-
PDF
- Split View
-
Views
-
Cite
Cite
Clementina López-Medina, Rafaela Ortega-Castro, M Carmen Castro-Villegas, Pilar Font-Ugalde, M Ángeles Puche-Larrubia, Ignacio Gómez-García, Iván Arias-de la Rosa, Nuria Barbarroja, Ruxandra Schiotis, Eduardo Collantes-Estévez, Axial and peripheral spondyloarthritis: does psoriasis influence the clinical expression and disease burden? Data from REGISPONSER registry, Rheumatology, Volume 60, Issue 3, March 2021, Pages 1125–1136, https://doi.org/10.1093/rheumatology/keaa398
Close - Share Icon Share
Abstract
To evaluate whether the presence of psoriasis influences the clinical expression, disease activity and disease burden in both axial and peripheral phenotypes of spondyloarthritis (SpA).
Patients from the Spanish REGISPONSER registry classified as having SpA according to the ESSG criteria were included. Patients were classified as psoriatic or non-psoriatic depending on the presence of cutaneous or nail psoriasis; thereafter, they were classified as having either axial [presence of radiographic sacroiliitis OR inflammatory back pain (IBP)] or peripheral phenotype (absence of radiographic sacroiliitis AND absence of IBP AND presence of peripheral involvement). Pair-wise univariate and multivariate analyses among the four groups (psoriatic/non-psoriatic axial phenotypes and psoriatic/non-psoriatic peripheral phenotypes) were performed with adjustment for treatment intake.
A total of 2296 patients were included in the analysis. Among patients with axial phenotype, psoriasis was independently associated (P < 0.05) with HLA-B27+ [odds ratio (OR) 0.27], uveitis (OR 0.46), synovitis (ever) (OR 2.59), dactylitis (OR 2.78) and the use of conventional synthetic DMARDs (csDMARDs) (OR 1.47) in comparison with non-psoriatic patients. Among patients with peripheral phenotype and adjusting for csDMARD intake, psoriasis was independently associated with higher age at disease onset (OR 1.05), HLA-B27+ (OR 0.14) and heel enthesitis (OR 0.22). Higher scores for patient-reported outcomes and greater use of treatment at the time of the study visit were observed in psoriatic patients with either axial or peripheral phenotype.
These findings suggest that, among all patients with SpA, psoriasis is associated with differences in clinical expression of SpA, a greater disease burden and increased use of drugs.
Psoriasis plays a key role in the clinical expression of spondyloarthritis.
There is a strong association between psoriasis and peripheral manifestations even in patients with axial phenotype.
Patients with psoriasis showed a more severe disease at the time of the study visit.
Introduction
The term spondyloarthritis (SpA) encompasses a group of inflammatory rheumatic disorders that mainly affect the axial skeleton and sacroiliac joints. Classically, SpA patients have been classified into several subtypes depending on the presence of peripheral and/or extra-musculoskeletal manifestations, such as ankylosing spondylitis (AS), PsA, IBD-associated SpA, reactive arthritis (ReA) and undifferentiated SpA (u-SpA) [1]. In 2011, the Assessment of Spondyloarthritis International Society (ASAS) developed a new set of criteria and introduced the concept of axial SpA (axSpA) and peripheral SpA (pSpA), for predominantly axial or peripheral involvement, respectively, and considered psoriasis as an extra-musculoskeletal characteristic associated with SpA [2]. Recently, the concept of axial PsA has emerged based on the fact that patients with PsA can also be classified as having axial disease. However, many rheumatologists consider AS and axial PsA as different entities on the basis of their clinical presentation: sacroiliitis is commonly bilateral in AS (or axSpA) but is either unilateral or bilateral in axial PsA [3]. Moreover, a higher prevalence of peripheral manifestations (such as peripheral arthritis and dactylitis) and a lower prevalence of HLA-B27 positivity have been reported in axial PsA patients [4]. On the other hand, patients classified as having AS (or axSpA) may also have concomitant psoriasis that is associated with a greater prevalence of peripheral manifestations, making it indistinguishable from axial PsA. For patients with pure peripheral phenotypes (without axial involvement), it is also unknown whether PsA and pSpA represent an overlap of the same disease or represent different entities.
Currently, the debate is focused on the concept of axial PsA, and whether this is a different entity from AS (or axSpA). A few studies have evaluated clinical similarities and differences between these groups of patients. However, some of them have classified patients either as AS or as axial PsA according to the modified New York (mNY) criteria or CASPAR criteria, respectively [5–7], introducing an artificial classification of patients who, in many cases, may fulfil both classification sets. For this reason, we think that the ideal approach to evaluate the influence of psoriasis in SpA disease is to compare patients across the full spectrum of SpA, irrespective of their fulfilment of mNY or CASPAR criteria.
Based on this, we decided to conduct this study with patients from a large Spanish national registry, with the aim to evaluate whether the presence of psoriasis influences the clinical expression, disease activity and disease burden in both axial and peripheral phenotypes of SpA. It should be noted that, with this study, we do not intend to demonstrate whether axial PsA or AS are the same disease or different diseases, but to evaluate the similarities and differences in SpA patients with regard to the presence of psoriasis.
Methods
Patients
This is a cross-sectional study including 2367 patients from the national, multicentre, Spanish REGISPONSER registry, who were recruited between March 2004 and March 2007. Patients had to fulfil the ESSG criteria for SpA [8] and have a diagnosis of any subtype of SpA according to their rheumatologist. In the ESSG criteria, patients had to fulfil the presence of either inflammatory back pain (IBP) or peripheral arthritis as entry criterion and at least one other minor criterion to be classified as having SpA [8]. More information about methodology and data inclusion for the REGISPONSER registry have been previously described [9]. All patients gave their consent to participate in the REGISPONSER registry, which was approved centrally by the ethics committee of the Reina Sofia University Hospital, Córdoba (Spain).
In this study, we verified the fulfilment of the ESSG criteria, and 61 patients were excluded because this fulfilment was not confirmed. Among the 2306 patients who fulfilled the ESSG criteria and had a positive diagnosis of SpA according to their rheumatologist, we classified patients into two groups depending on the presence of a positive personal history of psoriasis (either cutaneous or nail involvement) (Fig. 1). Ten patients were excluded from the analysis because information concerning personal history of psoriasis was missing. Finally, we classified patients as having either axial phenotype (defined as the presence of definite radiographic sacroiliitis according to the local reader OR presence of IBP according to the Calin criteria [10]) or peripheral phenotype [defined as absence of radiographic sacroiliitis AND absence of IBP AND presence of peripheral involvement (either arthritis, or enthesitis or dactylitis)]. Thus, we ultimately obtained four groups of patients depending on the presence of psoriasis and depending on the phenotype: psoriatic axial phenotype, psoriatic peripheral phenotype, non-psoriatic axial phenotype and non-psoriatic peripheral phenotype.
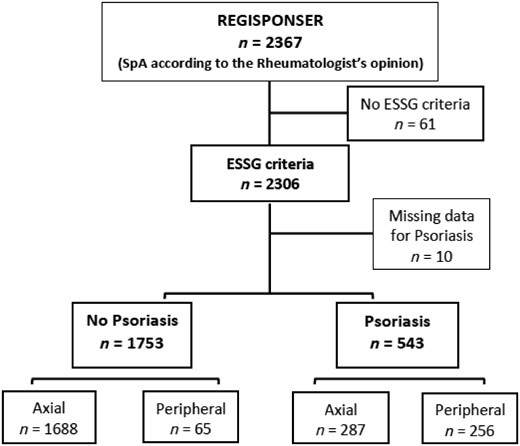
Flow-chart showing the selection of patients included in the analysis
SpA: spondyloarthritis.
Moreover, a sensitivity analysis was conducted considering the axial phenotype as only patients with definite radiographic sacroiliitis according to the local reader, and with the peripheral phenotype as patients without radiographic sacroiliitis AND with peripheral involvement (either arthritis, or dactylitis or enthesitis).
Patient and public involvement
Patients were not involved in the design of the study, conduct of the study, development or dissemination of study results.
Collected data
A case-report form was used to collect clinical data during a face-to-face interview. Information concerning clinical events that occurred before the study visit were collected retrospectively by asking patients or checking their medical records. The study included the following variables:
Sociodemographic characteristics: age, sex, university education, employment status, smoker (ever) and BMI (kg/m2).
Clinical characteristics: age at disease onset, disease duration since symptoms onset (years), diagnosis delay (years), IBP (ever), definite radiographic sacroiliitis according to the local reader, family history of SpA, good NSAIDs response (ever), heel enthesitis (ever and current), dactylitis (ever), uveitis (ever), IBD (ever), urethritis, cervicitis or diarrhoea (ever), moderate or severe hip involvement on X-ray according to the local reader, and shoulder involvement defined as pain or limitation with the movement (ever). During the study visit, the number of swollen joints (NSJ), the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) and the presence of pain and/or limitation of the hip on physical examination were also evaluated.
Treatments: information was collected on oral corticosteroid intake (ever and current); synthetic disease-modifying antirheumatic drugs [conventional synthetic DMARDs (csDMARDs; ever and current)], such as methotrexate, sulfasalazine, leflunomide and azathioprine; anti-TNF treatment (ever and current); and hip replacement.
Laboratory analysis: HLA-B27 status CRP (mg/l) and ESR (mm/h).
