-
PDF
- Split View
-
Views
-
Cite
Cite
Dario Camellino, Christina Duftner, Christian Dejaco, New insights into the role of imaging in polymyalgia rheumatica, Rheumatology, Volume 60, Issue 3, March 2021, Pages 1016–1033, https://doi.org/10.1093/rheumatology/keaa646
Close - Share Icon Share
Abstract
PMR is an inflammatory rheumatic disease of elderly people characterized by pain and stiffness in the neck, shoulder and pelvic girdles. No specific diagnostic confirmatory tests exist and clinical symptoms, as well as increased acute phase reactants, are unspecific. The diagnostic value of imaging including ultrasound, MRI and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) with/without CT for PMR is increasingly studied. These techniques, particularly FDG-PET/CT, may help to detect underlying GCA in PMR patients with an incomplete response to glucocorticoids and/or recurrent relapses. Recent imaging studies provide novel insights into the anatomical basis of inflammation in PMR, particularly at hip and spine, which may help to distinguish this disease from other mimicking conditions. In this review, we discuss novel insights into the pathoanatomy of PMR, compare the diagnostic values of different imaging techniques and summarize current data on the role of imaging for monitoring and outcome prediction.
• Imaging techniques, particularly MRI and PET, provide novel insights into the anatomical basis of PMR.
• Novel lesions in PMR include inflammation of peritendons at hips and interspinous bursae.
• The role of subclinical vasculitis (30–60% of PMR patients) for treatment and outcomes is unknown.
Introduction
PMR is an inflammatory rheumatic disease of elderly people characterized by pain and stiffness in the neck, shoulder and pelvic girdles [1]. No specific diagnostic confirmatory tests exist for this disease, and clinical symptoms, as well as increased acute phase reactants, are unspecific. The diagnostic value of imaging, including ultrasound, MRI and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) with or without CT, is increasingly studied. The provisional ACR/EULAR classification criteria for PMR were the first criteria in rheumatology that included ultrasound in the algorithm [2]. In the study to develop these criteria, ultrasound was more specific to distinguish PMR from non-inflammatory shoulder conditions than to differentiate it from RA [2].
Although most patients with PMR initially respond well to glucocorticoid treatment, up to 50% of them relapse once glucocorticoids are tapered [3]. Imaging, particularly FDG-PET/CT, may help to detect underlying GCA in those with an incomplete response to glucocorticoid therapy and/or recurrent relapses [4]. Monitoring of treatment response and disease relapse, particularly in patients with unspecific or doubtful symptoms, as well as the characterization of those who are at risk for prolonged treatment, are additional applications of imaging that are increasingly investigated [5, 6].
Recent imaging studies provided novel insights into the anatomical basis of inflammation in PMR patients. These data mainly result from MRI and FDG-PET, as ultrasound is limited in its penetration to the body and restricted in the acoustic window with difficulties to assess deep bursae and tendinous structures, particularly at the hips. In addition, contrast or tracer accumulation in MRI or FDG-PET scans, respectively, allows the detection of mild inflammatory changes that are hardly visible by grey-scale or Power Doppler (PD) sonography.
In the present review, we discuss novel insights into the pathoanatomy of PMR, compare the diagnostic values of different imaging techniques and summarize current data on the role of imaging for monitoring and outcome prediction.
‘Traditional’ imaging lesions in PMR
Early ultrasound and MRI studies indicated that subacromial/subdeltoid (SAD) bursitis, as well as biceps tenosynovitis (even though this is probably not a true tenosynovitis because of communication of the tendon sheath with the glenohumeral joint), usually detected bilaterally, are the most common lesions in PMR (bilateral SAD bursitis occurring in 32–93% and biceps tenosynovitis in 37–60% of patients) [7]. At the pelvis, trochanteric bursitis was deemed as being the most important lesion, observed in up to 100% of patients unilaterally and 90% bilaterally with ultrasound or MRI [8]. Bilateral glenohumeral and coxofemoral synovitis were found in 26–52% and 18–32% of patients, respectively [7]. Experience from clinical practice and data from the study to develop the provisional ACR/EULAR classification criteria for PMR, however, indicated that trochanteric bursitis is much less common, detected in only about 20% of PMR patients by ultrasound [2]. The reason for this divergence is unknown, but selection bias might have played a role.
Bursitis in PMR is usually mild, whereas extensive synovial proliferation should prompt suspicion for an alternative diagnosis, particularly RA [9]. While contrast enhancement or tracer uptake in extra-synovial tissues, using MRI or FDG-PET, are characteristic for PMR, the relevance of PD signals at these sites has only occasionally been investigated. In one study, one-third of PMR patients revealed a positive PD in SAD bursae, and the presence of PD signals at diagnosis was associated with relapses/recurrences at follow-up [10]. In another study, PD at shoulders occurred in 7% of PMR patients only; however, the presence of PD at peripheral joints such as wrists made a diagnosis of PMR unlikely [11].
Synovitis in PMR is usually non-erosive, whereas destructive arthritis is a strong indicator for RA. Similarly, calcium pyrophosphate (CPP) deposition in cartilage, the rotator cuff or other tendons are not typically seen in PMR but indicate underlying chondrocalcinosis [11, 12].
Modern imaging indicates novel anatomical targets of inflammation in PMR
Blockmans et al. were the first to describe FDG uptake around the vertebral spinous processes reflecting interspinous bursitis in ∼50% out of a cohort of 35 PMR patients [13]. See Fig. 1 for representative image examples. This finding was later confirmed by others using FDG-PET [14–16] or ultrasound [17]. Surprisingly, inflammation of interspinous bursae at the cervical and lumbar spine was only inconsistently associated with neck and lumbar pain or stiffness [16].
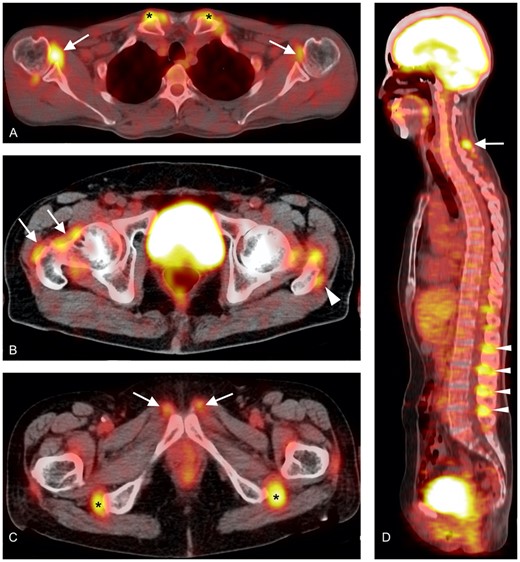
Characteristics of inflammatory lesions detected by FDG-PET/CT
(A): inflammation of the shoulders (white arrows) and the sternoclavicular joints (black asterisks). (B): inflammation around the hip joint (white arrows) and of the trochanteric bursa (white arrowhead). (C): inflammation at the level of the symphysis pubis (white arrows) and ischiatic tuberosities (black asterisks). (D): inflammation of the cervical (white arrow) and lumbar (white arrowheads) interspinous bursae.
Other targets of inflammation identified by FDG-PET include sternoclavicular joints, knees and extracapsular sites at shoulders and hips [14, 18–20]. Given the limited special resolution of FDG-PET/CT, the exact localization of inflammatory changes to the anatomical structure has been challenging. In one study, three out of 22 patients with new-onset PMR, who were investigated with FDG-PET/CT, also underwent an MRI scan of pelvis, knees and hands to overcome this problem [21]. At the hips, bilateral symmetric oedema surrounding the proximal hamstring tendons origin was observed in correspondence to FDG uptake at the ischial tuberosity. These changes were confined to the paratenon, with only minimally increased signal noted in the tendons themselves. There was no abnormality at the musculotendinous junctions, nor was there any bone oedema. The authors concluded that hamstring peritendonitis might be the anatomical correlate of FDG uptake at this site, rather than ischiatic bursitis as previously thought. At the knees, inflammation was also limited to the paratenon of the semimembranous and gracilis tendons and did not primarily involve synovia or bursae.
In another retrospective study of 40 PMR patients, MRI of the pelvis was performed [22]. Ten of these 40 patients were later re-classified as RA; however, the exclusion of these patients from the primary analysis did not impact the results. Peri- and intra-tendinous enhancement of tendons of the gluteus medius and minimus muscles at the insertion into the major trochanter, of the rectus femoris muscle at the proximal insertion and of the adductor origins at the inferomedial pubic symphysis were the most common findings (see Fig. 2 for image examples). Isolated synovial inflammation was not observed, neither at joints nor at bursae [22, 23]. Particularly at the greater trochanter, where inflammation was common, it was even difficult to differentiate bursitis from peritendinous enhancement. The authors concluded that the outer lining of the tendinous and capsular structures, which may correspond to the peritendineum externum and internum that continues into the perimysium, is the most likely primary site of inflammation in PMR that only secondarily extends to bursae and joints.
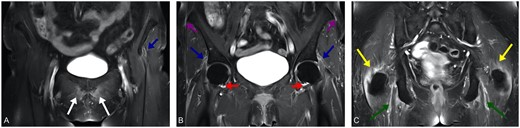
Characteristics inflammatory lesions detected by MRI
Coronal contrast-enhanced T1w TSE FS MRI of the pelvis at the level of the pubic symphysis (A), hip joint (B) and ischial tuberosity (C) reveals symmetrical peritendinous inflammation of numerous pelvic girdle muscles including straight and reflected head of rectus femoris muscle (blue arrows) as well as adductor longus muscle origins (white arrows), hamstring tendon at the ischial tuberosity (green arrows), iliotibial tract at the iliac crest (violet arrows) and gluteus medius muscle tendon at greater trochanter (yellow arrows). The extensive extracapsular inflammation is accompanied by only little synovitis of the hip joint (red arrows). With courtesy from Martin Fruth, blikk Radiologie am Rheumazentrum Ruhrgebiet, Herne, Germany.
Using whole-body multiple-joint MRI, two different patterns were observed in a cohort of 22 PMR patients: a predominant ‘extracapsular’ uptake suggesting inflammation mainly at extracapsular sites adjacent to the joint capsule and soft tissues, and a ‘non-extracapsular’ pattern with predominant synovial inflammation [24]. While both groups were similar regarding clinical manifestations and initial disease activity, patients with an extracapsular pattern had a more favourable response to glucocorticoid treatment as outlined below. In an earlier MRI study, predominant extracapsular inflammation adjacent to the joint capsule and soft tissues at shoulders was the only finding that distinguished PMR from RA patients, whereas joint effusion and tenosynovitis were comparable in both groups [25].
A comparison of the prevalence of different inflammatory lesions in PMR according to different imaging techniques is depicted in Table 1.
