7 (1.4) | 1146 (1.8) |
| Acute stroke | — | — | 198 (0.4) | — | 160 (0.2) |
| Atherosclerosis of arteries of extremities | — | — | 1942 (4.1) | — | 1146 (1.8) |
| Liver disease | 17 (2.9) | — | — | 30 (6.1) | 1557 (2.4) |
| Comorbidities . | Husted et al. [7], University of Toronto Psoriatic Arthritis Clinic . | Jafri et al. [22], Health Improvement Network . | Shah et al. [8], Truven Health Analytics MarketScan Database . | Sinnathurai et al. [21], ARAD . | Pina Vegas et al., SNDS (this study) . |
|---|---|---|---|---|---|
| Study design | Prospective, observational cohort | Longitudinal database | Health insurance claims | National observational cohort | National administrative database |
| N | 611 | 12 548 | 47 438 | 490 | 63 598 |
| Period | 2006–10 | 1994–2014 | 2008–15 | 2003–15 | 2015–18 |
| Country | Canada | UK | USA | Australia | France |
| Comorbidities, n (%) | |||||
| Hypertension | 226 (37.1) | 2807 (22.4) | 23 811 (47.3) | 187 (38.2) | 24 785 (39.0) |
| Essential hypertension | — | — | — | — | 13 809 (21.7) |
| Diabetes | 73 (12.0) | 973 (7.8) | 10 036 (21.2) | 63 (12.9) | 7707 (12.1) |
| COPD | 68 (11.2) | — | — | — | 5771 (9.1) |
| Dyslipidaemia | 126 (20.7) | 1240 (9.9) | 23 811 (50.2) | 124 (25.3) | 12 153 (19.1) |
| Dyslipidaemia without associated pathology | — | — | — | — | 5658 (8.9) |
| Cardiovascular disease | 50 (8.2) | ||||
| Chronic ischaemic heart disease | — | — | 6005 (11.6) | 37 (7.6) | 3503 (5.5) |
| Acute myocardial infarction | — | — | 292 (0.6) | — | 142 (0.2) |
| Sequelae of stroke | — | — | — | 7 (1.4) | 1146 (1.8) |
| Acute stroke | — | — | 198 (0.4) | — | 160 (0.2) |
| Atherosclerosis of arteries of extremities | — | — | 1942 (4.1) | — | 1146 (1.8) |
| Liver disease | 17 (2.9) | — | — | 30 (6.1) | 1557 (2.4) |
| Comorbidities . | Husted et al. [7], University of Toronto Psoriatic Arthritis Clinic . | Jafri et al. [22], Health Improvement Network . | Shah et al. [8], Truven Health Analytics MarketScan Database . | Sinnathurai et al. [21], ARAD . | Pina Vegas et al., SNDS (this study) . |
|---|---|---|---|---|---|
| Study design | Prospective, observational cohort | Longitudinal database | Health insurance claims | National observational cohort | National administrative database |
| N | 611 | 12 548 | 47 438 | 490 | 63 598 |
| Period | 2006–10 | 1994–2014 | 2008–15 | 2003–15 | 2015–18 |
| Country | Canada | UK | USA | Australia | France |
| Comorbidities, n (%) | |||||
| Hypertension | 226 (37.1) | 2807 (22.4) | 23 811 (47.3) | 187 (38.2) | 24 785 (39.0) |
| Essential hypertension | — | — | — | — | 13 809 (21.7) |
| Diabetes | 73 (12.0) | 973 (7.8) | 10 036 (21.2) | 63 (12.9) | 7707 (12.1) |
| COPD | 68 (11.2) | — | — | — | 5771 (9.1) |
| Dyslipidaemia | 126 (20.7) | 1240 (9.9) | 23 811 (50.2) | 124 (25.3) | 12 153 (19.1) |
| Dyslipidaemia without associated pathology | — | — | — | — | 5658 (8.9) |
| Cardiovascular disease | 50 (8.2) | ||||
| Chronic ischaemic heart disease | — | — | 6005 (11.6) | 37 (7.6) | 3503 (5.5) |
| Acute myocardial infarction | — | — | 292 (0.6) | — | 142 (0.2) |
| Sequelae of stroke | — | — | — | 7 (1.4) | 1146 (1.8) |
| Acute stroke | — | — | 198 (0.4) | — | 160 (0.2) |
| Atherosclerosis of arteries of extremities | — | — | 1942 (4.1) | — | 1146 (1.8) |
| Liver disease | 17 (2.9) | — | — | 30 (6.1) | 1557 (2.4) |
| Comorbidities . | Husted et al. [7], University of Toronto Psoriatic Arthritis Clinic . | Jafri et al. [22], Health Improvement Network . | Shah et al. [8], Truven Health Analytics MarketScan Database . | Sinnathurai et al. [21], ARAD . | Pina Vegas et al., SNDS (this study) . |
|---|---|---|---|---|---|
| Study design | Prospective, observational cohort | Longitudinal database | Health insurance claims | National observational cohort | National administrative database |