Patient-reported outcomes (PROs): visual analogue scale (VAS) nocturnal pain last week (0–10), VAS pain last week (0–10), patient global assessment last week (PtGA; 0–10), physician global assessment last week (PGA; 0–10), BASDAI (0–10) [11], BASFI (0–10) [12] and the mental and physical component of the SF-12 questionnaire (short form of the Medical Outcomes Study Short Form-36, with a scale 0–100, 100 being the worst score) [13]. The Ankylosing Spondylitis Disease Activity Score (ASDAS-CRP) was also calculated [14].
Statistical analysis
Descriptive data are presented as the mean (s.d.) for continuous variables and as absolute frequencies and percentages for categorical ones. Univariate pair-wise comparisons among the four groups (psoriatic/non-psoriatic axial phenotypes and psoriatic/non-psoriatic peripheral phenotypes) were performed using the χ2 and t test for categorical and continuous variables, respectively.
For each of the pair-wise comparisons, two multivariate logistic regression analyses were conducted (using backward stepwise procedures and including variables with a P-value <0.10 in the univariate analysis): one multivariate analysis for disease characteristics, and a second multivariate analysis for the current status of the disease (i.e. variables concerning the status of the disease at the time of the visit). Both multivariate analyses were adjusted for treatment intake (corticosteroids, csDMARDs or anti-TNF) in cases of significant differences in these variables to avoid the potential bias caused by the effect of treatment on clinical manifestations. Interactions and confounding factors were tested, and all contrasts were bilateral and considered significant when the P-value was <0.05. Data were collected, processed and analysed using SPSS Statistics v.25 (IBM Corp., Armonk, NY, USA).
To confirm that differences across groups were similar regardless of the definition of axial and peripheral phenotypes, the same univariate analyses were conducted using the second definition of axial phenotype (i.e. definite radiographic sacroiliitis according to the local reader) and peripheral phenotype [i.e. patients without definite radiographic sacroiliitis AND with peripheral involvement (either arthritis, or dactylitis or enthesitis)].
Results
Overall population
A total of 2296 patients were included in the final analysis. Among these, 543 (23.6%) had a personal history of psoriasis (287 and 256 showed axial and peripheral phenotypes, respectively), and 1753 (76.4%) did not have psoriasis (1688 and 65 showed axial and peripheral phenotypes, respectively). Table 1 shows the clinical characteristics of the overall population included in the study.
Clinical characteristics of axial and peripheral phenotypes regarding the presence of psoriasis. Univariate analysis
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Age, mean (s.d.), years | 47.6 (13.3) | 50.0 (13.0) | 46.7 (13.0) | <0.001 | 52.3 (13.8) | 41.2 (14.7) | <0.001 | 0.050 | 0.003 |
| Gender (male), n/n (%) | 1572/2296 (68.5) | 201/287 (70.0) | 1200/1688 (71.1) | 0.716 | 136/256 (53.1) | 35/65 (53.8) | 0.917 | <0.001 | 0.003 |
| University education, n/n (%) | 207/2296 (9.0) | 31/287 (10.8) | 156/1688 (9.2) | 0.404 | 15/256 (5.9) | 5/65 (7.7) | 0.585 | 0.039 | 0.671 |
| Employee, n/n (%) | 1060/2296 (46.2) | 142/287 (49.5) | 803/1688 (47.6) | 0.550 | 92/256 (35.9) | 23/65 (35.4) | 0.934 | 0.001 | 0.053 |
| Smoking (ever), n/n (%) | 258/709 (36.4) | 39/108 (36.1) | 195/536 (36.4) | 0.958 | 19/48 (39.6) | 5/17 (29.4) | 0.455 | 0.679 | 0.556 |
| BMI, mean (s.d.), kg/m2 | 26.6 (4.5) | 27.4 (5.2) | 26.5 (4.4) | 0.004 | 27.2 (4.3) | 25.9 (4.2) | 0.043 | 0.634 | 0.337 |
| Age at disease onset, mean (s.d.), years | 29.6 (12.5) | 33.0 (13.7) | 27.2 (10.7) | <0.001 | 41.3 (13.8) | 32.8 (13.4) | <0.001 | <0.001 | 0.002 |
| Disease duration since symptoms onset, mean (s.d.), years | 18.0 (12.8) | 17.0 (12.1) | 19.6 (13.1) | 0.001 | 11.0 (8.7) | 8.5 (7.6) | 0.036 | <0.001 | <0.001 |
| Diagnosis delay, mean (s.d.), years | 6.3 (8.6) | 6.31 (8.8) | 7.1 (8.9) | 0.204 | 2.7 (5.3) | 2.5 (4.2) | 0.823 | <0.001 | <0.001 |
| IBP (ever), n/n (%) | 1875/2293 (81.8) | 244/286 (85.3) | 1631/1687 (96.7) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| Radiographic sacroiliitis, n/n (%) | 1800/2296 (78.4) | 239/287 (83.3) | 1561/1688 (92.5) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| HLA-B27 positivity, n/n (%) | 1403/1933 (72.6) | 97/199 (48.7) | 1255/1536 (81.7) | <0.001 | 19/146 (13.0) | 32/52 (61.5) | <0.001 | <0.001 | 0.001 |
| Family history of SpA, n/n (%) | 381/2114 (18.0) | 38/253 (15.0) | 316/1568 (20.2) | 0.056 | 18/230 (7.8) | 9/63 (14.3) | 0.116 | 0.014 | 0.253 |
| Good NSAIDs response (ever), n/n (%) | 1767/2274 (77.7) | 217/279 (77.8) | 1340/1676 (80.0) | 0.404 | 172/254 (67.7) | 38/65 (58.5) | 0.160 | 0.009 | <0.001 |
| Heel enthesitis (ever), n/n (%) | 699/2280 (30.7) | 99/284 (34.9) | 533/1676 (31.8) | 0.308 | 31/255 (12.2) | 36/65 (55.4) | <0.001 | <0.001 | <0.001 |
| Any synovitis (ever), n/n (%) | 1156/2289 (50.5) | 214/285 (75.1) | 612/1683 (36.9) | <0.001 | 256/256 (100) | 65/65 (100) | — | <0.001 | <0.001 |
| Dactylitis (ever), n/n (%) | 266/2287 (11.6) | 80/287 (28.0) | 90/1681 (5.4) | <0.001 | 84/255 (32.9) | 12/65 (18.5) | 0.023 | 0.209 | <0.001 |
| Uveitis (ever), n/n (%) | 379/2281 (16.6) | 22/284 (7.7) | 350/1677 (20.9) | <0.001 | 2/255 (0.8) | 5/65 (7.7) | 0.001 | <0.001 | 0.010 |
| IBD (ever), n/n (%) | 118/2288 (5.2) | 10/286 (3.5) | 97/1683 (5.8) | 0.118 | 0/254 (0) | 11/65 (16.9) | <0.001 | 0.003 | <0.001 |
| Urethritis, cervicitis or diarrhoea (ever), n/n (%) | 58/2282 (2.5) | 0/284 (0) | 49/1678 (2.9) | 0.004 | 0/254 (0) | 9/65 (13.8) | <0.001 | — | <0.001 |
| Moderate or severe hip involvement on X-ray, n/n (%) | 169/2205 (7.7) | 22/274 (8.0) | 141/1620 (8.7) | 0.713 | 5/247 (2.0) | 1/64 (1.6) | 0.811 | 0.002 | 0.044 |
| Shoulder involvement (ever), n/n (%) | 315/2268 (13.9) | 50/282 (17.7) | 230/1669 (13.8) | 0.080 | 34/253 (13.4) | 1/64 (1.6) | 0.007 | 0.173 | 0.005 |
| Oral corticosteroids (ever), n/n (%) | 639/2268 (28.2) | 138/281 (49.1) | 325/1668 (19.5) | <0.001 | 149/254 (58.7) | 27/65 (41.5) | 0.013 | 0.027 | <0.001 |
| csDMARDs (ever), n/n (%) | 815/2271 (35.9) | 154/282 (54.6) | 473/1672 (28.3) | <0.001 | 157/253 (62.1) | 31/64 (48.4) | 0.048 | 0.081 | <0.001 |
| Anti-TNF treatment (ever), n/n (%) | 406/2265 (17.9) | 69/287 (24.7) | 295/1668 (17.7) | 0.005 | 33/254 (13.0) | 9/64 (14.1) | 0.821 | 0.001 | 0.455 |