Prevalence of different imaging findings from selected studies published from 2015 to 2020
| Author . | Year . | Imaging technique . | Patients (n) . | Controls (n) . | Main diagnoses in controls . | Anatomic lesion . | Prevalence in patients at baseline . | Prevalence in controls at baseline . | Prevalence in patients during follow-up . | Prevalence in controls during follow-up . | Follow-up (weeks) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ottaviani [26] | 2020 | US | 27 | 25 | CPPDa | Subacromial-subdeltoid bursitis | 96% | 68% | N/A | N/A | N/A |
| Long-head biceps tenosynovitis | 85% | 72% | N/A | N/A | N/A | ||||||
| Glenohumeral effusion | 48% | 68% | N/A | N/A | N/A | ||||||
| Humeral erosion | 7% | 44% | N/A | N/A | N/A | ||||||
| Acromioclavicular synovitis | 7% | 64% | N/A | N/A | N/A | ||||||
| Acromioclavicular chondrocalcinosis | 4% | 92% | N/A | N/A | N/A | ||||||
| Laporte [27] | 2019 | MRI | 15 | N/A | N/A | Shoulder myofascial lesionsb | 71% | N/A | 53% | N/A | 12 |
| Hip myofascial lesionsb | 87% | N/A | 33% | N/A | 12 | ||||||
| Pubic symphysis myofascial lesionsb | 80% | N/A | 20% | N/A | 12 | ||||||
| Ischial tuberosities myofascial lesionsb | 60% | N/A | 0% | N/A | 12 | ||||||
| Fruth [22] | 2020 | MRI (contrast-enhanced) | 40 | 80 | Mixed (Deg 26%; RA 26%; SpA 20%) | Common ischiocrural tendon origin at the ischial tuberosity | 100% | 56% | N/A | N/A | N/A |
| Glutaeus medius and minimus muscles tendons at the greater trochanter | 99.5% | 88% | N/A | N/A | N/A | ||||||
| Origin of the rectus femoris muscle | 96.5% | 4.5% | N/A | N/A | N/A | ||||||
| Origin of the adductor longus muscle | 92.5% | 5% | N/A | N/A | N/A | ||||||
| Interspinous bursae and origin of the paraspinous muscles | 68% | 24% | N/A | N/A | N/A | ||||||
| Nakamura [28] | 2020 | MRI (contrast-enhanced) | 58 | 79 | Mixed (RA 35%; Rot 13%; ReA 13%) | Enhancement of rotator cuff tendon | 72% | 32% | N/A | N/A | N/A |
| Enhancement of shoulder joint capsule | 69% | 35% | N/A | N/A | N/A | ||||||
| Shoulder joint effusion | 64% | 44% | N/A | N/A | N/A | ||||||
| Focal bone oedema in humerus head | 59% | 19% | N/A | N/A | N/A | ||||||
| Enhancement of biceps tendon | 12% | 6% | N/A | N/A | N/A | ||||||
| Shoulder synovial hypertrophy | 12% | 15% | N/A | N/A | N/A | ||||||
| Enhancement of glenohumeral joint | 5% | 10% | N/A | N/A | N/A | ||||||
| Diffuse bone oedema in humerus head | 2% | 9% | N/A | N/A | N/A | ||||||
| US | Biceps tenosynovitis | 43% | 23% | N/A | N/A | N/A | |||||
| Subdeltoid bursitis | 32% | 12% | N/A | N/A | N/A | ||||||
| Glenohumeral synovitis | 11% | 11% | N/A | N/A | N/A | ||||||
| Takahashi [20] | 2015 | FDG-PET/CT | 27 | 10 | Elderly-onset RA | Circular/linear uptake around the humeral headsc | 30% | 90% | N/A | N/A | N/A |
| Interspinous bursae/spinous processesc | 81% | 65% | N/A | N/A | N/A | ||||||
| Wristsc | 41% | 100% | N/A | N/A | N/A | ||||||
| Iliopectineal bursaec | 41% | 10% | N/A | N/A | N/A | ||||||
| Isolated uptake in front of the femoral headsc | 59% | 10% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 96% | 60% | N/A | N/A | N/A | ||||||
| Rehak [29] | 2015 | FDG-PET or FDG-PET/CT | 67 | Shouldersd | 87% | N/A | N/A | N/A | N/A | ||
| Sternoclavicular jointsd | 46% | N/A | N/A | N/A | N/A | ||||||
| Cervical interspinous bursaed | 19% | N/A | N/A | N/A | N/A | ||||||
| Lumbar interspinous bursaed | 57% | N/A | N/A | N/A | N/A | ||||||
| Hipsd | 70% | N/A | N/A | N/A | N/A | ||||||
| Ischiatic tuberositiesd | 52% | N/A | N/A | N/A | N/A | ||||||
| Vasculard | 40% | N/A | N/A | N/A | N/A | ||||||
| Sondag [30] | 2016 | FDG-PET/CT | 50 | 53 | Neoplasia | Shouldersc | 58% | 15% | N/A | N/A | N/A |
| Acromioclavicular jointsc | 26% | 7% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsc | 24% | 5% | N/A | N/A | N/A | ||||||
| Spinous processesc | 58% | 11% | N/A | N/A | N/A | ||||||
| Greater trochantersc | 40% | 15% | N/A | N/A | N/A | ||||||
| Hipsc | 38% | 15% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 55% | 8% | N/A | N/A | N/A | ||||||
| Symphysis pubis enthesesc | 22% | 1% | N/A | N/A | N/A | ||||||
| Iliopectinal bursaec | 31% | 7% | N/A | N/A | N/A | ||||||
| Palard-Novello [31] | 2016 | FDG-PET/CT | 18 | Shoulderse | 89% | N/A | N/A | N/A | N/A | ||
| Sternoclaviculare | 72% | N/A | N/A | N/A | N/A | ||||||
| Cervical spinous processese | 56% | N/A | N/A | N/A | N/A | ||||||
| Lumbar spinous processese | 72% | N/A | N/A | N/A | N/A | ||||||
| Hipse | 94% | N/A | N/A | N/A | N/A | ||||||
| Ischial tuberositiese | 94% | N/A | N/A | N/A | N/A | ||||||
| Vasculare | 0% | N/A | N/A | N/A | N/A | ||||||
| Henckaerts [14] | 2018 | FDG-PET or FDG-PET/CT | 67 | 32 | Mixed (Rot 25%; OA 16%; RA 9%) | Shouldersf | 96% | 87% | N/A | N/A | N/A |
| Sternoclavicular jointsf | 41% | 11% | N/A | N/A | N/A | ||||||
| Cervical spinous processesf | 75% | 13% | N/A | N/A | N/A | ||||||
| Lumbar spinous processesf | 46% | 0% | N/A | N/A | N/A | ||||||
| Hipsf | 65% | 12% | N/A | N/A | N/A | ||||||
| Greater trochantersf | 78% | 44% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesf | 65% | 10% | N/A | N/A | N/A | ||||||
| Carotid arteriesf | 4% | 0% | N/A | N/A | N/A | ||||||
| Subclavian arteriesf | 12% | 6% | N/A | N/A | N/A | ||||||
| Thoracic aortaf | 6% | 0% | N/A | N/A | N/A | ||||||
| Abdominal aortaf | 0% | 0% | N/A | N/A | N/A | ||||||
| Owen [32] | 2020 | FDG-PET/CT | 33 | 132 | Mixed (Neo 64%, RA 5%) | Shoulders (peri-articular)g | 97% | 78% | N/A | N/A | N/A |
| Glenohumeral jointsg | 73% | 4% | N/A | N/A | N/A | ||||||
| Acromioclavicular jointsg | 100% | 66% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsg | 88% | 57% | N/A | N/A | N/A | ||||||
| Interspinous bursaeg | 91% | 17% | N/A | N/A | N/A | ||||||
| Hips (peri-articular)g | 97% | 61% | N/A | N/A | N/A | ||||||
| Hip jointsg | 61% | 20% | N/A | N/A | N/A | ||||||
| Trochanteric bursaeg | 100% | 75% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesg | 100% | 58% | N/A | N/A | N/A | ||||||
| Knee jointsg | 53% | 54% | N/A | N/A | N/A | ||||||
| Knees (posteromedial)g | 73% | 20% | N/A | N/A | N/A |
| Author . | Year . | Imaging technique . | Patients (n) . | Controls (n) . | Main diagnoses in controls . | Anatomic lesion . | Prevalence in patients at baseline . | Prevalence in controls at baseline . | Prevalence in patients during follow-up . | Prevalence in controls during follow-up . | Follow-up (weeks) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ottaviani [26] | 2020 | US | 27 | 25 | CPPDa | Subacromial-subdeltoid bursitis | 96% | 68% | N/A | N/A | N/A |
| Long-head biceps tenosynovitis | 85% | 72% | N/A | N/A | N/A | ||||||
| Glenohumeral effusion | 48% | 68% | N/A | N/A | N/A | ||||||
| Humeral erosion | 7% | 44% | N/A | N/A | N/A | ||||||
| Acromioclavicular synovitis | 7% | 64% | N/A | N/A | N/A | ||||||
| Acromioclavicular chondrocalcinosis | 4% | 92% | N/A | N/A | N/A | ||||||
| Laporte [27] | 2019 | MRI | 15 | N/A | N/A | Shoulder myofascial lesionsb | 71% | N/A | 53% | N/A | 12 |
| Hip myofascial lesionsb | 87% | N/A | 33% | N/A | 12 | ||||||
| Pubic symphysis myofascial lesionsb | 80% | N/A | 20% | N/A | 12 | ||||||
| Ischial tuberosities myofascial lesionsb | 60% | N/A | 0% | N/A | 12 | ||||||
| Fruth [22] | 2020 | MRI (contrast-enhanced) | 40 | 80 | Mixed (Deg 26%; RA 26%; SpA 20%) | Common ischiocrural tendon origin at the ischial tuberosity | 100% | 56% | N/A | N/A | N/A |
| Glutaeus medius and minimus muscles tendons at the greater trochanter | 99.5% | 88% | N/A | N/A | N/A | ||||||
| Origin of the rectus femoris muscle | 96.5% | 4.5% | N/A | N/A | N/A | ||||||
| Origin of the adductor longus muscle | 92.5% | 5% | N/A | N/A | N/A | ||||||
| Interspinous bursae and origin of the paraspinous muscles | 68% | 24% | N/A | N/A | N/A | ||||||
| Nakamura [28] | 2020 | MRI (contrast-enhanced) | 58 | 79 | Mixed (RA 35%; Rot 13%; ReA 13%) | Enhancement of rotator cuff tendon | 72% | 32% | N/A | N/A | N/A |
| Enhancement of shoulder joint capsule | 69% | 35% | N/A | N/A | N/A | ||||||
| Shoulder joint effusion | 64% | 44% | N/A | N/A | N/A | ||||||
| Focal bone oedema in humerus head | 59% | 19% | N/A | N/A | N/A | ||||||
| Enhancement of biceps tendon | 12% | 6% | N/A | N/A | N/A | ||||||
| Shoulder synovial hypertrophy | 12% | 15% | N/A | N/A | N/A | ||||||
| Enhancement of glenohumeral joint | 5% | 10% | N/A | N/A | N/A | ||||||
| Diffuse bone oedema in humerus head | 2% | 9% | N/A | N/A | N/A | ||||||
| US | Biceps tenosynovitis | 43% | 23% | N/A | N/A | N/A | |||||
| Subdeltoid bursitis | 32% | 12% | N/A | N/A | N/A | ||||||
| Glenohumeral synovitis | 11% | 11% | N/A | N/A | N/A | ||||||
| Takahashi [20] | 2015 | FDG-PET/CT | 27 | 10 | Elderly-onset RA | Circular/linear uptake around the humeral headsc | 30% | 90% | N/A | N/A | N/A |
| Interspinous bursae/spinous processesc | 81% | 65% | N/A | N/A | N/A | ||||||
| Wristsc | 41% | 100% | N/A | N/A | N/A | ||||||
| Iliopectineal bursaec | 41% | 10% | N/A | N/A | N/A | ||||||
| Isolated uptake in front of the femoral headsc | 59% | 10% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 96% | 60% | N/A | N/A | N/A | ||||||
| Rehak [29] | 2015 | FDG-PET or FDG-PET/CT | 67 | Shouldersd | 87% | N/A | N/A | N/A | N/A | ||
| Sternoclavicular jointsd | 46% | N/A | N/A | N/A | N/A | ||||||
| Cervical interspinous bursaed | 19% | N/A | N/A | N/A | N/A | ||||||
| Lumbar interspinous bursaed | 57% | N/A | N/A | N/A | N/A | ||||||
| Hipsd | 70% | N/A | N/A | N/A | N/A | ||||||
| Ischiatic tuberositiesd | 52% | N/A | N/A | N/A | N/A | ||||||
| Vasculard | 40% | N/A | N/A | N/A | N/A | ||||||
| Sondag [30] | 2016 | FDG-PET/CT | 50 | 53 | Neoplasia | Shouldersc | 58% | 15% | N/A | N/A | N/A |
| Acromioclavicular jointsc | 26% | 7% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsc | 24% | 5% | N/A | N/A | N/A | ||||||
| Spinous processesc | 58% | 11% | N/A | N/A | N/A | ||||||
| Greater trochantersc | 40% | 15% | N/A | N/A | N/A | ||||||
| Hipsc | 38% | 15% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 55% | 8% | N/A | N/A | N/A | ||||||
| Symphysis pubis enthesesc | 22% | 1% | N/A | N/A | N/A | ||||||
| Iliopectinal bursaec | 31% | 7% | N/A | N/A | N/A | ||||||
| Palard-Novello [31] | 2016 | FDG-PET/CT | 18 | Shoulderse | 89% | N/A | N/A | N/A | N/A | ||
| Sternoclaviculare | 72% | N/A | N/A | N/A | N/A | ||||||
| Cervical spinous processese | 56% | N/A | N/A | N/A | N/A | ||||||
| Lumbar spinous processese | 72% | N/A | N/A | N/A | N/A | ||||||
| Hipse | 94% | N/A | N/A | N/A | N/A | ||||||
| Ischial tuberositiese | 94% | N/A | N/A | N/A | N/A | ||||||
| Vasculare | 0% | N/A | N/A | N/A | N/A | ||||||
| Henckaerts [14] | 2018 | FDG-PET or FDG-PET/CT | 67 | 32 | Mixed (Rot 25%; OA 16%; RA 9%) | Shouldersf | 96% | 87% | N/A | N/A | N/A |
| Sternoclavicular jointsf | 41% | 11% | N/A | N/A | N/A | ||||||
| Cervical spinous processesf | 75% | 13% | N/A | N/A | N/A | ||||||
| Lumbar spinous processesf | 46% | 0% | N/A | N/A | N/A | ||||||
| Hipsf | 65% | 12% | N/A | N/A | N/A | ||||||
| Greater trochantersf | 78% | 44% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesf | 65% | 10% | N/A | N/A | N/A | ||||||
| Carotid arteriesf | 4% | 0% | N/A | N/A | N/A | ||||||
| Subclavian arteriesf | 12% | 6% | N/A | N/A | N/A | ||||||
| Thoracic aortaf | 6% | 0% | N/A | N/A | N/A | ||||||
| Abdominal aortaf | 0% | 0% | N/A | N/A | N/A | ||||||
| Owen [32] | 2020 | FDG-PET/CT | 33 | 132 | Mixed (Neo 64%, RA 5%) | Shoulders (peri-articular)g | 97% | 78% | N/A | N/A | N/A |
| Glenohumeral jointsg | 73% | 4% | N/A | N/A | N/A | ||||||
| Acromioclavicular jointsg | 100% | 66% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsg | 88% | 57% | N/A | N/A | N/A | ||||||
| Interspinous bursaeg | 91% | 17% | N/A | N/A | N/A | ||||||
| Hips (peri-articular)g | 97% | 61% | N/A | N/A | N/A | ||||||
| Hip jointsg | 61% | 20% | N/A | N/A | N/A | ||||||
| Trochanteric bursaeg | 100% | 75% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesg | 100% | 58% | N/A | N/A | N/A | ||||||
| Knee jointsg | 53% | 54% | N/A | N/A | N/A | ||||||
| Knees (posteromedial)g | 73% | 20% | N/A | N/A | N/A |
Results originally reported separately for the right and left side, are presented as the mean of the two values. aPatients with CPPD also fulfilled the ACR/EULAR classification criteria for PMR. bThe reported data refer specifically to myofascial lesions, defined as high T2 STIR signal surrounding the muscle or diffuse within it. Examined muscles were, in the shoulder girdle, the infraspinatus, supraspinatus, subscapularis, and teres minor; in the pelvic girdle, the glutaeal, iliopsoas and pelvitrochanteric; at the level of ischial tuberosities, the semitendinosus, semimembranosus, and long head of the biceps femoris; at the pubic symphysis, the adductors. This study did not evaluate enthesitis, tendinitis, bursitis. cThe uptake of the examined structures was considered abnormal if similar or higher than the uptake of the liver (i.e. ≥2 on a 4-point scale). dThe uptake of the examined structures was considered abnormal if higher than the uptake of the liver. Assessed arteries were the ascending aorta, descending aorta, abdominal aorta, brachiocephalic trunk, subclavian, common iliac, and common femoral arteries. eThe uptake within a region of interest (ROI) was considered abnormal if its maximal standardized uptake value (SUV) was higher than the maximal SUV of the reference ROI of the liver. The reported prevalence refers to bilateral findings, except for the spinal spinous processes and the arteries. fThe uptake of the examined structures was considered abnormal if similar or higher than the uptake of the liver (i.e. 2 on a 3-point scale). gThe uptake of the examined structures was considered abnormal even if lower in intensity than the uptake of the liver (i.e. ≥1 on a 4-point scale). Deg: degenerative disc/joint disorder; N/A: not available; Neo: neoplasia; ReA: reactive arthritis; Rot: rotator cuff disease; SpA: spondyloarthritis; US: ultrasonography.