| N | 611 | 12 548 | 47 438 | 490 | 63 598 |
| Period | 2006–10 | 1994–2014 | 2008–15 | 2003–15 | 2015–18 |
| Country | Canada | UK | USA | Australia | France |
| Comorbidities, n (%) | |||||
| Hypertension | 226 (37.1) | 2807 (22.4) | 23 811 (47.3) | 187 (38.2) | 24 785 (39.0) |
| Essential hypertension | — | — | — | — | 13 809 (21.7) |
| Diabetes | 73 (12.0) | 973 (7.8) | 10 036 (21.2) | 63 (12.9) | 7707 (12.1) |
| COPD | 68 (11.2) | — | — | — | 5771 (9.1) |
| Dyslipidaemia | 126 (20.7) | 1240 (9.9) | 23 811 (50.2) | 124 (25.3) | 12 153 (19.1) |
| Dyslipidaemia without associated pathology | — | — | — | — | 5658 (8.9) |
| Cardiovascular disease | 50 (8.2) | ||||
| Chronic ischaemic heart disease | — | — | 6005 (11.6) | 37 (7.6) | 3503 (5.5) |
| Acute myocardial infarction | — | — | 292 (0.6) | — | 142 (0.2) |
| Sequelae of stroke | — | — | — | 7 (1.4) | 1146 (1.8) |
| Acute stroke | — | — | 198 (0.4) | — | 160 (0.2) |
| Atherosclerosis of arteries of extremities | — | — | 1942 (4.1) | — | 1146 (1.8) |
| Liver disease | 17 (2.9) | — | — | 30 (6.1) | 1557 (2.4) |
The strengths of the present study include the large sample from a nationally representative database and the 4 year study period. Our definition of PsA was based on either ICD-10 codes for PsA applied to inpatients or patients with fully reimbursed care procedures related to PsA. Unfortunately, ICD-10 codes are not recorded for outpatients in the French database except for patients with fully reimbursed care procedures. Thus our study probably underestimated the number of cases of PsA. However, we are confident that our definition is specific. First, our population received both NSAIDs and cs/b/bsDMARDs that are reimbursed in the context of PsA, thus minimizing selection bias. Second, our study population had much the same characteristics as previously described populations of patients with moderate to severe PsA [7, 21, 22]. Thus we succeed in identifying a large number of PsA patients who need systemic treatments from NSAIDs to cs/b/bsDMARDs. Because of inclusion criteria, national cohorts involve selected populations, usually based on tertiary care centre patients, and thus can lead to results with limited generalizability. Moreover, large cohorts are needed to highlight rare but severe adverse events, and national cohorts often fail to show an association between adverse events and drugs because of a lack of power. Thus, performing a large, exhaustive study of a non-selected population by using insurance databases allows for a complementary estimation of epidemiologic data.
Conclusion
Our results provide us with a better understanding of PsA and its care in France, allowing for estimating the burden of this severe disease. Results were consistent with previous published studies using other data sources, especially well-recorded PsA cohorts, which underscores the robustness of the use of insurance databases.
Acknowledgements
L.P.V. received a Master 2 grant from the French Society of Rheumatology (Bourse Master 2ème Année 2019).
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: P.C. has received consulting fees from AbbVie, Pfizer, Roche-Chugai Bristol-Myers Squibb, MSD, UCB, Novartis, Janssen, Eli Lilly and Celgene (<$10 000 each) and has been an investigator for Roche-Chugai, Sanofi Aventis, Celgene, Pfizer, MSD, Novartis and Bristol-Myers Squibb. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- dyslipidemias
- hypertension
- diabetes mellitus
- chronic obstructive airway disease
- diabetes mellitus, type 2
- antirheumatic drugs, disease-modifying
- adult
- arthritis, psoriatic
- comorbidity
- epidemiologic studies
- health insurance
- methotrexate
- patient discharge
- prostate-specific antigen
- international classification of diseases
- adalimumab
- apremilast




Comments