| Hip replacement, n/n (%) | 75/2265 (3.3) | 13/280 (4.6) | 60/1665 (3.6) | 0.397 | 2/255 (0.8) | 0/65 (0) | 0.474 | 0.007 | 0.168 |
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Age, mean (s.d.), years | 47.6 (13.3) | 50.0 (13.0) | 46.7 (13.0) | <0.001 | 52.3 (13.8) | 41.2 (14.7) | <0.001 | 0.050 | 0.003 |
| Gender (male), n/n (%) | 1572/2296 (68.5) | 201/287 (70.0) | 1200/1688 (71.1) | 0.716 | 136/256 (53.1) | 35/65 (53.8) | 0.917 | <0.001 | 0.003 |
| University education, n/n (%) | 207/2296 (9.0) | 31/287 (10.8) | 156/1688 (9.2) | 0.404 | 15/256 (5.9) | 5/65 (7.7) | 0.585 | 0.039 | 0.671 |
| Employee, n/n (%) | 1060/2296 (46.2) | 142/287 (49.5) | 803/1688 (47.6) | 0.550 | 92/256 (35.9) | 23/65 (35.4) | 0.934 | 0.001 | 0.053 |
| Smoking (ever), n/n (%) | 258/709 (36.4) | 39/108 (36.1) | 195/536 (36.4) | 0.958 | 19/48 (39.6) | 5/17 (29.4) | 0.455 | 0.679 | 0.556 |
| BMI, mean (s.d.), kg/m2 | 26.6 (4.5) | 27.4 (5.2) | 26.5 (4.4) | 0.004 | 27.2 (4.3) | 25.9 (4.2) | 0.043 | 0.634 | 0.337 |
| Age at disease onset, mean (s.d.), years | 29.6 (12.5) | 33.0 (13.7) | 27.2 (10.7) | <0.001 | 41.3 (13.8) | 32.8 (13.4) | <0.001 | <0.001 | 0.002 |
| Disease duration since symptoms onset, mean (s.d.), years | 18.0 (12.8) | 17.0 (12.1) | 19.6 (13.1) | 0.001 | 11.0 (8.7) | 8.5 (7.6) | 0.036 | <0.001 | <0.001 |
| Diagnosis delay, mean (s.d.), years | 6.3 (8.6) | 6.31 (8.8) | 7.1 (8.9) | 0.204 | 2.7 (5.3) | 2.5 (4.2) | 0.823 | <0.001 | <0.001 |
| IBP (ever), n/n (%) | 1875/2293 (81.8) | 244/286 (85.3) | 1631/1687 (96.7) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| Radiographic sacroiliitis, n/n (%) | 1800/2296 (78.4) | 239/287 (83.3) | 1561/1688 (92.5) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| HLA-B27 positivity, n/n (%) | 1403/1933 (72.6) | 97/199 (48.7) | 1255/1536 (81.7) | <0.001 | 19/146 (13.0) | 32/52 (61.5) | <0.001 | <0.001 | 0.001 |
| Family history of SpA, n/n (%) | 381/2114 (18.0) | 38/253 (15.0) | 316/1568 (20.2) | 0.056 | 18/230 (7.8) | 9/63 (14.3) | 0.116 | 0.014 | 0.253 |
| Good NSAIDs response (ever), n/n (%) | 1767/2274 (77.7) | 217/279 (77.8) | 1340/1676 (80.0) | 0.404 | 172/254 (67.7) | 38/65 (58.5) | 0.160 | 0.009 | <0.001 |
| Heel enthesitis (ever), n/n (%) | 699/2280 (30.7) | 99/284 (34.9) | 533/1676 (31.8) | 0.308 | 31/255 (12.2) | 36/65 (55.4) | <0.001 | <0.001 | <0.001 |
| Any synovitis (ever), n/n (%) | 1156/2289 (50.5) | 214/285 (75.1) | 612/1683 (36.9) | <0.001 | 256/256 (100) | 65/65 (100) | — | <0.001 | <0.001 |
| Dactylitis (ever), n/n (%) | 266/2287 (11.6) | 80/287 (28.0) | 90/1681 (5.4) | <0.001 | 84/255 (32.9) | 12/65 (18.5) | 0.023 | 0.209 | <0.001 |
| Uveitis (ever), n/n (%) | 379/2281 (16.6) | 22/284 (7.7) | 350/1677 (20.9) | <0.001 | 2/255 (0.8) | 5/65 (7.7) | 0.001 | <0.001 | 0.010 |
| IBD (ever), n/n (%) | 118/2288 (5.2) | 10/286 (3.5) | 97/1683 (5.8) | 0.118 | 0/254 (0) | 11/65 (16.9) | <0.001 | 0.003 | <0.001 |
| Urethritis, cervicitis or diarrhoea (ever), n/n (%) | 58/2282 (2.5) | 0/284 (0) | 49/1678 (2.9) | 0.004 | 0/254 (0) | 9/65 (13.8) | <0.001 | — | <0.001 |
| Moderate or severe hip involvement on X-ray, n/n (%) | 169/2205 (7.7) | 22/274 (8.0) | 141/1620 (8.7) | 0.713 | 5/247 (2.0) | 1/64 (1.6) | 0.811 | 0.002 | 0.044 |
| Shoulder involvement (ever), n/n (%) | 315/2268 (13.9) | 50/282 (17.7) | 230/1669 (13.8) | 0.080 | 34/253 (13.4) | 1/64 (1.6) | 0.007 | 0.173 | 0.005 |
| Oral corticosteroids (ever), n/n (%) | 639/2268 (28.2) | 138/281 (49.1) | 325/1668 (19.5) | <0.001 | 149/254 (58.7) | 27/65 (41.5) | 0.013 | 0.027 | <0.001 |
| csDMARDs (ever), n/n (%) | 815/2271 (35.9) | 154/282 (54.6) | 473/1672 (28.3) | <0.001 | 157/253 (62.1) | 31/64 (48.4) | 0.048 | 0.081 | <0.001 |
| Anti-TNF treatment (ever), n/n (%) | 406/2265 (17.9) | 69/287 (24.7) | 295/1668 (17.7) | 0.005 | 33/254 (13.0) | 9/64 (14.1) | 0.821 | 0.001 | 0.455 |
| Hip replacement, n/n (%) | 75/2265 (3.3) | 13/280 (4.6) | 60/1665 (3.6) | 0.397 | 2/255 (0.8) | 0/65 (0) | 0.474 | 0.007 | 0.168 |
Values shown in bold are considered significant: P-value <0.05. aAxial phenotype: defined as the presence of definite radiographic sacroiliitis according to the local reader OR presence of IBP according to the Calin criteria. bPeripheral phenotype: defined as absence of definite radiographic sacroiliitis according to the local reader AND absence of IBP AND presence of peripheral involvement (either arthritis, or enthesitis or dactylitis). Values shown in bold are considered significant. csDMARD: conventional synthetic DMARD; IBP: inflammatory back pain; SpA: spondyloarthritis.
Clinical characteristics of axial and peripheral phenotypes regarding the presence of psoriasis. Univariate analysis
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Age, mean (s.d.), years | 47.6 (13.3) | 50.0 (13.0) | 46.7 (13.0) | <0.001 | 52.3 (13.8) | 41.2 (14.7) | <0.001 | 0.050 | 0.003 |
| Gender (male), n/n (%) | 1572/2296 (68.5) | 201/287 (70.0) | 1200/1688 (71.1) | 0.716 | 136/256 (53.1) | 35/65 (53.8) | 0.917 | <0.001 | 0.003 |
| University education, n/n (%) | 207/2296 (9.0) | 31/287 (10.8) | 156/1688 (9.2) | 0.404 | 15/256 (5.9) | 5/65 (7.7) | 0.585 | 0.039 | 0.671 |
| Employee, n/n (%) | 1060/2296 (46.2) | 142/287 (49.5) | 803/1688 (47.6) | 0.550 | 92/256 (35.9) | 23/65 (35.4) | 0.934 | 0.001 | 0.053 |
| Smoking (ever), n/n (%) | 258/709 (36.4) | 39/108 (36.1) | 195/536 (36.4) | 0.958 | 19/48 (39.6) | 5/17 (29.4) | 0.455 | 0.679 | 0.556 |
| BMI, mean (s.d.), kg/m2 | 26.6 (4.5) | 27.4 (5.2) | 26.5 (4.4) | 0.004 | 27.2 (4.3) | 25.9 (4.2) | 0.043 | 0.634 | 0.337 |
| Age at disease onset, mean (s.d.), years | 29.6 (12.5) | 33.0 (13.7) | 27.2 (10.7) | <0.001 | 41.3 (13.8) | 32.8 (13.4) | <0.001 | <0.001 | 0.002 |
| Disease duration since symptoms onset, mean (s.d.), years | 18.0 (12.8) | 17.0 (12.1) | 19.6 (13.1) | 0.001 | 11.0 (8.7) | 8.5 (7.6) | 0.036 | <0.001 | <0.001 |
| Diagnosis delay, mean (s.d.), years | 6.3 (8.6) | 6.31 (8.8) | 7.1 (8.9) | 0.204 | 2.7 (5.3) | 2.5 (4.2) | 0.823 | <0.001 | <0.001 |
| IBP (ever), n/n (%) | 1875/2293 (81.8) | 244/286 (85.3) | 1631/1687 (96.7) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| Radiographic sacroiliitis, n/n (%) | 1800/2296 (78.4) | 239/287 (83.3) | 1561/1688 (92.5) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| HLA-B27 positivity, n/n (%) | 1403/1933 (72.6) | 97/199 (48.7) | 1255/1536 (81.7) | <0.001 | 19/146 (13.0) | 32/52 (61.5) | <0.001 | <0.001 | 0.001 |