Prevalence of different imaging findings from selected studies published from 2015 to 2020
| Author . | Year . | Imaging technique . | Patients (n) . | Controls (n) . | Main diagnoses in controls . | Anatomic lesion . | Prevalence in patients at baseline . | Prevalence in controls at baseline . | Prevalence in patients during follow-up . | Prevalence in controls during follow-up . | Follow-up (weeks) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ottaviani [26] | 2020 | US | 27 | 25 | CPPDa | Subacromial-subdeltoid bursitis | 96% | 68% | N/A | N/A | N/A |
| Long-head biceps tenosynovitis | 85% | 72% | N/A | N/A | N/A | ||||||
| Glenohumeral effusion | 48% | 68% | N/A | N/A | N/A | ||||||
| Humeral erosion | 7% | 44% | N/A | N/A | N/A | ||||||
| Acromioclavicular synovitis | 7% | 64% | N/A | N/A | N/A | ||||||
| Acromioclavicular chondrocalcinosis | 4% | 92% | N/A | N/A | N/A | ||||||
| Laporte [27] | 2019 | MRI | 15 | N/A | N/A | Shoulder myofascial lesionsb | 71% | N/A | 53% | N/A | 12 |
| Hip myofascial lesionsb | 87% | N/A | 33% | N/A | 12 | ||||||
| Pubic symphysis myofascial lesionsb | 80% | N/A | 20% | N/A | 12 | ||||||
| Ischial tuberosities myofascial lesionsb | 60% | N/A | 0% | N/A | 12 | ||||||
| Fruth [22] | 2020 | MRI (contrast-enhanced) | 40 | 80 | Mixed (Deg 26%; RA 26%; SpA 20%) | Common ischiocrural tendon origin at the ischial tuberosity | 100% | 56% | N/A | N/A | N/A |
| Glutaeus medius and minimus muscles tendons at the greater trochanter | 99.5% | 88% | N/A | N/A | N/A | ||||||
| Origin of the rectus femoris muscle | 96.5% | 4.5% | N/A | N/A | N/A | ||||||
| Origin of the adductor longus muscle | 92.5% | 5% | N/A | N/A | N/A | ||||||
| Interspinous bursae and origin of the paraspinous muscles | 68% | 24% | N/A | N/A | N/A | ||||||
| Nakamura [28] | 2020 | MRI (contrast-enhanced) | 58 | 79 | Mixed (RA 35%; Rot 13%; ReA 13%) | Enhancement of rotator cuff tendon | 72% | 32% | N/A | N/A | N/A |
| Enhancement of shoulder joint capsule | 69% | 35% | N/A | N/A | N/A | ||||||
| Shoulder joint effusion | 64% | 44% | N/A | N/A | N/A | ||||||
| Focal bone oedema in humerus head | 59% | 19% | N/A | N/A | N/A | ||||||
| Enhancement of biceps tendon | 12% | 6% | N/A | N/A | N/A | ||||||
| Shoulder synovial hypertrophy | 12% | 15% | N/A | N/A | N/A | ||||||
| Enhancement of glenohumeral joint | 5% | 10% | N/A | N/A | N/A | ||||||
| Diffuse bone oedema in humerus head | 2% | 9% | N/A | N/A | N/A | ||||||
| US | Biceps tenosynovitis | 43% | 23% | N/A | N/A | N/A | |||||
| Subdeltoid bursitis | 32% | 12% | N/A | N/A | N/A | ||||||
| Glenohumeral synovitis | 11% | 11% | N/A | N/A | N/A | ||||||
| Takahashi [20] | 2015 | FDG-PET/CT | 27 | 10 | Elderly-onset RA | Circular/linear uptake around the humeral headsc | 30% | 90% | N/A | N/A | N/A |
| Interspinous bursae/spinous processesc | 81% | 65% | N/A | N/A | N/A | ||||||
| Wristsc | 41% | 100% | N/A | N/A | N/A | ||||||
| Iliopectineal bursaec | 41% | 10% | N/A | N/A | N/A | ||||||
| Isolated uptake in front of the femoral headsc | 59% | 10% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 96% | 60% | N/A | N/A | N/A | ||||||
| Rehak [29] | 2015 | FDG-PET or FDG-PET/CT | 67 | Shouldersd | 87% | N/A | N/A | N/A | N/A | ||
| Sternoclavicular jointsd | 46% | N/A | N/A | N/A | N/A | ||||||
| Cervical interspinous bursaed | 19% | N/A | N/A | N/A | N/A | ||||||
| Lumbar interspinous bursaed | 57% | N/A | N/A | N/A | N/A | ||||||
| Hipsd | 70% | N/A | N/A | N/A | N/A | ||||||
| Ischiatic tuberositiesd | 52% | N/A | N/A | N/A | N/A | ||||||
| Vasculard | 40% | N/A | N/A | N/A | N/A | ||||||
| Sondag [30] | 2016 | FDG-PET/CT | 50 | 53 | Neoplasia | Shouldersc | 58% | 15% | N/A | N/A | N/A |
| Acromioclavicular jointsc | 26% | 7% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsc | 24% | 5% | N/A | N/A | N/A | ||||||
| Spinous processesc | 58% | 11% | N/A | N/A | N/A | ||||||
| Greater trochantersc | 40% | 15% | N/A | N/A | N/A | ||||||
| Hipsc | 38% | 15% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 55% | 8% | N/A | N/A | N/A | ||||||
| Symphysis pubis enthesesc | 22% | 1% | N/A | N/A | N/A | ||||||
| Iliopectinal bursaec | 31% | 7% | N/A | N/A | N/A | ||||||
| Palard-Novello [31] | 2016 | FDG-PET/CT | 18 | Shoulderse | 89% | N/A | N/A | N/A | N/A | ||
| Sternoclaviculare | 72% | N/A | N/A | N/A | N/A | ||||||
| Cervical spinous processese | 56% | N/A | N/A | N/A | N/A | ||||||
| Lumbar spinous processese | 72% | N/A | N/A | N/A | N/A | ||||||
| Hipse | 94% | N/A | N/A | N/A | N/A | ||||||
| Ischial tuberositiese | 94% | N/A | N/A | N/A | N/A | ||||||
| Vasculare | 0% | N/A | N/A | N/A | N/A | ||||||
| Henckaerts [14] | 2018 | FDG-PET or FDG-PET/CT | 67 | 32 | Mixed (Rot 25%; OA 16%; RA 9%) | Shouldersf | 96% | 87% | N/A | N/A | N/A |
| Sternoclavicular jointsf | 41% | 11% | N/A | N/A | N/A | ||||||
| Cervical spinous processesf | 75% | 13% | N/A | N/A | N/A | ||||||
| Lumbar spinous processesf | 46% | 0% | N/A | N/A | N/A | ||||||
| Hipsf | 65% | 12% | N/A | N/A | N/A | ||||||
| Greater trochantersf | 78% | 44% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesf | 65% | 10% | N/A | N/A | N/A | ||||||
| Carotid arteriesf | 4% | 0% | N/A | N/A | N/A | ||||||
| Subclavian arteriesf | 12% | 6% | N/A | N/A | N/A | ||||||
| Thoracic aortaf | 6% | 0% | N/A | N/A | N/A | ||||||
| Abdominal aortaf | 0% | 0% | N/A | N/A | N/A | ||||||
| Owen [32] | 2020 | FDG-PET/CT | 33 | 132 | Mixed (Neo 64%, RA 5%) | Shoulders (peri-articular)g | 97% | 78% | N/A | N/A | N/A |
| Glenohumeral jointsg | 73% | 4% | N/A | N/A | N/A | ||||||
| Acromioclavicular jointsg | 100% | 66% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsg | 88% | 57% | N/A | N/A | N/A | ||||||
| Interspinous bursaeg | 91% | 17% | N/A | N/A | N/A | ||||||
| Hips (peri-articular)g | 97% | 61% | N/A | N/A | N/A | ||||||
| Hip jointsg | 61% | 20% | N/A | N/A | N/A | ||||||
| Trochanteric bursaeg | 100% | 75% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesg | 100% | 58% | N/A | N/A | N/A | ||||||
| Knee jointsg | 53% | 54% | N/A | N/A | N/A | ||||||
| Knees (posteromedial)g | 73% | 20% | N/A | N/A | N/A |
| Author . | Year . | Imaging technique . | Patients (n) . | Controls (n) . | Main diagnoses in controls . | Anatomic lesion . | Prevalence in patients at baseline . | Prevalence in controls at baseline . | Prevalence in patients during follow-up . | Prevalence in controls during follow-up . | Follow-up (weeks) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ottaviani [26] | 2020 | US | 27 | 25 | CPPDa | Subacromial-subdeltoid bursitis | 96% | 68% | N/A | N/A | N/A |
| Long-head biceps tenosynovitis | 85% | 72% | N/A | N/A | N/A | ||||||
| Glenohumeral effusion | 48% | 68% | N/A | N/A | N/A | ||||||
| Humeral erosion | 7% | 44% | N/A | N/A | N/A | ||||||
| Acromioclavicular synovitis | 7% | 64% | N/A | N/A | N/A | ||||||
| Acromioclavicular chondrocalcinosis | 4% | 92% | N/A | N/A | N/A | ||||||
| Laporte [27] | 2019 | MRI | 15 | N/A | N/A | Shoulder myofascial lesionsb | 71% | N/A | 53% | N/A | 12 |
| Hip myofascial lesionsb | 87% | N/A | 33% | N/A | 12 | ||||||
| Pubic symphysis myofascial lesionsb | 80% | N/A | 20% | N/A | 12 | ||||||
| Ischial tuberosities myofascial lesionsb | 60% | N/A | 0% | N/A | 12 | ||||||
| Fruth [22] | 2020 | MRI (contrast-enhanced) | 40 | 80 | Mixed (Deg 26%; RA 26%; SpA 20%) | Common ischiocrural tendon origin at the ischial tuberosity | 100% | 56% | N/A | N/A | N/A |
| Glutaeus medius and minimus muscles tendons at the greater trochanter | 99.5% | 88% | N/A | N/A | N/A | ||||||
| Origin of the rectus femoris muscle | 96.5% | 4.5% | N/A | N/A | N/A | ||||||
| Origin of the adductor longus muscle | 92.5% | 5% | N/A | N/A | N/A | ||||||
| Interspinous bursae and origin of the paraspinous muscles | 68% | 24% | N/A | N/A | N/A | ||||||
| Nakamura [28] | 2020 | MRI (contrast-enhanced) | 58 | 79 | Mixed (RA 35%; Rot 13%; ReA 13%) | Enhancement of rotator cuff tendon | 72% | 32% | N/A | N/A | N/A |
| Enhancement of shoulder joint capsule | 69% | 35% | N/A | N/A | N/A | ||||||
| Shoulder joint effusion | 64% | 44% | N/A | N/A | N/A | ||||||
| Focal bone oedema in humerus head | 59% | 19% | N/A | N/A | N/A | ||||||
| Enhancement of biceps tendon | 12% | 6% | N/A | N/A | N/A | ||||||
| Shoulder synovial hypertrophy | 12% | 15% | N/A | N/A | N/A | ||||||
| Enhancement of glenohumeral joint | 5% | 10% | N/A | N/A | N/A | ||||||
| Diffuse bone oedema in humerus head | 2% | 9% | N/A | N/A | N/A | ||||||
| US | Biceps tenosynovitis | 43% | 23% | N/A | N/A | N/A | |||||
| Subdeltoid bursitis | 32% | 12% | N/A | N/A | N/A | ||||||
| Glenohumeral synovitis | 11% | 11% | N/A | N/A | N/A | ||||||
| Takahashi [20] | 2015 | FDG-PET/CT | 27 | 10 | Elderly-onset RA | Circular/linear uptake around the humeral headsc | 30% | 90% | N/A | N/A | N/A |
| Interspinous bursae/spinous processesc | 81% | 65% | N/A | N/A | N/A | ||||||
| Wristsc | 41% | 100% | N/A | N/A | N/A | ||||||
| Iliopectineal bursaec | 41% | 10% | N/A | N/A | N/A | ||||||
| Isolated uptake in front of the femoral headsc | 59% | 10% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 96% | 60% | N/A | N/A | N/A | ||||||
| Rehak [29] | 2015 | FDG-PET or FDG-PET/CT | 67 | Shouldersd | 87% | N/A | N/A | N/A | N/A | ||
| Sternoclavicular jointsd | 46% | N/A | N/A | N/A | N/A | ||||||
| Cervical interspinous bursaed | 19% | N/A | N/A | N/A | N/A | ||||||
| Lumbar interspinous bursaed | 57% | N/A | N/A | N/A | N/A | ||||||
| Hipsd | 70% | N/A | N/A | N/A | N/A | ||||||
| Ischiatic tuberositiesd | 52% | N/A | N/A | N/A | N/A | ||||||
| Vasculard | 40% | N/A | N/A | N/A | N/A | ||||||
| Sondag [30] | 2016 | FDG-PET/CT | 50 | 53 | Neoplasia | Shouldersc | 58% | 15% | N/A | N/A | N/A |
| Acromioclavicular jointsc | 26% | 7% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsc | 24% | 5% | N/A | N/A | N/A | ||||||
| Spinous processesc | 58% | 11% | N/A | N/A | N/A | ||||||
| Greater trochantersc | 40% | 15% | N/A | N/A | N/A | ||||||
| Hipsc | 38% | 15% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesc | 55% | 8% | N/A | N/A | N/A | ||||||
| Symphysis pubis enthesesc | 22% | 1% | N/A | N/A | N/A | ||||||
| Iliopectinal bursaec | 31% | 7% | N/A | N/A | N/A | ||||||
| Palard-Novello [31] | 2016 | FDG-PET/CT | 18 | Shoulderse | 89% | N/A | N/A | N/A | N/A | ||
| Sternoclaviculare | 72% | N/A | N/A | N/A | N/A | ||||||
| Cervical spinous processese | 56% | N/A | N/A | N/A | N/A | ||||||
| Lumbar spinous processese | 72% | N/A | N/A | N/A | N/A | ||||||
| Hipse | 94% | N/A | N/A | N/A | N/A | ||||||
| Ischial tuberositiese | 94% | N/A | N/A | N/A | N/A | ||||||
| Vasculare | 0% | N/A | N/A | N/A | N/A | ||||||
| Henckaerts [14] | 2018 | FDG-PET or FDG-PET/CT | 67 | 32 | Mixed (Rot 25%; OA 16%; RA 9%) | Shouldersf | 96% | 87% | N/A | N/A | N/A |
| Sternoclavicular jointsf | 41% | 11% | N/A | N/A | N/A | ||||||
| Cervical spinous processesf | 75% | 13% | N/A | N/A | N/A | ||||||
| Lumbar spinous processesf | 46% | 0% | N/A | N/A | N/A | ||||||
| Hipsf | 65% | 12% | N/A | N/A | N/A | ||||||
| Greater trochantersf | 78% | 44% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesf | 65% | 10% | N/A | N/A | N/A | ||||||
| Carotid arteriesf | 4% | 0% | N/A | N/A | N/A | ||||||
| Subclavian arteriesf | 12% | 6% | N/A | N/A | N/A | ||||||
| Thoracic aortaf | 6% | 0% | N/A | N/A | N/A | ||||||
| Abdominal aortaf | 0% | 0% | N/A | N/A | N/A | ||||||
| Owen [32] | 2020 | FDG-PET/CT | 33 | 132 | Mixed (Neo 64%, RA 5%) | Shoulders (peri-articular)g | 97% | 78% | N/A | N/A | N/A |
| Glenohumeral jointsg | 73% | 4% | N/A | N/A | N/A | ||||||
| Acromioclavicular jointsg | 100% | 66% | N/A | N/A | N/A | ||||||
| Sternoclavicular jointsg | 88% | 57% | N/A | N/A | N/A | ||||||
| Interspinous bursaeg | 91% | 17% | N/A | N/A | N/A | ||||||
| Hips (peri-articular)g | 97% | 61% | N/A | N/A | N/A | ||||||
| Hip jointsg | 61% | 20% | N/A | N/A | N/A | ||||||
| Trochanteric bursaeg | 100% | 75% | N/A | N/A | N/A | ||||||
| Ischial tuberositiesg | 100% | 58% | N/A | N/A | N/A | ||||||
| Knee jointsg | 53% | 54% | N/A | N/A | N/A | ||||||
| Knees (posteromedial)g | 73% | 20% | N/A | N/A | N/A |
Results originally reported separately for the right and left side, are presented as the mean of the two values. aPatients with CPPD also fulfilled the ACR/EULAR classification criteria for PMR. bThe reported data refer specifically to myofascial lesions, defined as high T2 STIR signal surrounding the muscle or diffuse within it. Examined muscles were, in the shoulder girdle, the infraspinatus, supraspinatus, subscapularis, and teres minor; in the pelvic girdle, the glutaeal, iliopsoas and pelvitrochanteric; at the level of ischial tuberosities, the semitendinosus, semimembranosus, and long head of the biceps femoris; at the pubic symphysis, the adductors. This study did not evaluate enthesitis, tendinitis, bursitis. cThe uptake of the examined structures was considered abnormal if similar or higher than the uptake of the liver (i.e. ≥2 on a 4-point scale). dThe uptake of the examined structures was considered abnormal if higher than the uptake of the liver. Assessed arteries were the ascending aorta, descending aorta, abdominal aorta, brachiocephalic trunk, subclavian, common iliac, and common femoral arteries. eThe uptake within a region of interest (ROI) was considered abnormal if its maximal standardized uptake value (SUV) was higher than the maximal SUV of the reference ROI of the liver. The reported prevalence refers to bilateral findings, except for the spinal spinous processes and the arteries. fThe uptake of the examined structures was considered abnormal if similar or higher than the uptake of the liver (i.e. 2 on a 3-point scale). gThe uptake of the examined structures was considered abnormal even if lower in intensity than the uptake of the liver (i.e. ≥1 on a 4-point scale). Deg: degenerative disc/joint disorder; N/A: not available; Neo: neoplasia; ReA: reactive arthritis; Rot: rotator cuff disease; SpA: spondyloarthritis; US: ultrasonography.
Subclinical vascular inflammation in PMR
Reports on the relevance of subclinical large vessel vasculitis in patients with PMR have been controversial. FDG-PET studies suggest that vasculitis occurred in up to 30% of individuals with PMR, particularly in those with anaemia, markedly elevated inflammatory markers and/or incomplete response to glucocorticoids or relapsing course [13, 16, 29, 33]. Others reported that large vessel vasculitis in PMR patients is much less common or even absent [14, 32]. Apart from the different case selections, one factor that limits the comparison of different FDG-PET studies is the lack of standardization of image analysis and scoring [34, 35]. Another important technical aspect that might have contributed to divergent results is the inhomogeneous time of image acquisition after tracer injection. While most studies recorded images after 45–60 min (which is the ‘oncological’ protocol), a high prevalence of subclinical large vessel vasculitis was found in one study where images were acquired only 180 min after FDG administration [36]. In that study, FDG-PET/CT scans from 100 PMR patients were analysed [36]. Sixteen of these patients were considered atypical because they were either below the age of 50 years or had prominent pain at the pelvis and proximal thighs. Patients with headache and other characteristic symptoms of GCA were excluded, and temporal artery biopsy, which was available from 36 cases, was always negative. Sixty-one per cent of the 84 patients with typical PMR yielded tracer accumulation at the aorta and/or large arteries, particularly those who had symptoms at the pelvic girdle, inflammatory low back pain or diffuse lower limb pain. Vasculitis was even more common in patients with atypical PMR (75%).
In one of the first studies on FDG-PET in PMR, no difference was found between patients with positive and negative PET concerning constitutional symptoms and laboratory markers of inflammation [13]. These results have recently been confirmed [37]. The high prevalence of large vessel vasculitis in PMR supports the long-existing concept of common pathophysiology of PMR and GCA [6].
Given the results of the aforementioned study, future PET studies in PMR (and GCA) should foresee longer time intervals between tracer administration and scanning. A minimum of 60 min has recently been recommended by a joint position paper of the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the and the PET Interest Group; however, the sensitivity of different time intervals for the diagnosis of vasculitis still needs to be compared [35]. New tracers targeting immune cells (e.g. 1C-PK11195 targeting the translocator protein receptor, which is highly expressed in activated cells of the mononuclear phagocyte lineage, see [38] for review) may possibly uncover novel insights into the pathogenesis of PMR and possibly identify common targets in large arteries, peritendineum, bursae and joints.
Imaging detected inflammation is different in PMR and mimicking diseases
Elderly onset RA (EORA) and degenerative shoulder disorders are among the most common differential diagnoses of PMR [1]. Ultrasound seems to be of limited value to differentiate EORA from PMR as both diseases involve similar structures [2]. FDG-PET indicated that the overall amount of tracer uptake in shoulders and hips was comparable between PMR and EORA patients, whereas the pattern of inflammation showed some differences: in EORA, a circular and linear tracer uptake around the humeral head was found while in PMR, tracer accumulation was focal and non-linear [20]. Also, overall tracer accumulation at greater trochanters was similar in both groups, whereas focal uptake at the iliopectineal bursa, as well as in the area of ischial tuberosities were characteristic for PMR [20].
Others confirmed the observation that inflammatory burden at shoulders is comparable between EORA and PMR, whereas extracapsular inflammation at (peri-) tendons adjacent to the ischial tuberosity, in the periacetabular area (without involving the hip joint itself) and inferior to the symphysis pubis helped to differentiate the two conditions [24]. Severe rotator cuff tendinopathy with swelling of the supraspinatus tendon >6 mm was commonly observed in PMR patients but did not occur in RA [12].
A detailed comparison of the MRI patterns at hands was conducted in 10 PMR and 10 early-RA patients assuming that synovitis at peripheral joints is characteristic for RA but may occasionally also occur in PMR [39]. No difference between the groups was observed concerning the volume of synovitis, degree of flexor tenosynovitis, periarticular erosions and bone oedema. Only the proportion of extracapsular enhancement at the level of metacarpophalangeal joints was higher in PMR than in RA patients [39]. One of the main concerns of this study is selection bias, as RA and PMR patients were both recruited from an early arthritis clinic that might have favoured the inclusion of patients with peripheral involvement. Another surprising finding was that 80% of PMR patients suffered from erosive joint disease. Given that PMR is normally non-erosive [40], the patients of this study probably resembled more closely seronegative RA with polymyalgic onset rather than true PMR.
The differentiation of PMR from chondrocalcinosis may be less challenging. While microscopic confirmation of CPP crystals in synovial fluid or tissue remains the gold standard to diagnose chondrocalcinosis, x-rays and particularly ultrasound may help to detect CPP deposition in cartilage, tendons and soft tissue. One study on 52 patients with suspected PMR demonstrated that patients with CPP deposition disease (CPPD) more commonly revealed humeral bone erosions, acromioclavicular synovitis and CPP deposition but less frequently SAD bursitis. The frequencies of biceps tenosynovitis and glenohumeral joint synovitis were comparable between the groups [26].
Imaging for diagnosing PMR in clinical practice
The best imaging method for diagnosing PMR is elusive so far. While ultrasound is the only imaging method included in the 2012 ACR/EULAR classification criteria [2], ultrasound, MRI and FDG-PET/CT have all been tested for diagnostic purposes as reported above and in a recent meta-analysis [7]. Among ultrasound studies, bilateral SAD bursitis yielded the best combination of sensitivity (mean 66%) and specificity (89%) for the diagnosis of PMR, whereas other lesions such as glenohumeral synovitis (62% and 58%, respectively) or hip synovitis (33% and 78%, respectively) were less sensitive and specific. Data for trochanteric bursitis were heterogeneous with sensitivities ranging from 21% to 98% and specificities from 70% to 91%.
Direct comparisons between different imaging techniques in PMR are scarce. In one of the first studies comparing ultrasound and MRI, published in 2001, 57 PMR patients and 114 controls were examined by ultrasound and 24 of these PMR patients also underwent MRI [41]. The authors concluded that ultrasound is highly sensitive and specific for PMR and that both techniques were equally effective in detecting bilateral SAD bursitis. In a more recent study on 137 patients with shoulder pain fulfilling Bird’s criteria for PMR, bilateral ultrasound and one-sided contrast-enhanced MRI of the shoulder were conducted [28]. A final clinical diagnosis of PMR was made in 58 out of these 137 patients. Two out of the three of the following MRI findings—enhancement at the joint capsule, enhancement of the rotator cuff tendon and focal bone oedema in humeral heads—revealed a sensitivity of 76% and a specificity of 85%, whereas one out of the following three ultrasound lesions—biceps tenosynovitis, SAD bursitis and glenohumeral synovitis—had a lower sensitivity (50%) and specificity (72%).
MRI seems to be particularly useful at sites where ultrasound is difficult to perform due to the restricted acoustic window and limited penetration of the ultrasound beam, such as at the spine and the hip [22, 42, 43]. In a retrospective study (which is in part described above) of contrast-enhanced MRI of the pelvic girdle conducted in 40 patients with PMR and 80 controls with a variety of diagnoses including RA [22], the presence of bilateral inflammation at the rectus femoris muscle or the adductor longus origins differentiated patients from controls with a sensitivity of 100% and a specificity of 96%. These sites would in principle also be accessible by ultrasound; however, a formal comparison between the two techniques has not been conducted yet. Because all patients of this study underwent pelvic girdle MRI for unclear hip pain and 10/40 PMR patients were eventually re-classified as RA, the findings of this study cannot be generalized to all PMR patients, particularly because prominent shoulder pain and stiffness are the most common clinical features of PMR. In the study using whole-body MRI mentioned above, a characteristic pattern of symmetrical, extracapsular inflammation, adjacent to the greater trochanter, acetabulum, ischial tuberosity and/or symphysis pubis, was observed in 14/22 of the PMR cases but only in 1/16 patients with RA [24].
Despite these promising results for MRI, this technique is usually not the first imaging method in clinical practice due to its lower availability, higher costs, and longer execution time compared with sonography. Another challenge is to get a rapid appointment for MRI given that symptoms rapidly resolve under glucocorticoids, and that treatment cannot be withheld for a prolonged time period. Ultrasound, in contrast, can be performed during the first visit as an extension of clinical examination. Moreover, the use of MRI is limited to the evaluation of one anatomical region at time except in those centres where whole-body MRI is performed routinely.