| Family history of SpA, n/n (%) | 381/2114 (18.0) | 38/253 (15.0) | 316/1568 (20.2) | 0.056 | 18/230 (7.8) | 9/63 (14.3) | 0.116 | 0.014 | 0.253 |
| Good NSAIDs response (ever), n/n (%) | 1767/2274 (77.7) | 217/279 (77.8) | 1340/1676 (80.0) | 0.404 | 172/254 (67.7) | 38/65 (58.5) | 0.160 | 0.009 | <0.001 |
| Heel enthesitis (ever), n/n (%) | 699/2280 (30.7) | 99/284 (34.9) | 533/1676 (31.8) | 0.308 | 31/255 (12.2) | 36/65 (55.4) | <0.001 | <0.001 | <0.001 |
| Any synovitis (ever), n/n (%) | 1156/2289 (50.5) | 214/285 (75.1) | 612/1683 (36.9) | <0.001 | 256/256 (100) | 65/65 (100) | — | <0.001 | <0.001 |
| Dactylitis (ever), n/n (%) | 266/2287 (11.6) | 80/287 (28.0) | 90/1681 (5.4) | <0.001 | 84/255 (32.9) | 12/65 (18.5) | 0.023 | 0.209 | <0.001 |
| Uveitis (ever), n/n (%) | 379/2281 (16.6) | 22/284 (7.7) | 350/1677 (20.9) | <0.001 | 2/255 (0.8) | 5/65 (7.7) | 0.001 | <0.001 | 0.010 |
| IBD (ever), n/n (%) | 118/2288 (5.2) | 10/286 (3.5) | 97/1683 (5.8) | 0.118 | 0/254 (0) | 11/65 (16.9) | <0.001 | 0.003 | <0.001 |
| Urethritis, cervicitis or diarrhoea (ever), n/n (%) | 58/2282 (2.5) | 0/284 (0) | 49/1678 (2.9) | 0.004 | 0/254 (0) | 9/65 (13.8) | <0.001 | — | <0.001 |
| Moderate or severe hip involvement on X-ray, n/n (%) | 169/2205 (7.7) | 22/274 (8.0) | 141/1620 (8.7) | 0.713 | 5/247 (2.0) | 1/64 (1.6) | 0.811 | 0.002 | 0.044 |
| Shoulder involvement (ever), n/n (%) | 315/2268 (13.9) | 50/282 (17.7) | 230/1669 (13.8) | 0.080 | 34/253 (13.4) | 1/64 (1.6) | 0.007 | 0.173 | 0.005 |
| Oral corticosteroids (ever), n/n (%) | 639/2268 (28.2) | 138/281 (49.1) | 325/1668 (19.5) | <0.001 | 149/254 (58.7) | 27/65 (41.5) | 0.013 | 0.027 | <0.001 |
| csDMARDs (ever), n/n (%) | 815/2271 (35.9) | 154/282 (54.6) | 473/1672 (28.3) | <0.001 | 157/253 (62.1) | 31/64 (48.4) | 0.048 | 0.081 | <0.001 |
| Anti-TNF treatment (ever), n/n (%) | 406/2265 (17.9) | 69/287 (24.7) | 295/1668 (17.7) | 0.005 | 33/254 (13.0) | 9/64 (14.1) | 0.821 | 0.001 | 0.455 |
| Hip replacement, n/n (%) | 75/2265 (3.3) | 13/280 (4.6) | 60/1665 (3.6) | 0.397 | 2/255 (0.8) | 0/65 (0) | 0.474 | 0.007 | 0.168 |
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Age, mean (s.d.), years | 47.6 (13.3) | 50.0 (13.0) | 46.7 (13.0) | <0.001 | 52.3 (13.8) | 41.2 (14.7) | <0.001 | 0.050 | 0.003 |
| Gender (male), n/n (%) | 1572/2296 (68.5) | 201/287 (70.0) | 1200/1688 (71.1) | 0.716 | 136/256 (53.1) | 35/65 (53.8) | 0.917 | <0.001 | 0.003 |
| University education, n/n (%) | 207/2296 (9.0) | 31/287 (10.8) | 156/1688 (9.2) | 0.404 | 15/256 (5.9) | 5/65 (7.7) | 0.585 | 0.039 | 0.671 |
| Employee, n/n (%) | 1060/2296 (46.2) | 142/287 (49.5) | 803/1688 (47.6) | 0.550 | 92/256 (35.9) | 23/65 (35.4) | 0.934 | 0.001 | 0.053 |
| Smoking (ever), n/n (%) | 258/709 (36.4) | 39/108 (36.1) | 195/536 (36.4) | 0.958 | 19/48 (39.6) | 5/17 (29.4) | 0.455 | 0.679 | 0.556 |
| BMI, mean (s.d.), kg/m2 | 26.6 (4.5) | 27.4 (5.2) | 26.5 (4.4) | 0.004 | 27.2 (4.3) | 25.9 (4.2) | 0.043 | 0.634 | 0.337 |
| Age at disease onset, mean (s.d.), years | 29.6 (12.5) | 33.0 (13.7) | 27.2 (10.7) | <0.001 | 41.3 (13.8) | 32.8 (13.4) | <0.001 | <0.001 | 0.002 |
| Disease duration since symptoms onset, mean (s.d.), years | 18.0 (12.8) | 17.0 (12.1) | 19.6 (13.1) | 0.001 | 11.0 (8.7) | 8.5 (7.6) | 0.036 | <0.001 | <0.001 |
| Diagnosis delay, mean (s.d.), years | 6.3 (8.6) | 6.31 (8.8) | 7.1 (8.9) | 0.204 | 2.7 (5.3) | 2.5 (4.2) | 0.823 | <0.001 | <0.001 |
| IBP (ever), n/n (%) | 1875/2293 (81.8) | 244/286 (85.3) | 1631/1687 (96.7) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| Radiographic sacroiliitis, n/n (%) | 1800/2296 (78.4) | 239/287 (83.3) | 1561/1688 (92.5) | <0.001 | 0 (0) | 0 (0) | — | <0.001 | <0.001 |
| HLA-B27 positivity, n/n (%) | 1403/1933 (72.6) | 97/199 (48.7) | 1255/1536 (81.7) | <0.001 | 19/146 (13.0) | 32/52 (61.5) | <0.001 | <0.001 | 0.001 |
| Family history of SpA, n/n (%) | 381/2114 (18.0) | 38/253 (15.0) | 316/1568 (20.2) | 0.056 | 18/230 (7.8) | 9/63 (14.3) | 0.116 | 0.014 | 0.253 |
| Good NSAIDs response (ever), n/n (%) | 1767/2274 (77.7) | 217/279 (77.8) | 1340/1676 (80.0) | 0.404 | 172/254 (67.7) | 38/65 (58.5) | 0.160 | 0.009 | <0.001 |
| Heel enthesitis (ever), n/n (%) | 699/2280 (30.7) | 99/284 (34.9) | 533/1676 (31.8) | 0.308 | 31/255 (12.2) | 36/65 (55.4) | <0.001 | <0.001 | <0.001 |
| Any synovitis (ever), n/n (%) | 1156/2289 (50.5) | 214/285 (75.1) | 612/1683 (36.9) | <0.001 | 256/256 (100) | 65/65 (100) | — | <0.001 | <0.001 |
| Dactylitis (ever), n/n (%) | 266/2287 (11.6) | 80/287 (28.0) | 90/1681 (5.4) | <0.001 | 84/255 (32.9) | 12/65 (18.5) | 0.023 | 0.209 | <0.001 |
| Uveitis (ever), n/n (%) | 379/2281 (16.6) | 22/284 (7.7) | 350/1677 (20.9) | <0.001 | 2/255 (0.8) | 5/65 (7.7) | 0.001 | <0.001 | 0.010 |
| IBD (ever), n/n (%) | 118/2288 (5.2) | 10/286 (3.5) | 97/1683 (5.8) | 0.118 | 0/254 (0) | 11/65 (16.9) | <0.001 | 0.003 | <0.001 |
| Urethritis, cervicitis or diarrhoea (ever), n/n (%) | 58/2282 (2.5) | 0/284 (0) | 49/1678 (2.9) | 0.004 | 0/254 (0) | 9/65 (13.8) | <0.001 | — | <0.001 |
| Moderate or severe hip involvement on X-ray, n/n (%) | 169/2205 (7.7) | 22/274 (8.0) | 141/1620 (8.7) | 0.713 | 5/247 (2.0) | 1/64 (1.6) | 0.811 | 0.002 | 0.044 |
| Shoulder involvement (ever), n/n (%) | 315/2268 (13.9) | 50/282 (17.7) | 230/1669 (13.8) | 0.080 | 34/253 (13.4) | 1/64 (1.6) | 0.007 | 0.173 | 0.005 |
| Oral corticosteroids (ever), n/n (%) | 639/2268 (28.2) | 138/281 (49.1) | 325/1668 (19.5) | <0.001 | 149/254 (58.7) | 27/65 (41.5) | 0.013 | 0.027 | <0.001 |
| csDMARDs (ever), n/n (%) | 815/2271 (35.9) | 154/282 (54.6) | 473/1672 (28.3) | <0.001 | 157/253 (62.1) | 31/64 (48.4) | 0.048 | 0.081 | <0.001 |
| Anti-TNF treatment (ever), n/n (%) | 406/2265 (17.9) | 69/287 (24.7) | 295/1668 (17.7) | 0.005 | 33/254 (13.0) | 9/64 (14.1) | 0.821 | 0.001 | 0.455 |
| Hip replacement, n/n (%) | 75/2265 (3.3) | 13/280 (4.6) | 60/1665 (3.6) | 0.397 | 2/255 (0.8) | 0/65 (0) | 0.474 | 0.007 | 0.168 |
Values shown in bold are considered significant: P-value <0.05. aAxial phenotype: defined as the presence of definite radiographic sacroiliitis according to the local reader OR presence of IBP according to the Calin criteria. bPeripheral phenotype: defined as absence of definite radiographic sacroiliitis according to the local reader AND absence of IBP AND presence of peripheral involvement (either arthritis, or enthesitis or dactylitis). Values shown in bold are considered significant. csDMARD: conventional synthetic DMARD; IBP: inflammatory back pain; SpA: spondyloarthritis.