The value of FDG-PET for the diagnosis of PMR has recently been evaluated in a prospective study involving 99 patients with a clinical suspicion of PMR [14]. The gold standard for the diagnosis was the confirmation of PMR by an expert clinician, six months after the inclusion into the study, based on all clinical, laboratory and imaging information available, including the PET result. Sixty-seven patients were diagnosed with PMR, whereas 32 patients had another disease such as rotator cuff pathology, OA or RA. FDG-PET uptake was assessed at 12 skeletal regions (cervical spinous processes, lumbar spinous processes, bilateral sternoclavicular joints, ischial tuberosity, greater trochanter, hip and shoulder), and scored semiquantitatively per site (0–2) resulting in a global score ranging from 0–24. While PMR patients had tendentially more intense FDG uptake in all predefined skeletal regions compared with controls, except for shoulders, none of the individual sites was useful as a diagnostic tool on its own. Quantitative assessment of the global skeletal score with a cut-off of 16, however, revealed a sensitivity of 85% and a specificity of 88% for PMR diagnosis.
Other studies, which were either retrospective and/or had a case-control design, revealed divergent diagnostic values for FDG-PET: one retrospective study reported that an increased tracer uptake in at least three out of 17 musculoskeletal sites (shoulders, acromioclavicular and sternoclavicular joints, greater trochanter, hips, ischial tuberosities, iliopectineal bursitis, symphysis pubis enthesis and the most inflammatory interspinous bursa) had a sensitivity of 74% and a specificity of 79% for the differentiation of PMR cases from controls (who underwent FDG-PET/CT for neoplastic disease) [30]. A case-control study involving 33 PMR patients and 132 controls observed that the presence grade ≥2 uptake (range 0–3) at the ischial tuberosities and either the periarticular area of shoulders or interspinous bursae performed best for the diagnosis of PMR revealing a sensitivity of 91% and a specificity of 92% [32]. A comparison of 27 PMR and 10 EORA patients investigated by FDG-PET/CT yielded a sensitivity of 93% and a specificity of 90% to diagnose PMR when at least three of the following five items were satisfied: focal and non-linear uptake pattern at shoulders, iliopectineal bursitis, FDG uptake in ischial tuberosities and spinal spinous processes, and lack of FDG uptake at the wrists [20].
These studies indicate a relatively good diagnostic value of FDG-PET for PMR; however, this technique is costly, exposes patients to considerable ionising radiation, and its availability is limited. Thus, FDG-PET cannot be applied in the routine workup of patients with suspected PMR, but it might be retained to those patients with systemic features, in whom neoplasia or suspected large vessel vasculitis should be ruled out. Sequential application of different imaging techniques (as detailed in Fig. 3) starting with ultrasound and reserving MRI for detailed anatomical localization of inflammation, particularly in case of prominent hip pain, and FDG-PET for patients with atypical PMR symptoms or clues of large vessel vasculitis such as prominent constitutional symptoms might be a reasonable approach in clinical practice.
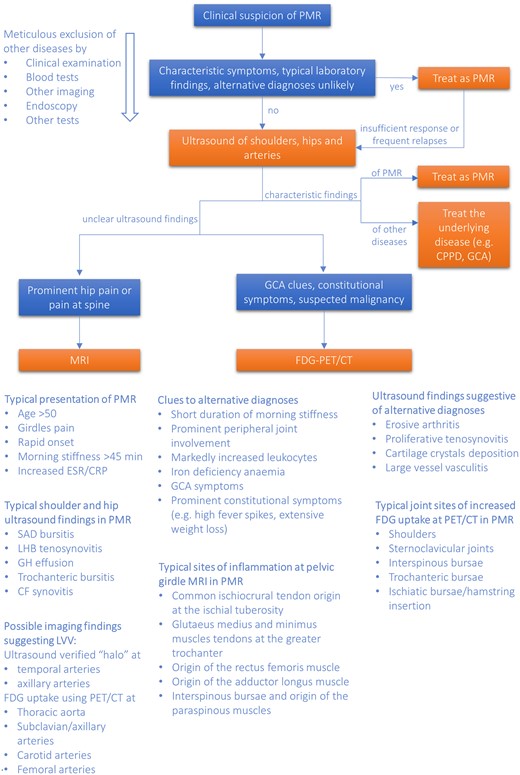
A proposed algorithm for the use of imaging in patients with PMR in clinical practice
For the purpose of this flow-chart, GCA is considered a differential diagnosis rather than a manifestation of the GCA-PMR disease spectrum (see Dejaco C, Duftner C, Buttgereit F, Matteson. EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology 2017;56:506 for detailed discussion). The halo sign is an ultrasound lesion defined as a hypoechoic thickening of the vessel wall. Blue boxes contain clinical scenarios; orange boxes, diagnostic/therapeutic strategies. CF: coxofemoral; CPPD: calcium pyrophosphate deposition disease; FDG-PET/CT: 18 F-fluorodeoxyglucose positron emission tomography/CT; GH: glenohumeral; LHB: long head of the biceps; LVV: large vessel vasculitis; SAD: subacromial/subdeltoid.
Imaging for monitoring and outcome prediction of PMR patients
Monitoring of disease activity in PMR patients currently relies on clinical and laboratory parameters while studies investigating the role of imaging for this purpose are scarce (see Tables 2 and 3 for summary).
| Author . | Year . | Imaging technique . | inclusion criteria . | Patients at FU [n, (%)] . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Devauchelle-Pensec [44] | 2016 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 19/20 (95) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitisb bilateral hips: trochanteric bursitis, synovitisb | Δ proportion pat scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 no difference at hips from baseline to wk 2 and 12 |
| Suzuki [9] | 2017 | US | PMR like symptomsc + US examination w/o GC-treatment + clinical FU after 1 year | 3/15 (20) | after 0.9–8.7m | bilateral shoulders: Bil-HSScTSd | Δ Bil-HSScTS | none | decrease of Bil-HSScTS in 3/3 pate |
| Huwart [45] | 2018 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusionf bilateral hips: trochanteric and/or iliopsoas bursitis, synovitis/effusionf | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 improvement of bursitis at shoulders from 2 to 0.5 (P =0.018) at wk 12g no difference of synovitis/effusion at shoulders from baseline to wk 12 no difference of bursitis and synovitis/effusion at hips from baseline to wk 2 improvement of synovitis/effusion at hips from 2 to 0 (P =0.018) at wk 12g no difference of bursitis at hips from baseline to wk 12 |
| Devauchelle-Pensec [44] | 2016 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–14/20 (70) wk 2–12/20 (60) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: SAD bursitis and/or synovitish bilateral hips: trochanteric bursitis and/or synovitish | Δ proportion patients scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 decrease of pat scored 0–1 at hips from baseline to wk 2 from 87% to 46% (P =0.031) increase of pat scored 0–1 at hips from baseline to wk 12 from 13% to 69% (P =0.005) decrease of pat scored >1 at hips from baseline to wk 12 from 87% to 31% (P =0.023) |
| Huwart [45] | 2018 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusioni bilateral hips: trochanteric and/or iliopsoas bursitis, effusioni | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 12 improvement of bursitis from baseline to wk 2 at hips from 4 to 3 (P <0.001)g improvement of bursitis at hips from baseline to wk 12 from 4 to 2 (P <0.001)g no difference of synovitis/effusion at hips from baseline to wk 2 and 12 |
| Laporte [27] | 2019 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 2–13/18 (72) wk 12–15/18 (83) | wk 0, 2, 12 | bilateral shouldersj: supraspinatus, infraspinatus, subscapularis, teres minor bilateral hipj: glutaeal, iliopsoas, pelvitrochanteric muscles bilateral ischial tuberosityj: semitendinosus, semimembranosus, long head biceps femoris bilateral pubic symphysisj: adductors | Δ proportion myofascial lesions | none | decline from 70 to 60 lesions from baseline to wk 12 (P = 0.002) improvement of 66 (64.1%), worsening of 2 (1.9%) and unchanged 35 (34%) muscle groups (P =0.034) at wk 12 |
| Devauchelle-Pensec [44] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/20 (90) wk 2–14/20 (70) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: global SUVk bilateral hips: global SUVk | comparison global SUV | none | no difference of global SUV at shoulders from baseline to wk 2 and 12 decline of global SUV at hips from baseline to wk 2 from 6.4–5.3 (P =0.007)g decrease of global SUV at hips from baseline to wk 12 from 6.4–4.9, P <0.001g decline of global SUV at hips from wk 2 to wk 12 from 5.3–4.9, P =0.017g |
| Palard-Novello [31] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/18 (100) wk 2–16/18 (70) wk 12–16/18 (80) | wk 0, 2, 12 | bilateral shoulders: SUVmaxl bilateral sternoclavicular joints: SUVmaxl bilateral hips: SUVmaxl bilateral ischial tuberosities: SUVmaxl cervical and lumbar spinous processes: SUVmaxl | comparison SUVmax | ΔPMR-AS, ΔCRP and ΔESR | no correlation of ΔSUVmax with ΔPMR-AS, ΔCRP or ΔESR in patient-based analysis at wk 2 and wk 12 decline of SUVmax from baseline to wk 2 from 5.8–5.2 (P =0.026) and from baseline to wk 12 with 5.8–4.7 (P <0.001) in patient-based analysisg decline of SUVmax at left hip from baseline to wk 2 from 6.4–4.8 (P =0.024)g improvements of SUVmax at both hips, ischial tuberosities and lumbar spinous processes from baseline to wk 12 (all with P <0.05)m |
| Author . | Year . | Imaging technique . | inclusion criteria . | Patients at FU [n, (%)] . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Devauchelle-Pensec [44] | 2016 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 19/20 (95) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitisb bilateral hips: trochanteric bursitis, synovitisb | Δ proportion pat scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 no difference at hips from baseline to wk 2 and 12 |
| Suzuki [9] | 2017 | US | PMR like symptomsc + US examination w/o GC-treatment + clinical FU after 1 year | 3/15 (20) | after 0.9–8.7m | bilateral shoulders: Bil-HSScTSd | Δ Bil-HSScTS | none | decrease of Bil-HSScTS in 3/3 pate |
| Huwart [45] | 2018 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusionf bilateral hips: trochanteric and/or iliopsoas bursitis, synovitis/effusionf | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 improvement of bursitis at shoulders from 2 to 0.5 (P =0.018) at wk 12g no difference of synovitis/effusion at shoulders from baseline to wk 12 no difference of bursitis and synovitis/effusion at hips from baseline to wk 2 improvement of synovitis/effusion at hips from 2 to 0 (P =0.018) at wk 12g no difference of bursitis at hips from baseline to wk 12 |
| Devauchelle-Pensec [44] | 2016 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–14/20 (70) wk 2–12/20 (60) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: SAD bursitis and/or synovitish bilateral hips: trochanteric bursitis and/or synovitish | Δ proportion patients scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 decrease of pat scored 0–1 at hips from baseline to wk 2 from 87% to 46% (P =0.031) increase of pat scored 0–1 at hips from baseline to wk 12 from 13% to 69% (P =0.005) decrease of pat scored >1 at hips from baseline to wk 12 from 87% to 31% (P =0.023) |
| Huwart [45] | 2018 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusioni bilateral hips: trochanteric and/or iliopsoas bursitis, effusioni | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 12 improvement of bursitis from baseline to wk 2 at hips from 4 to 3 (P <0.001)g improvement of bursitis at hips from baseline to wk 12 from 4 to 2 (P <0.001)g no difference of synovitis/effusion at hips from baseline to wk 2 and 12 |
| Laporte [27] | 2019 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 2–13/18 (72) wk 12–15/18 (83) | wk 0, 2, 12 | bilateral shouldersj: supraspinatus, infraspinatus, subscapularis, teres minor bilateral hipj: glutaeal, iliopsoas, pelvitrochanteric muscles bilateral ischial tuberosityj: semitendinosus, semimembranosus, long head biceps femoris bilateral pubic symphysisj: adductors | Δ proportion myofascial lesions | none | decline from 70 to 60 lesions from baseline to wk 12 (P = 0.002) improvement of 66 (64.1%), worsening of 2 (1.9%) and unchanged 35 (34%) muscle groups (P =0.034) at wk 12 |
| Devauchelle-Pensec [44] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/20 (90) wk 2–14/20 (70) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: global SUVk bilateral hips: global SUVk | comparison global SUV | none | no difference of global SUV at shoulders from baseline to wk 2 and 12 decline of global SUV at hips from baseline to wk 2 from 6.4–5.3 (P =0.007)g decrease of global SUV at hips from baseline to wk 12 from 6.4–4.9, P <0.001g decline of global SUV at hips from wk 2 to wk 12 from 5.3–4.9, P =0.017g |
| Palard-Novello [31] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/18 (100) wk 2–16/18 (70) wk 12–16/18 (80) | wk 0, 2, 12 | bilateral shoulders: SUVmaxl bilateral sternoclavicular joints: SUVmaxl bilateral hips: SUVmaxl bilateral ischial tuberosities: SUVmaxl cervical and lumbar spinous processes: SUVmaxl | comparison SUVmax | ΔPMR-AS, ΔCRP and ΔESR | no correlation of ΔSUVmax with ΔPMR-AS, ΔCRP or ΔESR in patient-based analysis at wk 2 and wk 12 decline of SUVmax from baseline to wk 2 from 5.8–5.2 (P =0.026) and from baseline to wk 12 with 5.8–4.7 (P <0.001) in patient-based analysisg decline of SUVmax at left hip from baseline to wk 2 from 6.4–4.8 (P =0.024)g improvements of SUVmax at both hips, ischial tuberosities and lumbar spinous processes from baseline to wk 12 (all with P <0.05)m |
Summary of characteristics and main findings of selected studies published between 2015–2020 on ultrasound, MRI and 18 F-fluorodeoxyglucose positron emission tomography (18 F-FDG-PET) in PMR. aFulfilling Chuang criteria. bB-mode assessment by a semi-quantitative score (0–3; 0 = normal, 1 = slight, 2 = moderate, 3 = severe). cFinal PMR based on the 2012 EULAR/ACR provisional criteria. dEvaluated by bilateral assessment of the hyperaemia adjacent to the anterior aspect of the subscapularis tendon (0–3): 0 = absent or minimal flow; 1 = single vessel dots or confluent linear shape signals shorter than half the length of the region; 2 = confluent linear shape signals longer than half the length of the region to be evaluated or confluent zone shape signals shorter than half the length of the region to be evaluated; 3 = confluent zone shape signals longer than half the length of the region eOnly descriptive results, no interference reported. fB-mode or power Doppler imaging were graded using the OMERACT semi-quantitative scale (0–3): 0 = no abnormalities; 1 = mild; 2 = moderate; 3 = marked gMedian values. hEvaluated by a 3 T superconductive magnet system for bursa and joint effusion and scored semi-quantitatively (0–3): 0 = normal; 1 = slight; 2 = moderate; 3 = severe. iBursitis and effusion were defined as a high T2 signal, as no contrast injection was used a high T2 signal could indicate synovitis and/or effusion, abnormalities were scored by the semi-quantitative OMERACT scale (0–3): 0 = no abnormalities; 1 = mild; 2 = moderate; 3 = marked. jMyofascial lesions were defined as a high T2 STIR signal either diffuse within the muscle or forming a line surrounding the muscle, a muscle group was considered positive if at least one muscle in the group revealed a lesion. kEvaluation of the median global standardized uptake values (SUVs) at the shoulders and pelvic girdle. lThe maximal SUV [SUVmax, SUV with the maximal intensity among the pixels of the region of interest (ROI)] of each ROI were calculated and compared with the liver reference SUVmax, only ROIs with SUVmax higher than that of the liver reference SUVmax were considered abnormal. mOriginal data provided median values of SUVmax for each site of bilateral investigated structures Δ: change; Bil-HSScTS: bilateral hyperemia on subscapularis tendon score; FU: follow-up; GC: glucocorticoid; LHBT: long head biceps tendon; m: month; max: maximal; n: number; n pat FU: number of patients in follow-up; N/A: not applicable; pat: patients; PET: 18F-FDG positron emission tomography; PMR-AS: PMR activity score; SAD: subacromial/subdeltoid; SUV: standardized uptake values; US: ultrasound; vs: versus ; wk: week.