Patients with axial phenotype: psoriatic vs non-psoriatic
In this analysis, we compared the psoriatic axial phenotype (n = 287) with the non-psoriatic axial phenotype (n = 1688). Table 1 represents the univariate analysis of clinical characteristics associated with the presence of psoriasis, while Table 2 represents the univariate analysis for variables concerning the current status of the disease at the time of the study visit. Supplementary Tables S1 and S2, available at Rheumatology online, show the sensitivity analysis using a different definition of axial phenotype, which showed similar results to the main analysis. Multivariate analysis (Fig. 2A) shows that, among patients with axial phenotype, the presence of psoriasis was independently associated (P < 0.05) with a lower prevalence of HLA-B27 positivity [odds ratio (OR) 0.27, 95% CI: 0.19, 0.39] and uveitis (ever) (OR 0.46, 95% CI: 0.27, 0.80), a higher prevalence of synovitis (ever) (OR 2.59, 95% CI: 1.75, 3.82) and dactylitis (ever) (OR 2.78, 95% CI: 1.70, 4.56), and a greater use of csDMARDs (ever) (OR 1.47, 95% CI: 1.08, 2.27) in comparison with non-psoriatic patients. Moreover, BMI was found to be a confounding factor (OR 1.03, 95% CI: 0.99, 1.07) in this model. Concerning the current status of the disease (Fig. 2B), psoriatic patients showed higher NSJ (OR 1.10, 95% CI: 1.04, 1.16), higher BASDAI scores (OR 1.07, 95% CI: 1.10, 1.13) and a greater current treatment intake [OR 2.92 (95% CI: 2.21, 3.85) and OR 1.47 (95% CI: 1.06, 2.04) for csDMARDs and anti-TNF, respectively] in comparison with non-psoriatic patients.

Comparison of axial phenotype patients with regard to the presence of psoriasis. Multivariate analysis
csDMARD: conventional synthetic DMARD; NSJ: number of swollen joints; ref.: reference.
Disease status and disease burden at the moment of the study visit of axial and peripheral phenotypes with regard to the presence of psoriasis. Univariate analysis
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Number of swollen joints (current), mean (s.d.) | 0.5 (1.8) | 1.0 (2.7) | 0.4 (1.7) | <0.001 | 1.8 (3.7) | 0.9 (1.8) | 0.056 | 0.010 | 0.046 |
| MASES enthesis index (current), mean (s.d.) | 1.7 (1.9) | 2.3 (2.1) | 1.6 (1.9) | 0.005 | 0.7 (1.3) | 0.8 (0.8) | 0.871 | <0.001 | <0.001 |
| Heel enthesitis (current), n/n (%) | 144/2296 (6.3) | 24/287 (8.4) | 103/1688 (6.1) | 0.149 | 7/256 (2.7) | 10/65 (15.4) | <0.001 | 0.005 | 0.007 |
| Pain or limitation of the hip on physical examination, n/n (%) | 406/2265 (17.9) | 48/280 (17.1) | 340/1667 (20.4) | 0.207 | 15/253 (5.9) | 3/65 (4.6) | 0.683 | <0.001 | 0.002 |
| Oral corticosteroids (current), n/n (%) | 256/2264 (11.3) | 37/279 (13.3) | 145/1666 (8.7) | 0.016 | 59/254 (23.2) | 15/65 (23.1) | 0.979 | 0.003 | <0.001 |
| csDMARDs (current), n/n (%) | 661/2269 (29.1) | 130/281 (46.3) | 353/1672 (21.1) | <0.001 | 148/252 (58.7) | 30/64 (46.9) | 0.088 | 0.004 | <0.001 |
| Anti-TNF treatment (current), n/n (%) | 364/2263 (16.1) | 61/278 (21.9) | 265/1667 (15.9) | 0.012 | 30/254 (11.8) | 8/64 (12.5) | 0.879 | 0.002 | 0.464 |
| CRP, mean (s.d.), mg/l | 8.6 (13.4) | 9.0 (15.0) | 8.7 (13.7) | 0.770 | 7.9 (10.2) | 5.8 (7.4) | 0.138 | 0.332 | 0.005 |
| ESR, mean (s.d.), mm/h | 18.0 (15.9) | 17.6 (16.4) | 18.0 (16.1) | 0.718 | 19.8 (15.2) | 14.0 (10.7) | 0.004 | 0.123 | 0.005 |
| ASDAS-CRP, mean (s.d.) | 2.5 (1.1) | 2.6 (1.1) | 2.5 (1.1) | 0.254 | 2.4 (1.1) | 1.9 (1.2) | 0.001 | 0.044 | <0.001 |
| VAS nocturnal pain last week, mean (s.d.), 0–10 | 3.6 (3.0) | 3.6 (2.9) | 3.8 (2.9) | 0.174 | 2.3 (2.9) | 1.9 (2.6) | 0.224 | <0.001 | <0.001 |
| VAS pain last week, mean (s.d.), 0–10 | 3.8 (2.8) | 3.8 (2.8) | 4.0 (2.7) | 0.197 | 2.5 (2.9) | 2.2 (2.7) | 0.430 | <0.001 | <0.001 |
| Patient global assessment last week, mean (s.d.), 0–10 | 4.4 (2.7) | 4.6 (2.6) | 4.5 (2.7) | 0.406 | 4.2 (2.8) | 3.3 (2.9) | 0.014 | 0.104 | <0.001 |
| Physician global assessment last week, mean (s.d.), 0–10 | 3.0 (2.1) | 3.4 (2.2) | 3.1 (2.1) | 0.053 | 2.5 (2.1) | 2.3 (2.2) | 0.450 | <0.001 | 0.002 |
| BASDAI, mean (s.d.), 0–10 | 4.0 (2.4) | 4.3 (2.4) | 4.0 (2.4) | 0.038 | 4.0 (2.5) | 2.9 (2.5) | 0.004 | 0.087 | <0.001 |
| BASFI, mean (s.d.), 0–10 | 3.5 (2.7) | 3.8 (2.6) | 3.6 (2.7) | 0.373 | 2.8 (2.6) | 1.9 (2.3) | 0.013 | <0.001 | <0.001 |
| SF-12 mental component, mean (s.d.), 0–100 | 47.8 (12.4) | 47.3 (13.5) | 47.9 (12.5) | 0.455 | 48.3 (1.9) | 47.5 (10.1) | 0.042 | 0.338 | 0.802 |
| SF-12 physical component, mean (s.d.), 0–100 | 35.7 (11.1) | 34.9 (11.7) | 35.6 (11.1) | 0.328 | 36.8 (10.5) | 39.7 (10.0) | 0.589 | 0.046 | 0.003 |
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Number of swollen joints (current), mean (s.d.) | 0.5 (1.8) | 1.0 (2.7) | 0.4 (1.7) | <0.001 | 1.8 (3.7) | 0.9 (1.8) | 0.056 | 0.010 | 0.046 |
| MASES enthesis index (current), mean (s.d.) | 1.7 (1.9) | 2.3 (2.1) | 1.6 (1.9) | 0.005 | 0.7 (1.3) | 0.8 (0.8) | 0.871 | <0.001 | <0.001 |
| Heel enthesitis (current), n/n (%) | 144/2296 (6.3) | 24/287 (8.4) | 103/1688 (6.1) | 0.149 | 7/256 (2.7) | 10/65 (15.4) | <0.001 | 0.005 | 0.007 |
| Pain or limitation of the hip on physical examination, n/n (%) | 406/2265 (17.9) | 48/280 (17.1) | 340/1667 (20.4) | 0.207 | 15/253 (5.9) | 3/65 (4.6) | 0.683 | <0.001 | 0.002 |
| Oral corticosteroids (current), n/n (%) | 256/2264 (11.3) | 37/279 (13.3) | 145/1666 (8.7) | 0.016 | 59/254 (23.2) | 15/65 (23.1) | 0.979 | 0.003 | <0.001 |
| csDMARDs (current), n/n (%) | 661/2269 (29.1) | 130/281 (46.3) | 353/1672 (21.1) | <0.001 | 148/252 (58.7) | 30/64 (46.9) | 0.088 | 0.004 | <0.001 |
| Anti-TNF treatment (current), n/n (%) | 364/2263 (16.1) | 61/278 (21.9) | 265/1667 (15.9) | 0.012 | 30/254 (11.8) | 8/64 (12.5) | 0.879 | 0.002 | 0.464 |
| CRP, mean (s.d.), mg/l | 8.6 (13.4) | 9.0 (15.0) | 8.7 (13.7) | 0.770 | 7.9 (10.2) | 5.8 (7.4) | 0.138 | 0.332 | 0.005 |
| ESR, mean (s.d.), mm/h | 18.0 (15.9) | 17.6 (16.4) | 18.0 (16.1) | 0.718 | 19.8 (15.2) | 14.0 (10.7) | 0.004 | 0.123 | 0.005 |
| ASDAS-CRP, mean (s.d.) | 2.5 (1.1) | 2.6 (1.1) | 2.5 (1.1) | 0.254 | 2.4 (1.1) | 1.9 (1.2) | 0.001 | 0.044 | <0.001 |
| VAS nocturnal pain last week, mean (s.d.), 0–10 | 3.6 (3.0) | 3.6 (2.9) | 3.8 (2.9) | 0.174 | 2.3 (2.9) | 1.9 (2.6) | 0.224 | <0.001 | <0.001 |
| VAS pain last week, mean (s.d.), 0–10 | 3.8 (2.8) | 3.8 (2.8) | 4.0 (2.7) | 0.197 | 2.5 (2.9) | 2.2 (2.7) | 0.430 | <0.001 | <0.001 |
| Patient global assessment last week, mean (s.d.), 0–10 | 4.4 (2.7) | 4.6 (2.6) | 4.5 (2.7) | 0.406 | 4.2 (2.8) | 3.3 (2.9) | 0.014 | 0.104 | <0.001 |
| Physician global assessment last week, mean (s.d.), 0–10 | 3.0 (2.1) | 3.4 (2.2) | 3.1 (2.1) | 0.053 | 2.5 (2.1) | 2.3 (2.2) | 0.450 | <0.001 | 0.002 |
| BASDAI, mean (s.d.), 0–10 | 4.0 (2.4) | 4.3 (2.4) | 4.0 (2.4) | 0.038 | 4.0 (2.5) | 2.9 (2.5) | 0.004 | 0.087 | <0.001 |
| BASFI, mean (s.d.), 0–10 | 3.5 (2.7) | 3.8 (2.6) | 3.6 (2.7) | 0.373 | 2.8 (2.6) | 1.9 (2.3) | 0.013 | <0.001 | <0.001 |
| SF-12 mental component, mean (s.d.), 0–100 | 47.8 (12.4) | 47.3 (13.5) | 47.9 (12.5) | 0.455 | 48.3 (1.9) | 47.5 (10.1) | 0.042 | 0.338 | 0.802 |
| SF-12 physical component, mean (s.d.), 0–100 | 35.7 (11.1) | 34.9 (11.7) | 35.6 (11.1) | 0.328 | 36.8 (10.5) | 39.7 (10.0) | 0.589 | 0.046 | 0.003 |
Values shown in bold are considered significant: P-value <0.05. aAxial phenotype: defined as the presence of definite radiographic sacroiliitis according to the local reader OR presence of IBP according to the Calin criteria. bPeripheral phenotype: defined as absence of definite radiographic sacroiliitis according to the local reader AND absence of IBP AND presence of peripheral involvement (either arthritis, or enthesitis or dactylitis). Values shown in bold are considered significant. ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score; csDMARD: conventional synthetic DMARD; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; SF-12: short form of the Medical Outcomes Study Short Form-36; VAS: visual analogue scale.