| Author . | Year . | Imaging technique . | inclusion criteria . | Patients at FU [n, (%)] . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Devauchelle-Pensec [44] | 2016 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 19/20 (95) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitisb bilateral hips: trochanteric bursitis, synovitisb | Δ proportion pat scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 no difference at hips from baseline to wk 2 and 12 |
| Suzuki [9] | 2017 | US | PMR like symptomsc + US examination w/o GC-treatment + clinical FU after 1 year | 3/15 (20) | after 0.9–8.7m | bilateral shoulders: Bil-HSScTSd | Δ Bil-HSScTS | none | decrease of Bil-HSScTS in 3/3 pate |
| Huwart [45] | 2018 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusionf bilateral hips: trochanteric and/or iliopsoas bursitis, synovitis/effusionf | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 improvement of bursitis at shoulders from 2 to 0.5 (P =0.018) at wk 12g no difference of synovitis/effusion at shoulders from baseline to wk 12 no difference of bursitis and synovitis/effusion at hips from baseline to wk 2 improvement of synovitis/effusion at hips from 2 to 0 (P =0.018) at wk 12g no difference of bursitis at hips from baseline to wk 12 |
| Devauchelle-Pensec [44] | 2016 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–14/20 (70) wk 2–12/20 (60) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: SAD bursitis and/or synovitish bilateral hips: trochanteric bursitis and/or synovitish | Δ proportion patients scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 decrease of pat scored 0–1 at hips from baseline to wk 2 from 87% to 46% (P =0.031) increase of pat scored 0–1 at hips from baseline to wk 12 from 13% to 69% (P =0.005) decrease of pat scored >1 at hips from baseline to wk 12 from 87% to 31% (P =0.023) |
| Huwart [45] | 2018 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusioni bilateral hips: trochanteric and/or iliopsoas bursitis, effusioni | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 12 improvement of bursitis from baseline to wk 2 at hips from 4 to 3 (P <0.001)g improvement of bursitis at hips from baseline to wk 12 from 4 to 2 (P <0.001)g no difference of synovitis/effusion at hips from baseline to wk 2 and 12 |
| Laporte [27] | 2019 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 2–13/18 (72) wk 12–15/18 (83) | wk 0, 2, 12 | bilateral shouldersj: supraspinatus, infraspinatus, subscapularis, teres minor bilateral hipj: glutaeal, iliopsoas, pelvitrochanteric muscles bilateral ischial tuberosityj: semitendinosus, semimembranosus, long head biceps femoris bilateral pubic symphysisj: adductors | Δ proportion myofascial lesions | none | decline from 70 to 60 lesions from baseline to wk 12 (P = 0.002) improvement of 66 (64.1%), worsening of 2 (1.9%) and unchanged 35 (34%) muscle groups (P =0.034) at wk 12 |
| Devauchelle-Pensec [44] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/20 (90) wk 2–14/20 (70) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: global SUVk bilateral hips: global SUVk | comparison global SUV | none | no difference of global SUV at shoulders from baseline to wk 2 and 12 decline of global SUV at hips from baseline to wk 2 from 6.4–5.3 (P =0.007)g decrease of global SUV at hips from baseline to wk 12 from 6.4–4.9, P <0.001g decline of global SUV at hips from wk 2 to wk 12 from 5.3–4.9, P =0.017g |
| Palard-Novello [31] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/18 (100) wk 2–16/18 (70) wk 12–16/18 (80) | wk 0, 2, 12 | bilateral shoulders: SUVmaxl bilateral sternoclavicular joints: SUVmaxl bilateral hips: SUVmaxl bilateral ischial tuberosities: SUVmaxl cervical and lumbar spinous processes: SUVmaxl | comparison SUVmax | ΔPMR-AS, ΔCRP and ΔESR | no correlation of ΔSUVmax with ΔPMR-AS, ΔCRP or ΔESR in patient-based analysis at wk 2 and wk 12 decline of SUVmax from baseline to wk 2 from 5.8–5.2 (P =0.026) and from baseline to wk 12 with 5.8–4.7 (P <0.001) in patient-based analysisg decline of SUVmax at left hip from baseline to wk 2 from 6.4–4.8 (P =0.024)g improvements of SUVmax at both hips, ischial tuberosities and lumbar spinous processes from baseline to wk 12 (all with P <0.05)m |
| Author . | Year . | Imaging technique . | inclusion criteria . | Patients at FU [n, (%)] . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Devauchelle-Pensec [44] | 2016 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 19/20 (95) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitisb bilateral hips: trochanteric bursitis, synovitisb | Δ proportion pat scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 no difference at hips from baseline to wk 2 and 12 |
| Suzuki [9] | 2017 | US | PMR like symptomsc + US examination w/o GC-treatment + clinical FU after 1 year | 3/15 (20) | after 0.9–8.7m | bilateral shoulders: Bil-HSScTSd | Δ Bil-HSScTS | none | decrease of Bil-HSScTS in 3/3 pate |
| Huwart [45] | 2018 | US | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusionf bilateral hips: trochanteric and/or iliopsoas bursitis, synovitis/effusionf | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 improvement of bursitis at shoulders from 2 to 0.5 (P =0.018) at wk 12g no difference of synovitis/effusion at shoulders from baseline to wk 12 no difference of bursitis and synovitis/effusion at hips from baseline to wk 2 improvement of synovitis/effusion at hips from 2 to 0 (P =0.018) at wk 12g no difference of bursitis at hips from baseline to wk 12 |
| Devauchelle-Pensec [44] | 2016 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–14/20 (70) wk 2–12/20 (60) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: SAD bursitis and/or synovitish bilateral hips: trochanteric bursitis and/or synovitish | Δ proportion patients scored 0–1, >1 | none | no difference at shoulders from baseline to wk 2 and 12 decrease of pat scored 0–1 at hips from baseline to wk 2 from 87% to 46% (P =0.031) increase of pat scored 0–1 at hips from baseline to wk 12 from 13% to 69% (P =0.005) decrease of pat scored >1 at hips from baseline to wk 12 from 87% to 31% (P =0.023) |
| Huwart [45] | 2018 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | 14/18 (78) | wk 0, 2 and 12 | bilateral shoulders: SAD bursitis, tenosynovitis LHBT, synovitis/effusioni bilateral hips: trochanteric and/or iliopsoas bursitis, effusioni | Δ semi-quantitative scores | none | no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 2 no difference of bursitis and synovitis/effusion at shoulders from baseline to wk 12 improvement of bursitis from baseline to wk 2 at hips from 4 to 3 (P <0.001)g improvement of bursitis at hips from baseline to wk 12 from 4 to 2 (P <0.001)g no difference of synovitis/effusion at hips from baseline to wk 2 and 12 |
| Laporte [27] | 2019 | MRI | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 2–13/18 (72) wk 12–15/18 (83) | wk 0, 2, 12 | bilateral shouldersj: supraspinatus, infraspinatus, subscapularis, teres minor bilateral hipj: glutaeal, iliopsoas, pelvitrochanteric muscles bilateral ischial tuberosityj: semitendinosus, semimembranosus, long head biceps femoris bilateral pubic symphysisj: adductors | Δ proportion myofascial lesions | none | decline from 70 to 60 lesions from baseline to wk 12 (P = 0.002) improvement of 66 (64.1%), worsening of 2 (1.9%) and unchanged 35 (34%) muscle groups (P =0.034) at wk 12 |
| Devauchelle-Pensec [44] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/20 (90) wk 2–14/20 (70) wk 12–16/20 (80) | wk 0, 2, 12 | bilateral shoulders: global SUVk bilateral hips: global SUVk | comparison global SUV | none | no difference of global SUV at shoulders from baseline to wk 2 and 12 decline of global SUV at hips from baseline to wk 2 from 6.4–5.3 (P =0.007)g decrease of global SUV at hips from baseline to wk 12 from 6.4–4.9, P <0.001g decline of global SUV at hips from wk 2 to wk 12 from 5.3–4.9, P =0.017g |
| Palard-Novello [31] | 2016 | FDG-PET | GC-free PMRa, symptom-onset < 12m, PMR-AS > 10 | wk 0–18/18 (100) wk 2–16/18 (70) wk 12–16/18 (80) | wk 0, 2, 12 | bilateral shoulders: SUVmaxl bilateral sternoclavicular joints: SUVmaxl bilateral hips: SUVmaxl bilateral ischial tuberosities: SUVmaxl cervical and lumbar spinous processes: SUVmaxl | comparison SUVmax | ΔPMR-AS, ΔCRP and ΔESR | no correlation of ΔSUVmax with ΔPMR-AS, ΔCRP or ΔESR in patient-based analysis at wk 2 and wk 12 decline of SUVmax from baseline to wk 2 from 5.8–5.2 (P =0.026) and from baseline to wk 12 with 5.8–4.7 (P <0.001) in patient-based analysisg decline of SUVmax at left hip from baseline to wk 2 from 6.4–4.8 (P =0.024)g improvements of SUVmax at both hips, ischial tuberosities and lumbar spinous processes from baseline to wk 12 (all with P <0.05)m |
Summary of characteristics and main findings of selected studies published between 2015–2020 on ultrasound, MRI and 18 F-fluorodeoxyglucose positron emission tomography (18 F-FDG-PET) in PMR. aFulfilling Chuang criteria. bB-mode assessment by a semi-quantitative score (0–3; 0 = normal, 1 = slight, 2 = moderate, 3 = severe). cFinal PMR based on the 2012 EULAR/ACR provisional criteria. dEvaluated by bilateral assessment of the hyperaemia adjacent to the anterior aspect of the subscapularis tendon (0–3): 0 = absent or minimal flow; 1 = single vessel dots or confluent linear shape signals shorter than half the length of the region; 2 = confluent linear shape signals longer than half the length of the region to be evaluated or confluent zone shape signals shorter than half the length of the region to be evaluated; 3 = confluent zone shape signals longer than half the length of the region eOnly descriptive results, no interference reported. fB-mode or power Doppler imaging were graded using the OMERACT semi-quantitative scale (0–3): 0 = no abnormalities; 1 = mild; 2 = moderate; 3 = marked gMedian values. hEvaluated by a 3 T superconductive magnet system for bursa and joint effusion and scored semi-quantitatively (0–3): 0 = normal; 1 = slight; 2 = moderate; 3 = severe. iBursitis and effusion were defined as a high T2 signal, as no contrast injection was used a high T2 signal could indicate synovitis and/or effusion, abnormalities were scored by the semi-quantitative OMERACT scale (0–3): 0 = no abnormalities; 1 = mild; 2 = moderate; 3 = marked. jMyofascial lesions were defined as a high T2 STIR signal either diffuse within the muscle or forming a line surrounding the muscle, a muscle group was considered positive if at least one muscle in the group revealed a lesion. kEvaluation of the median global standardized uptake values (SUVs) at the shoulders and pelvic girdle. lThe maximal SUV [SUVmax, SUV with the maximal intensity among the pixels of the region of interest (ROI)] of each ROI were calculated and compared with the liver reference SUVmax, only ROIs with SUVmax higher than that of the liver reference SUVmax were considered abnormal. mOriginal data provided median values of SUVmax for each site of bilateral investigated structures Δ: change; Bil-HSScTS: bilateral hyperemia on subscapularis tendon score; FU: follow-up; GC: glucocorticoid; LHBT: long head biceps tendon; m: month; max: maximal; n: number; n pat FU: number of patients in follow-up; N/A: not applicable; pat: patients; PET: 18F-FDG positron emission tomography; PMR-AS: PMR activity score; SAD: subacromial/subdeltoid; SUV: standardized uptake values; US: ultrasound; vs: versus ; wk: week.