Disease status and disease burden at the moment of the study visit of axial and peripheral phenotypes with regard to the presence of psoriasis. Univariate analysis
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Number of swollen joints (current), mean (s.d.) | 0.5 (1.8) | 1.0 (2.7) | 0.4 (1.7) | <0.001 | 1.8 (3.7) | 0.9 (1.8) | 0.056 | 0.010 | 0.046 |
| MASES enthesis index (current), mean (s.d.) | 1.7 (1.9) | 2.3 (2.1) | 1.6 (1.9) | 0.005 | 0.7 (1.3) | 0.8 (0.8) | 0.871 | <0.001 | <0.001 |
| Heel enthesitis (current), n/n (%) | 144/2296 (6.3) | 24/287 (8.4) | 103/1688 (6.1) | 0.149 | 7/256 (2.7) | 10/65 (15.4) | <0.001 | 0.005 | 0.007 |
| Pain or limitation of the hip on physical examination, n/n (%) | 406/2265 (17.9) | 48/280 (17.1) | 340/1667 (20.4) | 0.207 | 15/253 (5.9) | 3/65 (4.6) | 0.683 | <0.001 | 0.002 |
| Oral corticosteroids (current), n/n (%) | 256/2264 (11.3) | 37/279 (13.3) | 145/1666 (8.7) | 0.016 | 59/254 (23.2) | 15/65 (23.1) | 0.979 | 0.003 | <0.001 |
| csDMARDs (current), n/n (%) | 661/2269 (29.1) | 130/281 (46.3) | 353/1672 (21.1) | <0.001 | 148/252 (58.7) | 30/64 (46.9) | 0.088 | 0.004 | <0.001 |
| Anti-TNF treatment (current), n/n (%) | 364/2263 (16.1) | 61/278 (21.9) | 265/1667 (15.9) | 0.012 | 30/254 (11.8) | 8/64 (12.5) | 0.879 | 0.002 | 0.464 |
| CRP, mean (s.d.), mg/l | 8.6 (13.4) | 9.0 (15.0) | 8.7 (13.7) | 0.770 | 7.9 (10.2) | 5.8 (7.4) | 0.138 | 0.332 | 0.005 |
| ESR, mean (s.d.), mm/h | 18.0 (15.9) | 17.6 (16.4) | 18.0 (16.1) | 0.718 | 19.8 (15.2) | 14.0 (10.7) | 0.004 | 0.123 | 0.005 |
| ASDAS-CRP, mean (s.d.) | 2.5 (1.1) | 2.6 (1.1) | 2.5 (1.1) | 0.254 | 2.4 (1.1) | 1.9 (1.2) | 0.001 | 0.044 | <0.001 |
| VAS nocturnal pain last week, mean (s.d.), 0–10 | 3.6 (3.0) | 3.6 (2.9) | 3.8 (2.9) | 0.174 | 2.3 (2.9) | 1.9 (2.6) | 0.224 | <0.001 | <0.001 |
| VAS pain last week, mean (s.d.), 0–10 | 3.8 (2.8) | 3.8 (2.8) | 4.0 (2.7) | 0.197 | 2.5 (2.9) | 2.2 (2.7) | 0.430 | <0.001 | <0.001 |
| Patient global assessment last week, mean (s.d.), 0–10 | 4.4 (2.7) | 4.6 (2.6) | 4.5 (2.7) | 0.406 | 4.2 (2.8) | 3.3 (2.9) | 0.014 | 0.104 | <0.001 |
| Physician global assessment last week, mean (s.d.), 0–10 | 3.0 (2.1) | 3.4 (2.2) | 3.1 (2.1) | 0.053 | 2.5 (2.1) | 2.3 (2.2) | 0.450 | <0.001 | 0.002 |
| BASDAI, mean (s.d.), 0–10 | 4.0 (2.4) | 4.3 (2.4) | 4.0 (2.4) | 0.038 | 4.0 (2.5) | 2.9 (2.5) | 0.004 | 0.087 | <0.001 |
| BASFI, mean (s.d.), 0–10 | 3.5 (2.7) | 3.8 (2.6) | 3.6 (2.7) | 0.373 | 2.8 (2.6) | 1.9 (2.3) | 0.013 | <0.001 | <0.001 |
| SF-12 mental component, mean (s.d.), 0–100 | 47.8 (12.4) | 47.3 (13.5) | 47.9 (12.5) | 0.455 | 48.3 (1.9) | 47.5 (10.1) | 0.042 | 0.338 | 0.802 |
| SF-12 physical component, mean (s.d.), 0–100 | 35.7 (11.1) | 34.9 (11.7) | 35.6 (11.1) | 0.328 | 36.8 (10.5) | 39.7 (10.0) | 0.589 | 0.046 | 0.003 |
| . | . | Axial phenotypea . | Peripheral phenotypeb . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Overall population (n = 2296) . | Psoriatic axial phenotype (a) (n = 287) . | Non-psoriatic axial phenotype (b) (n = 1688) . | P-value . | Psoriatic peripheral phenotype (c) (n = 256) . | Non-psoriatic peripheral phenotype (d) (n = 65) . | P-value . | P-value psoriatic axial phenotype vs psoriatic peripheral phenotype (a vs c) . | P-value non-psoriatic axial phenotype vs non-psoriatic peripheral phenotype (b vs d) . |
| Number of swollen joints (current), mean (s.d.) | 0.5 (1.8) | 1.0 (2.7) | 0.4 (1.7) | <0.001 | 1.8 (3.7) | 0.9 (1.8) | 0.056 | 0.010 | 0.046 |
| MASES enthesis index (current), mean (s.d.) | 1.7 (1.9) | 2.3 (2.1) | 1.6 (1.9) | 0.005 | 0.7 (1.3) | 0.8 (0.8) | 0.871 | <0.001 | <0.001 |
| Heel enthesitis (current), n/n (%) | 144/2296 (6.3) | 24/287 (8.4) | 103/1688 (6.1) | 0.149 | 7/256 (2.7) | 10/65 (15.4) | <0.001 | 0.005 | 0.007 |
| Pain or limitation of the hip on physical examination, n/n (%) | 406/2265 (17.9) | 48/280 (17.1) | 340/1667 (20.4) | 0.207 | 15/253 (5.9) | 3/65 (4.6) | 0.683 | <0.001 | 0.002 |
| Oral corticosteroids (current), n/n (%) | 256/2264 (11.3) | 37/279 (13.3) | 145/1666 (8.7) | 0.016 | 59/254 (23.2) | 15/65 (23.1) | 0.979 | 0.003 | <0.001 |
| csDMARDs (current), n/n (%) | 661/2269 (29.1) | 130/281 (46.3) | 353/1672 (21.1) | <0.001 | 148/252 (58.7) | 30/64 (46.9) | 0.088 | 0.004 | <0.001 |
| Anti-TNF treatment (current), n/n (%) | 364/2263 (16.1) | 61/278 (21.9) | 265/1667 (15.9) | 0.012 | 30/254 (11.8) | 8/64 (12.5) | 0.879 | 0.002 | 0.464 |
| CRP, mean (s.d.), mg/l | 8.6 (13.4) | 9.0 (15.0) | 8.7 (13.7) | 0.770 | 7.9 (10.2) | 5.8 (7.4) | 0.138 | 0.332 | 0.005 |
| ESR, mean (s.d.), mm/h | 18.0 (15.9) | 17.6 (16.4) | 18.0 (16.1) | 0.718 | 19.8 (15.2) | 14.0 (10.7) | 0.004 | 0.123 | 0.005 |
| ASDAS-CRP, mean (s.d.) | 2.5 (1.1) | 2.6 (1.1) | 2.5 (1.1) | 0.254 | 2.4 (1.1) | 1.9 (1.2) | 0.001 | 0.044 | <0.001 |
| VAS nocturnal pain last week, mean (s.d.), 0–10 | 3.6 (3.0) | 3.6 (2.9) | 3.8 (2.9) | 0.174 | 2.3 (2.9) | 1.9 (2.6) | 0.224 | <0.001 | <0.001 |
| VAS pain last week, mean (s.d.), 0–10 | 3.8 (2.8) | 3.8 (2.8) | 4.0 (2.7) | 0.197 | 2.5 (2.9) | 2.2 (2.7) | 0.430 | <0.001 | <0.001 |
| Patient global assessment last week, mean (s.d.), 0–10 | 4.4 (2.7) | 4.6 (2.6) | 4.5 (2.7) | 0.406 | 4.2 (2.8) | 3.3 (2.9) | 0.014 | 0.104 | <0.001 |
| Physician global assessment last week, mean (s.d.), 0–10 | 3.0 (2.1) | 3.4 (2.2) | 3.1 (2.1) | 0.053 | 2.5 (2.1) | 2.3 (2.2) | 0.450 | <0.001 | 0.002 |
| BASDAI, mean (s.d.), 0–10 | 4.0 (2.4) | 4.3 (2.4) | 4.0 (2.4) | 0.038 | 4.0 (2.5) | 2.9 (2.5) | 0.004 | 0.087 | <0.001 |
| BASFI, mean (s.d.), 0–10 | 3.5 (2.7) | 3.8 (2.6) | 3.6 (2.7) | 0.373 | 2.8 (2.6) | 1.9 (2.3) | 0.013 | <0.001 | <0.001 |
| SF-12 mental component, mean (s.d.), 0–100 | 47.8 (12.4) | 47.3 (13.5) | 47.9 (12.5) | 0.455 | 48.3 (1.9) | 47.5 (10.1) | 0.042 | 0.338 | 0.802 |
| SF-12 physical component, mean (s.d.), 0–100 | 35.7 (11.1) | 34.9 (11.7) | 35.6 (11.1) | 0.328 | 36.8 (10.5) | 39.7 (10.0) | 0.589 | 0.046 | 0.003 |
Values shown in bold are considered significant: P-value <0.05. aAxial phenotype: defined as the presence of definite radiographic sacroiliitis according to the local reader OR presence of IBP according to the Calin criteria. bPeripheral phenotype: defined as absence of definite radiographic sacroiliitis according to the local reader AND absence of IBP AND presence of peripheral involvement (either arthritis, or enthesitis or dactylitis). Values shown in bold are considered significant. ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score; csDMARD: conventional synthetic DMARD; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; SF-12: short form of the Medical Outcomes Study Short Form-36; VAS: visual analogue scale.
Patients with peripheral phenotype: psoriatic vs non-psoriatic
The univariate analysis of patients with psoriatic peripheral phenotype (n = 256) compared with non-psoriatic peripheral phenotype (n = 65) is shown in Tables 1 and 2. Supplementary Tables S1 and S2, available at Rheumatology online, show the sensitivity analysis using a different definition of peripheral phenotype, which showed similar results to the main analysis. The multivariate analysis (Fig. 3A) showed that, among patients with peripheral phenotype and with adjustment for csDMARD intake, the presence of psoriasis is independently associated with a higher age at disease onset (years) (OR 1.05, 95% CI: 1.01, 1.08), a lower frequency of HLA-B27 positivity (OR 0.14, 95% CI: 0.06, 0.31) and a lower frequency of heel enthesitis (ever) (OR 0.22, 95% CI: 0.10, 0.50). Concerning the current status of the disease at the time of the study visit and adjusting for csDMARD intake (Fig. 3B), patients with psoriatic peripheral phenotype showed higher NSJ (OR 1.24, 95%CI: 1.01, 1.54), higher ESR (OR 1.03, 95% CI: 1.00, 1.06), higher ASDAS-CRP (OR 1.36, 95% CI: 1.00, 1.85) and lower prevalence of heel enthesitis (ever) (OR 0.11, 95% CI: 0.04, 0.36) compared with non-psoriatic peripheral patients.
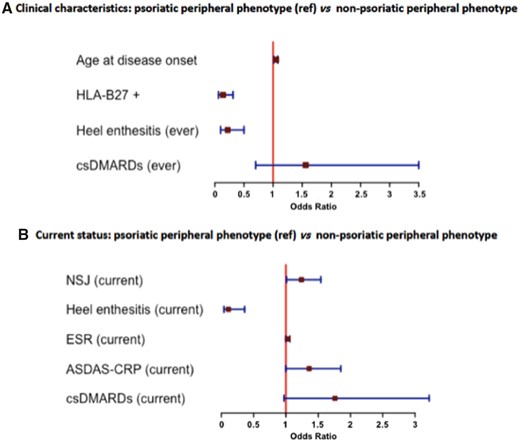
Comparison of peripheral phenotype patients with regard to the presence of psoriasis. Multivariate analysis
ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score; csDMARD: conventional synthetic DMARD; NSJ: number of swollen joints; ref.: reference.
Patients with psoriasis: axial vs peripheral phenotype
The axial and peripheral phenotypes among patients with psoriasis were also compared in Tables 1 and 2. Multivariate analysis (Fig. 4A) showed that, among patients with psoriasis, the axial phenotype was independently associated with being male (OR 2.04, 95% CI: 1.23, 3.71), longer diagnosis delay (OR 1.10, 95% CI: 1.04, 1.16), higher prevalence of employees (OR 1.86, 95% CI: 1.03, 3.38), HLA-B27 positivity (OR 5.25, 95% CI: 2.71, 10.17), positive family history of SpA (OR 2.79, 95% CI: 1.05, 7.44), heel enthesitis (OR 3.73, 95% CI: 1.86, 7.50), greater use of anti-TNF (OR 3.26, 95% CI: 1.44, 7.40) and lower use of csDMARDs (OR 0.40, 95% CI: 0.22, 0.73) compared with the peripheral phenotype. Multivariate analysis concerning the current status of the disease (Fig. 4B) showed that, among patients with psoriasis, the axial phenotype was independently associated with a higher MASES index (OR 1.50, 95% CI: 1.04, 2.16), higher ASDAS-CRP (OR 2.57, 95% CI: 1.49, 4.45), greater use of anti-TNF (OR 8.56, 95% CI: 1.54, 47.71) and lower use of corticosteroids (OR 0.08, 95% CI: 0.02, 0.39) at the time of the visit after adjusting for csDMARD intake, compared with the peripheral phenotype.
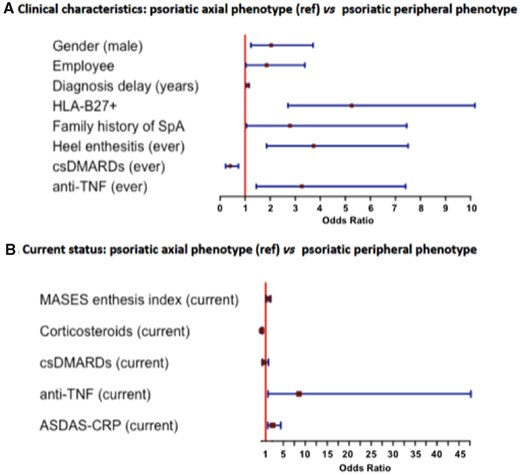
Comparison of psoriatic patients with regard to the phenotype. Multivariate analysis
ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score; csDMARD: conventional synthetic DMARD; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; ref.: reference.
Patients without psoriasis: axial vs peripheral phenotype
Finally, the axial and peripheral phenotypes among patients without psoriasis were also compared in Tables 1 and 2. Multivariate analysis (Supplementary Fig. S1A, available at Rheumatology online) demonstrated that, among non-psoriatic patients, the axial phenotype was independently associated with increased disease duration since symptoms onset (years) (OR 1.10, 95% CI: 1.06, 1.14), lower prevalence of heel enthesitis (OR 0.35, 95% CI: 0.20, 0.62), dactylitis (OR 0.38, 95% CI: 0.17, 0.82), IBD (OR 0.31, 95% CI: 0.15, 8.53), lower use of csDMARDs (OR 0.56, 95% CI: 0.32, 0.98), higher prevalence of good NSAIDs response (OR 3.23, 95% CI: 1.82, 5.73) and uveitis (OR 3.13, 95% CI: 1.15, 8.53). The multivariate analysis for the current status (Supplementary Fig. S1B, available at Rheumatology online) showed that, among non-psoriatic patients, the axial phenotype is associated with a higher MASES index (OR 2.28, 95% CI: 1.09, 4.79), higher ASDAS-CRP (OR 3.88, 95% CI: 1.29, 11.72), higher VAS nocturnal pain during the prior week (OR 1.45, 95% CI: 1.06, 1.99), poorer SF-12 physical component (OR 0.89, 95% CI: 0.81, 0.98), lower NSJ (OR 0.79, 95% CI: 0.63, 0.98), lower prevalence of heel enthesitis (OR 0.10, 95% CI: 0.02, 0.45), lower use of csDMARDs (OR 0.11, 95% CI: 0.04, 0.33) and lower levels of BASDAI (OR 0.48, 95% CI: 0.27, 0.85) compared with the peripheral phenotypes.