| Author . | Year . | Imaging technique . | Inclusion criteria . | n pat . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Miceli [46] | 2017 | US | new diagnosis PMRa | 66 | 0, 12 m | bilateral shouldersb: bursitis SAD, tenosynovitis LHBT | clinical and laboratory remission after 12 mc, GC-dosage < 5mg/d after 12 m | MSUS+ vs MSUS– pat | no difference in achieving clinical remission among MSUS+ vs MSUS– pat at 12 md no difference in achieving laboratory remission among MSUS+ (50%) vs MSUS– (45%; P = n.s.) pat at 12 m no difference in achieving a GC-daily dosage < 5mg among MSUS+ (93%) vs MSUS– pat (80%, P = n.s.) at 12 m |
| Ayano [47] | 2020 | US | new diagnosis. PMRe | 56f | 0 | bilateral shouldersg: bursitis SAD, tenosynovitis LHBT | GC treatment response at 12 mh | GC-responders vs GC-inadequate responders | association of serum LDH ≥ 175IU/l and an inadequate GC-response (OR 27, 95% CI: 3, 274, P =0.006) association of total GS-score ≥5 and an inadequate GC response (OR 12, 95% CI: 1, 130, P =0.045) |
| Mackie [24] | 2015 | MRI | new diagnosis of PMRi (RA imaging controls) | 22 (16) | 0 | bilateral shoulders, hips, hands, knees, feet, spinej | GC responsiveness during median FU of 22 m | ´extracapsulaŕ vs ´non-extracapsulaŕ pattern | pat with ´extracapsulaŕ pattern were less likely able to stop GC within first year (7%) vs those with ´non-extracapsulaŕ pattern (57%, P =0.030) no difference of relapse-free pat (50% vs 25%), pat with relapses on ≥5 mg prednisone (21% vs 43%) or pat with required initial dose increase >15 mg (14% vs 13%) between pat with ´extracapsulaŕ and ´non-extracapsulaŕ pattern (all with P = n.s.) |
| Nakamura [28] | 2020 | MRI | new diagnosis of PMRi with MRI and US shoulder examination at baseline | 54 | 0 | dominant shoulderk enhancement of joint capsule, glenohumeral joint, rotator cuff tendon, biceps tendon, synovial hypertrophy, shoulder joint effusion, focal and/or diffuse bone oedema of humeral head | PMR recurrence within median FU of 30 m | PMR pat with recurrence vs w/o recurrence | PMR pat with recurrence less frequently revealed enhancement of rotator cuff tendon than those w/o recurrence (58% vs 87%, P =0.01), OR 0.2 (95% CI: 0.1, 0.8) synovial hypertrophy at baseline was more frequently observed in PMR pat with recurrence than in those w/o recurrence (21% vs 3%, P =0.04), OR 7.6 (95% CI: 0.8, 70.5) no association with enhancement of shoulder joint capsule and biceps tendon, shoulder joint effusion, focal bone oedema in humeral head and PMR recurrence (all with P = n.s.) |
| Prieto-Peña [36] | 2019 | FDG-PET | ‘classic’ PMR and PET/CT during FUl | 84 | N/A | supraaortic trunks, thoracic and abdominal aorta, iliac arteries and femoral/tibio-peroneal arteries | LVV | PET+ Vs PET- | bilateral diffuse lower limb pain (OR: 8.8, 95% CI: 1.7, 46.3; P =0.01), pelvic girdle pain (OR = 4.9, 95% CI: 1.5, 16.5; P =0.01) and inflammatory low back pain (OR= 4.7, 95% CI: 1, 21.5; P =0.04) were associated with LVV |
| Author . | Year . | Imaging technique . | Inclusion criteria . | n pat . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Miceli [46] | 2017 | US | new diagnosis PMRa | 66 | 0, 12 m | bilateral shouldersb: bursitis SAD, tenosynovitis LHBT | clinical and laboratory remission after 12 mc, GC-dosage < 5mg/d after 12 m | MSUS+ vs MSUS– pat | no difference in achieving clinical remission among MSUS+ vs MSUS– pat at 12 md no difference in achieving laboratory remission among MSUS+ (50%) vs MSUS– (45%; P = n.s.) pat at 12 m no difference in achieving a GC-daily dosage < 5mg among MSUS+ (93%) vs MSUS– pat (80%, P = n.s.) at 12 m |
| Ayano [47] | 2020 | US | new diagnosis. PMRe | 56f | 0 | bilateral shouldersg: bursitis SAD, tenosynovitis LHBT | GC treatment response at 12 mh | GC-responders vs GC-inadequate responders | association of serum LDH ≥ 175IU/l and an inadequate GC-response (OR 27, 95% CI: 3, 274, P =0.006) association of total GS-score ≥5 and an inadequate GC response (OR 12, 95% CI: 1, 130, P =0.045) |
| Mackie [24] | 2015 | MRI | new diagnosis of PMRi (RA imaging controls) | 22 (16) | 0 | bilateral shoulders, hips, hands, knees, feet, spinej | GC responsiveness during median FU of 22 m | ´extracapsulaŕ vs ´non-extracapsulaŕ pattern | pat with ´extracapsulaŕ pattern were less likely able to stop GC within first year (7%) vs those with ´non-extracapsulaŕ pattern (57%, P =0.030) no difference of relapse-free pat (50% vs 25%), pat with relapses on ≥5 mg prednisone (21% vs 43%) or pat with required initial dose increase >15 mg (14% vs 13%) between pat with ´extracapsulaŕ and ´non-extracapsulaŕ pattern (all with P = n.s.) |
| Nakamura [28] | 2020 | MRI | new diagnosis of PMRi with MRI and US shoulder examination at baseline | 54 | 0 | dominant shoulderk enhancement of joint capsule, glenohumeral joint, rotator cuff tendon, biceps tendon, synovial hypertrophy, shoulder joint effusion, focal and/or diffuse bone oedema of humeral head | PMR recurrence within median FU of 30 m | PMR pat with recurrence vs w/o recurrence | PMR pat with recurrence less frequently revealed enhancement of rotator cuff tendon than those w/o recurrence (58% vs 87%, P =0.01), OR 0.2 (95% CI: 0.1, 0.8) synovial hypertrophy at baseline was more frequently observed in PMR pat with recurrence than in those w/o recurrence (21% vs 3%, P =0.04), OR 7.6 (95% CI: 0.8, 70.5) no association with enhancement of shoulder joint capsule and biceps tendon, shoulder joint effusion, focal bone oedema in humeral head and PMR recurrence (all with P = n.s.) |
| Prieto-Peña [36] | 2019 | FDG-PET | ‘classic’ PMR and PET/CT during FUl | 84 | N/A | supraaortic trunks, thoracic and abdominal aorta, iliac arteries and femoral/tibio-peroneal arteries | LVV | PET+ Vs PET- | bilateral diffuse lower limb pain (OR: 8.8, 95% CI: 1.7, 46.3; P =0.01), pelvic girdle pain (OR = 4.9, 95% CI: 1.5, 16.5; P =0.01) and inflammatory low back pain (OR= 4.7, 95% CI: 1, 21.5; P =0.04) were associated with LVV |
Summary of study characteristics and main findings for outcome prediction of ultrasound, MRI and 18 F-fluorodeoxyglucose positron emission tomography (18 F-FDG-PET) in PMR. aAccording to the ACR/EULAR 2012 provisional clinical criteria for PMR without considering the optional US criteria. bB-MODE evaluation with a dichotomous description (presence/absence), SAD bursitis was considered if the maximal thickness of hypoechoic signal within the bursa was >2 mm, LHB tenosynovitis was defined as thickness of the hypoechoic halo of fluid surrounding the biceps tendon >2 mm, Doppler modalities were not considered, the presence of at least monolateral SB or LHB tenosynovitis was considered as a positive pattern. cClinical remission was defined as the lack of girdle pain and laboratory remission as levels of ESR ≤40 mm/h and CRP ≤5 mg/l, a low prednisone dose in remission was considered if prednisone was taken at <5 mg/day. dOnly descriptive results. eBased on the Bird criteria, reclassified according to the 2012 ACR/EULAR classification criteria for PMR. fFrom two cohorts: 32 patients included in a derivation cohort and 24 patients included in a validation cohort. gGrey-scale evaluation of LHB tenosynovitis and SAD bursitis were scored semi-quantitatively on each side (0–3, 0 = absent, 1 = minimal accumulation of fluid, 2 = moderate distension without stretching of peripheral structures, 3 = significant accumulation of fluid to stretch the walls of the structure), a total grey-scale score was calculated as the sum of the GS scores of LHB tenosynovitis and SAD bursitis bilaterally ranging from 0 to 12, evaluation of power Doppler US and assessment of glenohumeral synovitis were not considered. hGC-responders were defined as patients receiving ≤5mg of prednisolone/day 1 year after diagnosis without any relapse, GC-inadequate responders were defined as patients who developed a relapse at any time or who were still taking >5mg prednisolone/day 1 year after diagnosis, a relapse was defined as the reappearance of symptoms along with increased ESR- and/or CRP-levels requiring an increased prednisolone dose or addition of immunosuppressive drugs. iAccording to Bird criteria. jWhole-body, multiple-joint, contrast-enhanced MRI with semi-quantitative scoring of inflammatory signs (0–3: 0 = no; 1 = mild; 2 = moderate; or 3 = severe inflammation), respectively, judged by two independent investigators, each MRI was additionally classified as ‘extracapsular pattern’ or ‘non-extracapsular pattern’. kGadolinum-enhanced MRI (1.5 T) of the dominant shoulder, evaluated by independent radiologists, no detailed definitions of pathologic findings given lBased on 2012 EULAR/ACR classification criteria, a PET/CT was performed because of (1) persistence of classic PMR symptoms, despite GC-treatment of at least 15 mg/day prednisone, (2) occurrence of unusual manifestations, as inflammatory low back pain or bilateral diffuse lower limb pain (including intermittent claudication pain on movement in the lower extremities), (3) development of marked constitutional symptoms (with or without fever) during FU despite receiving GC-therapy, (4) unexplained increase of acute phase proteins (ESR a/o CRP). mImages evaluated by two experienced nuclear medicine specialists according to the intensity of the 18 F-FDG uptake by the vessel wall, images were visually evaluated grading the vascular FDG uptake in comparison to liver-uptake, PET/CT scans were considered positive for active LVV when a pattern of linear uptake was found in the aorta wall and its branches with an intensity similar or higher than the liver. CI: confidence interval; FU: follow-up; GC: glucocorticoid; LDH: lactate dehydrogenase; LHBT: long head biceps tendon; LVV: large vessel vasculitis; m: month; MSUS+: patients presenting with PMR-related MSUS findings at baseline; MSUS: patients lacking PMR-related MSUS findings at baseline; n: number; N/A: not applicable; n.s.: not significant; OR: odds ratio; pat: patients; PET: 18F-FDG positron emission tomography; P: P-value; SAD: subacromial/subdeltoid; US: ultrasound; vs: versus; w/o: without.