Discussion
In this study, we evaluated similarities and differences in the axial and peripheral SpA phenotypes with regard to the presence of psoriasis. To date, most studies have compared axSpA or AS with the whole group of PsA, but this is one of the first studies that independently evaluated the axial and peripheral phenotypes with and without psoriasis irrespective of the fulfilment of the CASPAR or mNY criteria and irrespective of the rheumatologist’s opinion.
The prevalence of psoriasis in this study was 23.6%, similar to what has been reported in the overall SpA patients, compared with the 2.3% described in the general Spanish population [15, 16]. This study showed a clear influence of psoriasis in the clinical expression of SpA, especially in patients with axial phenotype. Among the whole group of patients with axial phenotype, those with psoriasis showed a lower prevalence of HLA-B27 positivity and uveitis but a higher frequency of dactylitis and synovitis and a greater use of csDMARDs. The association between HLA-B27 antigen and axial involvement has been well stablished [17]; however, we demonstrate here that, in psoriatic patients with axial phenotype, the prevalence of this antigen was nearly half that in non-psoriatic patients with axial phenotype (48.7% and 81.7%, respectively). Interestingly, uveitis (an extra-musculoskeletal manifestation classically associated with axial phenotypes) was also less prevalent among psoriatic patients than among non-psoriatic patients across the whole group of patients with axial phenotype. This can be explained by the low prevalence of HLA-B27 in psoriatic patients, since uveitis is associated with the presence of HLA-B27 antigen [18]. These results also confirm the strong association between psoriasis and peripheral manifestations (synovitis and dactylitis) even in patients with axial phenotype. To date, the association between psoriasis and dactylitis has been evident among ‘classic’ PsA cases, but here we confirm that dactylitis is also a hallmark of psoriasis within the full spectrum of SpA (both axial and peripheral). It should be noted that, in the axial phenotype, BMI acts as a confounding factor, which means that BMI is associated with both the presence of psoriasis and the clinical manifestations. It is well stablished that increased BMI is associated with skin diseases. In fact, obesity has been postulated as an independent risk factor for psoriasis [19], and increased BMI is directly related to the severity of the psoriasis [20]. Obesity can exacerbate the clinical manifestations or even trigger the disease, due to the role of adipose tissue releasing pro-inflammatory cytokines and specific molecules such as leptin, adiponectin and resistin, which could contribute to the overall inflammatory burden [21]. Moreover, obesity in axSpA patients represents an additional challenge in both managing and diagnosis of the rheumatic disease, since the incidence of back pain and mechanical-related sacroiliitis is more frequent in this population.
The comparison of peripheral phenotypes with and without psoriasis also confirmed the low prevalence of HLA-B27 antigen among psoriatic patients. Moreover, psoriatic patients showed a later disease onset than non-psoriatic patients, with a mean age of symptoms onset of 41.3 years compared with 32.8 years. This can be explained, on one hand, by cumulative microdamage and vascular changes in the enthesis over time [22], and on the other hand, by different inducers of the inflammatory response among the patients with non-psoriatic peripheral phenotype, such as IBD or genito-urinary infections, which usually appear in younger people. It should be noted that, among psoriatic patients, the disease onset was earlier in axial vs peripheral phenotypes, which could be dependent on the HLA-B27 positivity [23], more frequently found in the axial phenotype group (48.7% vs 13.0%). It has been described that psoriatic patients presenting nail dystrophy (specifically nail pitting and onycholysis) are more likely to develop PsA and certain clinical manifestations than patients without nail involvement [24]. Unfortunately, we do not have the distinction between cutaneous or nail psoriasis to prove that, since this information was collected as a single data.
Interestingly, we found that, among patients with peripheral phenotype, heel enthesitis was more prevalent among non-psoriatic than psoriatic patients. Although heel enthesitis has been classically associated with psoriatic patients, to our knowledge, no studies have compared this feature between psoriatic and non-psoriatic patients. For this reason, further studies focused on peripheral phenotype patients could confirm these results.
Classically, root joint (i.e. hip and shoulder) involvement has also been considered a clinical manifestation associated with axial phenotype [25]. Our results confirm that hip and shoulder involvement is mainly associated with axial phenotype irrespective of the presence of psoriasis, while hip replacement was more frequent among axial psoriatic patients.
Overall, patients with psoriasis showed a more severe disease at the time of the study visit: greater use of drugs, higher disease activity and poorer quality of life. A significant association between skin disease severity and joint involvement has been reported in previous studies [26]. However, this finding could not be confirmed in this study since the REGISPONSER registry did not include information concerning the severity of the psoriasis or the number of tender joints. The greater use of drugs found in this study could be explained by the presence of psoriasis and the necessity of specific csDMARDs or anti-TNF to treat cutaneous involvement; alternatively, it could be explained by the higher prevalence of peripheral manifestations, which have been demonstrated as an independent factor associated with anti-TNF initiation in other cohorts [27]. However, data concerning the specific indication of biologic DMARDs (bDMARDs; either for cutaneous or for rheumatic disease) was not available to demonstrate these two hypotheses. It should be noted that information about other new bDMARDs use (such as anti-interleukin 17 or anti-interleukin 12/23) was not collected since data were recorded between 2004 and 2007 when only anti-TNF drugs were available. These results suggest that patients with psoriasis are more likely to suffer more severe disease than non-psoriatic patients.
This study has some limitations in addition to its strengths. One limitation is the use of local X-ray reading to classify patients as positive or negative for X-ray sacroiliitis. However, this approach mimics clinical practice, in which only one rheumatologist evaluates X-ray images. A second limitation is that we did not analysed axial radiographic scores, such as the number of syndesmophytes or bone bridges, since this information was not collected in this registry. Another limitation is the cross-sectional nature of the study, which does not allow for comparison of the natural history between psoriatic and non-psoriatic patients. One weakness of this study is that we did not classify patients according to mNY or CASPAR criteria because this could induce an artificial classification of patients (who may fulfil both classification sets in many cases, as was demonstrated in some previous studies) [28]. For this reason, we decided not to consider these classification criteria and to group patients as having axial or peripheral phenotype using their clinical findings, which allowed for a better evaluation of how psoriasis is associated with the clinical expression of the disease within the full spectrum of SpA. Moreover, we excluded patients who did not fulfil the ESSG criteria from the analysis to ensure that all patients had SpA. Another weakness is that we conducted a sensitivity analysis considering as axial phenotype only patients with radiographic sacroiliitis, and we indeed found the same results as those in the main analysis (which considered as axial phenotype patients with radiographic sacroiliitis or IBP). Finally, another weakness is that the multivariate analyses were adjusted for treatment intake, since drugs can modify the prevalence of the clinical manifestations of the disease as well as the burden of disease at the time of the study visit.
In summary, this study suggests that, across the whole group of SpA, psoriasis is associated with differences in clinical disease expression, a greater disease burden and increased use of drugs. With these results, we cannot conclude that SpA with psoriasis is a distinct disease from SpA without psoriasis, since only genetic, epigenetic and transcriptomic studies can address this question. However, we can affirm that psoriasis plays a key role in the clinical disease expression in both the axial and peripheral phenotypes.
Acknowledgements
We dedicate this work to the memory of our colleague and friend Dr María del Carmen Castro-Villegas, co-author of this study, who passed away during the peer-review process of this manuscript. She will be present in her legacy.
REGISPONSER group—listed are the principal investigators: Hu. Reina Sofía: Eduardo Collantes Estévez; H. Bellvitge: Xavier Juanola Roura; Hu. Juan Canalejo: Jose Luis Fernández Sueiro; HU. Gregorio Marañón: Carlos González Fernández; H. Parc Taulí: Jordi Gratacós Masmitjá; Clínica Puerta Hierro: Juan Mulero Mendoza; H. Monte Naranco: Juan Carlos Torre Alonso; H. Doce de Octubre: Pilar Fernández Dapica; H. Ramón y Cajal: Ma Elia Brito Brito; HU. Alicante: Enrique Batlle Gualda; H. Virgen de la Arrixaca: Luis F. Linares Ferrando; H. Virgen del Perpetuo Socorro: Enrique Judez Navarro; H.G. San Jorge: Carlos Vázquez Galeano; H. de Palamós: Teresa Clavaguera Poch; H. Mostoles: M. Cruz Fernández Espartero; HU. Carlos Haya: Enrique Calero Secall; H. Mutua de Terrassa: Manuel Pujol Busquets; H. Doctor Negrín: Carlos Rodríguez Lozano; H. Santa María del Rosell: Manuel J. Moreno Ramos; HU. Príncipe de Asturias: Eduardo Cuende Quintana; HU. de Guadalajara: Manuel Fernández Prada; HU. Central de Asturias: Rubén Queiro Silva; H. San Rafael: Estefanía Moreno Ruzafa; HU Virgen de la Vega: Carlos Montilla Morales; H. Virgen del Rocío: Alicia García López; HU. Miguel Servet: Eugenio Giménez Úbeda; H. Fundación Son Llatzer: Antonio Juan Más; H. Internacional Merimar: Cristina Medrano Le Quement; HU. Navarra: Enrique Ornilla.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
Author notes
Clementina López-Medina, Rafaela Ortega-Castro, Ruxandra Schiotis and Eduardo Collantes-Estévez contributed equally to this manuscript.




Comments