| Author . | Year . | Imaging technique . | Inclusion criteria . | n pat . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Miceli [46] | 2017 | US | new diagnosis PMRa | 66 | 0, 12 m | bilateral shouldersb: bursitis SAD, tenosynovitis LHBT | clinical and laboratory remission after 12 mc, GC-dosage < 5mg/d after 12 m | MSUS+ vs MSUS– pat | no difference in achieving clinical remission among MSUS+ vs MSUS– pat at 12 md no difference in achieving laboratory remission among MSUS+ (50%) vs MSUS– (45%; P = n.s.) pat at 12 m no difference in achieving a GC-daily dosage < 5mg among MSUS+ (93%) vs MSUS– pat (80%, P = n.s.) at 12 m |
| Ayano [47] | 2020 | US | new diagnosis. PMRe | 56f | 0 | bilateral shouldersg: bursitis SAD, tenosynovitis LHBT | GC treatment response at 12 mh | GC-responders vs GC-inadequate responders | association of serum LDH ≥ 175IU/l and an inadequate GC-response (OR 27, 95% CI: 3, 274, P =0.006) association of total GS-score ≥5 and an inadequate GC response (OR 12, 95% CI: 1, 130, P =0.045) |
| Mackie [24] | 2015 | MRI | new diagnosis of PMRi (RA imaging controls) | 22 (16) | 0 | bilateral shoulders, hips, hands, knees, feet, spinej | GC responsiveness during median FU of 22 m | ´extracapsulaŕ vs ´non-extracapsulaŕ pattern | pat with ´extracapsulaŕ pattern were less likely able to stop GC within first year (7%) vs those with ´non-extracapsulaŕ pattern (57%, P =0.030) no difference of relapse-free pat (50% vs 25%), pat with relapses on ≥5 mg prednisone (21% vs 43%) or pat with required initial dose increase >15 mg (14% vs 13%) between pat with ´extracapsulaŕ and ´non-extracapsulaŕ pattern (all with P = n.s.) |
| Nakamura [28] | 2020 | MRI | new diagnosis of PMRi with MRI and US shoulder examination at baseline | 54 | 0 | dominant shoulderk enhancement of joint capsule, glenohumeral joint, rotator cuff tendon, biceps tendon, synovial hypertrophy, shoulder joint effusion, focal and/or diffuse bone oedema of humeral head | PMR recurrence within median FU of 30 m | PMR pat with recurrence vs w/o recurrence | PMR pat with recurrence less frequently revealed enhancement of rotator cuff tendon than those w/o recurrence (58% vs 87%, P =0.01), OR 0.2 (95% CI: 0.1, 0.8) synovial hypertrophy at baseline was more frequently observed in PMR pat with recurrence than in those w/o recurrence (21% vs 3%, P =0.04), OR 7.6 (95% CI: 0.8, 70.5) no association with enhancement of shoulder joint capsule and biceps tendon, shoulder joint effusion, focal bone oedema in humeral head and PMR recurrence (all with P = n.s.) |
| Prieto-Peña [36] | 2019 | FDG-PET | ‘classic’ PMR and PET/CT during FUl | 84 | N/A | supraaortic trunks, thoracic and abdominal aorta, iliac arteries and femoral/tibio-peroneal arteries | LVV | PET+ Vs PET- | bilateral diffuse lower limb pain (OR: 8.8, 95% CI: 1.7, 46.3; P =0.01), pelvic girdle pain (OR = 4.9, 95% CI: 1.5, 16.5; P =0.01) and inflammatory low back pain (OR= 4.7, 95% CI: 1, 21.5; P =0.04) were associated with LVV |
| Author . | Year . | Imaging technique . | Inclusion criteria . | n pat . | Time points of imaging . | Investigated structures/lesions . | Outcome . | Comparison performed . | Summary of findings . |
|---|---|---|---|---|---|---|---|---|---|
| Miceli [46] | 2017 | US | new diagnosis PMRa | 66 | 0, 12 m | bilateral shouldersb: bursitis SAD, tenosynovitis LHBT | clinical and laboratory remission after 12 mc, GC-dosage < 5mg/d after 12 m | MSUS+ vs MSUS– pat | no difference in achieving clinical remission among MSUS+ vs MSUS– pat at 12 md no difference in achieving laboratory remission among MSUS+ (50%) vs MSUS– (45%; P = n.s.) pat at 12 m no difference in achieving a GC-daily dosage < 5mg among MSUS+ (93%) vs MSUS– pat (80%, P = n.s.) at 12 m |
| Ayano [47] | 2020 | US | new diagnosis. PMRe | 56f | 0 | bilateral shouldersg: bursitis SAD, tenosynovitis LHBT | GC treatment response at 12 mh | GC-responders vs GC-inadequate responders | association of serum LDH ≥ 175IU/l and an inadequate GC-response (OR 27, 95% CI: 3, 274, P =0.006) association of total GS-score ≥5 and an inadequate GC response (OR 12, 95% CI: 1, 130, P =0.045) |
| Mackie [24] | 2015 | MRI | new diagnosis of PMRi (RA imaging controls) | 22 (16) | 0 | bilateral shoulders, hips, hands, knees, feet, spinej | GC responsiveness during median FU of 22 m | ´extracapsulaŕ vs ´non-extracapsulaŕ pattern | pat with ´extracapsulaŕ pattern were less likely able to stop GC within first year (7%) vs those with ´non-extracapsulaŕ pattern (57%, P =0.030) no difference of relapse-free pat (50% vs 25%), pat with relapses on ≥5 mg prednisone (21% vs 43%) or pat with required initial dose increase >15 mg (14% vs 13%) between pat with ´extracapsulaŕ and ´non-extracapsulaŕ pattern (all with P = n.s.) |
| Nakamura [28] | 2020 | MRI | new diagnosis of PMRi with MRI and US shoulder examination at baseline | 54 | 0 | dominant shoulderk enhancement of joint capsule, glenohumeral joint, rotator cuff tendon, biceps tendon, synovial hypertrophy, shoulder joint effusion, focal and/or diffuse bone oedema of humeral head | PMR recurrence within median FU of 30 m | PMR pat with recurrence vs w/o recurrence | PMR pat with recurrence less frequently revealed enhancement of rotator cuff tendon than those w/o recurrence (58% vs 87%, P =0.01), OR 0.2 (95% CI: 0.1, 0.8) synovial hypertrophy at baseline was more frequently observed in PMR pat with recurrence than in those w/o recurrence (21% vs 3%, P =0.04), OR 7.6 (95% CI: 0.8, 70.5) no association with enhancement of shoulder joint capsule and biceps tendon, shoulder joint effusion, focal bone oedema in humeral head and PMR recurrence (all with P = n.s.) |
| Prieto-Peña [36] | 2019 | FDG-PET | ‘classic’ PMR and PET/CT during FUl | 84 | N/A | supraaortic trunks, thoracic and abdominal aorta, iliac arteries and femoral/tibio-peroneal arteries | LVV | PET+ Vs PET- | bilateral diffuse lower limb pain (OR: 8.8, 95% CI: 1.7, 46.3; P =0.01), pelvic girdle pain (OR = 4.9, 95% CI: 1.5, 16.5; P =0.01) and inflammatory low back pain (OR= 4.7, 95% CI: 1, 21.5; P =0.04) were associated with LVV |
Summary of study characteristics and main findings for outcome prediction of ultrasound, MRI and 18 F-fluorodeoxyglucose positron emission tomography (18 F-FDG-PET) in PMR. aAccording to the ACR/EULAR 2012 provisional clinical criteria for PMR without considering the optional US criteria. bB-MODE evaluation with a dichotomous description (presence/absence), SAD bursitis was considered if the maximal thickness of hypoechoic signal within the bursa was >2 mm, LHB tenosynovitis was defined as thickness of the hypoechoic halo of fluid surrounding the biceps tendon >2 mm, Doppler modalities were not considered, the presence of at least monolateral SB or LHB tenosynovitis was considered as a positive pattern. cClinical remission was defined as the lack of girdle pain and laboratory remission as levels of ESR ≤40 mm/h and CRP ≤5 mg/l, a low prednisone dose in remission was considered if prednisone was taken at <5 mg/day. dOnly descriptive results. eBased on the Bird criteria, reclassified according to the 2012 ACR/EULAR classification criteria for PMR. fFrom two cohorts: 32 patients included in a derivation cohort and 24 patients included in a validation cohort. gGrey-scale evaluation of LHB tenosynovitis and SAD bursitis were scored semi-quantitatively on each side (0–3, 0 = absent, 1 = minimal accumulation of fluid, 2 = moderate distension without stretching of peripheral structures, 3 = significant accumulation of fluid to stretch the walls of the structure), a total grey-scale score was calculated as the sum of the GS scores of LHB tenosynovitis and SAD bursitis bilaterally ranging from 0 to 12, evaluation of power Doppler US and assessment of glenohumeral synovitis were not considered. hGC-responders were defined as patients receiving ≤5mg of prednisolone/day 1 year after diagnosis without any relapse, GC-inadequate responders were defined as patients who developed a relapse at any time or who were still taking >5mg prednisolone/day 1 year after diagnosis, a relapse was defined as the reappearance of symptoms along with increased ESR- and/or CRP-levels requiring an increased prednisolone dose or addition of immunosuppressive drugs. iAccording to Bird criteria. jWhole-body, multiple-joint, contrast-enhanced MRI with semi-quantitative scoring of inflammatory signs (0–3: 0 = no; 1 = mild; 2 = moderate; or 3 = severe inflammation), respectively, judged by two independent investigators, each MRI was additionally classified as ‘extracapsular pattern’ or ‘non-extracapsular pattern’. kGadolinum-enhanced MRI (1.5 T) of the dominant shoulder, evaluated by independent radiologists, no detailed definitions of pathologic findings given lBased on 2012 EULAR/ACR classification criteria, a PET/CT was performed because of (1) persistence of classic PMR symptoms, despite GC-treatment of at least 15 mg/day prednisone, (2) occurrence of unusual manifestations, as inflammatory low back pain or bilateral diffuse lower limb pain (including intermittent claudication pain on movement in the lower extremities), (3) development of marked constitutional symptoms (with or without fever) during FU despite receiving GC-therapy, (4) unexplained increase of acute phase proteins (ESR a/o CRP). mImages evaluated by two experienced nuclear medicine specialists according to the intensity of the 18 F-FDG uptake by the vessel wall, images were visually evaluated grading the vascular FDG uptake in comparison to liver-uptake, PET/CT scans were considered positive for active LVV when a pattern of linear uptake was found in the aorta wall and its branches with an intensity similar or higher than the liver. CI: confidence interval; FU: follow-up; GC: glucocorticoid; LDH: lactate dehydrogenase; LHBT: long head biceps tendon; LVV: large vessel vasculitis; m: month; MSUS+: patients presenting with PMR-related MSUS findings at baseline; MSUS: patients lacking PMR-related MSUS findings at baseline; n: number; N/A: not applicable; n.s.: not significant; OR: odds ratio; pat: patients; PET: 18F-FDG positron emission tomography; P: P-value; SAD: subacromial/subdeltoid; US: ultrasound; vs: versus; w/o: without.
Imaging-based follow-up of PMR patients was conducted in a prospective, open-label trial on the safety and efficacy of tocilizumab (TCZ). In the TENOR trial [44], 20 glucocorticoid-free active PMR patients were treated with three TCZ infusions at week 0, 4 and 8 without concurrent glucocorticoids. At week 12, all patients achieved clinical remission or low-disease activity, while only moderate changes of inflammatory lesions at shoulders and hips were observed by FDF-PET/CT and MRI [31, 44, 45]. Using FDG-PET/CT, a decreased maximal standardized uptake value (SUVmax, which is a method for the quantification of FDG uptake reflecting the radioactive concentration of the pixel with the maximal intensity among all pixels within a region of interest) was observed in regions typically involved in PMR such as the spinous processes, hips, shoulders, sternoclavicular region and ischial tuberosities after 12 weeks (range SUVmax values baseline 4.3–6.4, at 12 weeks 4.2–5.0) along with a sharp decline of bio-clinical markers. Inflammation detected by FDG-PET/CT, however, did not equally change at all musculoskeletal sites. For example, the uptake at the cervical spinous processes and shoulders remained relatively stable, while it improved at hips, in the sternoclavicular region and at ischial tuberosities. In addition, changes in the SUVmax and those of clinical or laboratory parameters did not correlate. Possible explanations for this observation are that the response of FDG-PET/CT findings to glucocorticoid therapy is delayed or that FDG accumulation is influenced by degenerative changes, which are common in PMR patients and which might not sufficiently respond to TCZ treatment [31]. Using ultrasound and MRI in the same study, subacromial, trochanteric and iliopsoas bursitis, as well as intraarticular glenohumeral and coxofemoral effusions/synovitis were evaluated at baseline, at week 2 and at week 12. Inflammatory signs persisted in the first two weeks, eventually improving at week 12. Residual imaging abnormalities, however, were present at the last follow-up visit in 42% and 37% of cases using MRI and ultrasound, respectively [45]. In another analysis of this study that was focused on muscle involvement by MRI, the presence of localized inflammatory myofascial lesions in 70 out of 103 muscle groups was revealed (at shoulders, hips, ischial tuberosity and pubic symphysis) at baseline, which improved in 64% and persisted or worsened in 36% of muscle groups at week 12 [27]. Given that all these patients had no or minimal disease activity at this time point, the relevance of residual imaging abnormalities for treatment decisions remains unclear and requires further investigations.
Whether imaging might help to predict the outcome of PMR patients is also unknown. In a 12-month prospective follow-up study of 66 patients with PMR, it was demonstrated that the presence of ultrasound verified SAD bursitis and/or biceps tenosynovitis at diagnosis was not a predictor of glucocorticoid response nor for the need of higher glucocorticoid doses for maintenance of remission [46]. Another study, comparing laboratory parameters and bilateral shoulder ultrasound findings among PMR patients responding and non-responding to glucocorticoid therapy showed that serum lactate dehydrogenase (LDH) levels, as well as the extent of SAD bursitis and biceps tenosynovitis, were higher in the latter as compared with the former group [47]. An MRI study indicated that PMR patients presenting with a characteristic pattern of symmetrical, extracapsular inflammation adjacent to the greater trochanter, acetabulum, ischial tuberosity and/or symphysis pubis in conjunction with elevated IL-6 and/or CRP levels revealed a more favourable disease course with a good response to glucocorticoids as compared with patients without these findings [24]. In another MRI study, patients with a relapse yielded less enhancement of rotator cuff tendon and more synovial hypertrophy in glenohumeral joints at baseline than patients without a flare [28].
The question whether subclinical large vessel vasculitis (LVV) has any impact on disease outcomes in PMR was already addressed in an FDG-PET/CT study by Blockmans et al. more than ten years ago [13]. LVV was observed in ∼30% of individuals with PMR at baseline. A positive PET, however, was not related to a worse response to glucocorticoids nor to a higher relapse rate. Besides, residual FDG uptake at vessels was observed 3 and 6 months after baseline despite clinical remission. Patients with a persistent positive FDG-PET/CT, however, had a similar course to those with a negative follow-up scan. In another study of 84 consecutive patients with classical PMR fulfilling the EULAR/ACR classification criteria [36], persisting symptoms despite glucocorticoid therapy was the most frequent reason for performing an FDG-PET/CT scan revealing LVV in 51 (61%) of PMR cases. Diffuse lower limb pain, pelvic girdle pain and inflammatory low back pain were positive predictors for the presence of LVV [36]. One might conclude from this study that subclinical LVV is associated with persisting symptoms of PMR, particularly at lower limbs; however, this still needs to be confirmed by a proper study.
Summary and conclusions
Modern imaging techniques, particularly MRI and PET, provided novel insights into the anatomical basis of PMR. In addition to long-known findings such as SAD bursitis and fluid accumulation along the biceps tendon, inflammation of the interspinous bursae at the cervical and lumbar spine, as well as inflammation in the peritendon of proximal muscle insertions at hips, are novel findings that may help to differentiate PMR from possible disease mimickers such as EORA. LVV might be detected in 30–60% of PMR patients; however, it is as yet unclear whether these patients should be treated as PMR or GCA cases. At least in one study, PMR patients with LVV did not have a worse response to glucocorticoid doses normally used to treat PMR, and these patients did not yield a higher risk of flare as compared with other PMR patients. Future studies are required to better characterise the value of different imaging lesions for the diagnosis of PMR, to compare the value of different imaging techniques and to investigate the prognostic value of imaging findings for short- and long-term clinical outcomes.
Acknowledgements
We thank Martin Fruth, blikk Radiologie am Rheumazentrum Ruhrgebiet, Herne, Germany for providing us the MRI images. The scientific work of C.Du. is supported by the ‘Verein zur Förderung von Hämatologie, Onkologie und Immunologie Innsbruck’.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data of the literature search are available upon request.




Comments