-
PDF
- Split View
-
Views
-
Cite
Cite
Evrydiki Kravvariti, George Konstantonis, Petros P Sfikakis, Maria G Tektonidou, Progression of subclinical atherosclerosis in systemic lupus erythematosus versus rheumatoid arthritis: the impact of low disease activity, Rheumatology, Volume 57, Issue 12, December 2018, Pages 2158–2166, https://doi.org/10.1093/rheumatology/key233
Close - Share Icon Share
Abstract
The progression of subclinical atherosclerosis in SLE and RA has not been comparatively assessed. We sought to investigate the impact of low disease activity and other disease-related factors on atherosclerosis progression in SLE vs RA.
We performed a 3-year follow-up carotid and femoral artery ultrasound in 101 patients with SLE, 85 with RA and 85 controls after a baseline examination in 115 SLE and 1:1 age- and gender-matched RA patients and controls. We used logistic regression to compare atherosclerosis progression (new plaque development) between SLE and RA vs controls, and assess determinants of progression in SLE patients with different lupus low disease activity state (LLDAS) durations, adjusting for disease-related factors, antihypertensives, antiplatelets, statins and the Systemic Coronary Risk Evaluation 10-year cardiovascular risk.
The odds ratio (OR) of plaque progression vs controls was significantly higher in SLE (OR = 2.81, P = 0.043), but not in RA (OR = 2.22, P = 0.109). Results were similar in patients with low disease activity (88% of SLE, 74% of RA). Multivariate determinants of progression in SLE included antiphospholipid antibodies (OR = 2.00, P = 0.043) and Systemic Coronary Risk Evaluation (OR = 2.87, P = 0.019) for all patients, and additionally cumulative corticosteroid dose during follow-up (OR = 1.38, P = 0.013) and disease duration (OR = 1.20, P = 0.022) for patients in LLDAS over entire follow-up. Results were similar for patients with shorter LLDAS durations (>75% or >50% of follow-up).
Plaque progression is accelerated in SLE regardless of disease activity, and is associated with antiphospholipid antibodies and the Systemic Coronary Risk Evaluation. In LLDAS, cumulative corticosteroid dose and disease duration are additional determinants of progression.
Subclinical atherosclerosis in SLE is accelerated even in the setting of low disease activity.
Antiphospholipid antibodies and traditional cardiovascular risk factor burden are independent predictors of plaque progression in SLE.
Cumulative corticosteroid dose contributes to plaque progression in patients maintaining lupus low disease activity state.
Introduction
SLE and RA are characterized by high burden of atherosclerosis and mortality due to cardiovascular disease (CVD) [1, 2]. In a previous case-control study from our centre, we showed a comparable extent of subclinical atherosclerotic plaques between SLE and RA patients [3]. Although several studies have shown a progression of atherosclerotic plaques in patients with RA during their follow-up [4–13], only a few studies examined the progression of subclinical atherosclerosis in SLE [14–21]. A direct comparison of atherosclerosis progression between patients with SLE and those with RA has never been undertaken.
Subclinical atherosclerosis progression in RA has been found analogous to disease activity, cumulative prednisone doses and traditional CVD risk factors, but lower in those treated with TNF inhibitors [7, 8, 10, 12, 13]. Prospective studies of atherosclerosis in SLE are limited and characterized by heterogeneity in design, measured outcomes, and adjustment for confounders. Of six studies in the literature using vascular ultrasound to detect progression of subclinical atherosclerosis in SLE [15, 16, 18–21], only three included a healthy control group [15, 18, 21], and only two investigated the impact of SLE-related factors on plaque progression while controlling for traditional CVD risk factors [19, 21]. Disease-related risk factors for the progression of subclinical atherosclerosis in SLE include disease duration [14, 16, 18–20] and baseline activity and damage [14, 21]. Recent studies in RA have shown that maintaining low disease activity during the follow-up protects against the progression of subclinical atherosclerosis [4, 5]. It is not known if maintaining low disease activity in patients with SLE, as measured by the newly validated construct of lupus low disease activity state (LLDAS), alters the odds of atherosclerosis progression in these patients.
The aim of this study was to compare the progression of ultrasound-detected atherosclerotic plaques in the carotid and femoral arteries between patients with SLE and RA vs healthy controls, over a 3-year follow-up. We also examined the impact of LLDAS and other disease-related and traditional CVD risk factors on the progression of subclinical atherosclerosis in SLE.
Methods
Study design and population
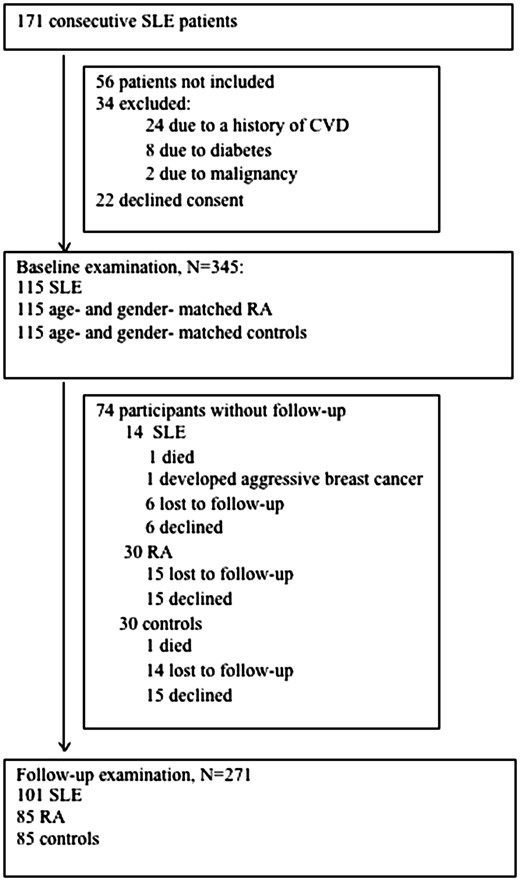
The study was conducted in the Cardiovascular Research Laboratory of the First Department of Propaedeutic Internal Medicine. Between 2012 and 2013, we performed the initial carotid and femoral artery ultrasound to identify subclinical atherosclerosis in 115 eligible, consecutive adult patients with SLE and 1:1 age- and sex- matched patients with RA and healthy controls from the local community [3]. The study flow chart is shown in Fig. 1. Three years after the initial examination, all participants were called to repeat the carotid and femoral ultrasound in order to evaluate the development of new atherosclerotic plaques. During the follow-up period, patients had routine clinical and laboratory evaluation every 3–6 months in our rheumatology outpatient clinic. In total, 271 participants had a follow-up examination (79% of initial sample), of which 101 patients with SLE, 85 patients with RA, and 85 healthy controls (Fig. 1). All participants provided informed consent in accordance to the declaration of Helsinki, and the study was approved by our hospital’s Institutional Review Board (Laikon General Hospital Scientific Council).
Recorded parameters
Besides the demographic characteristics (age, gender, ethnicity), the following data were recorded at baseline and 3-year follow-up ultrasound examination: laboratory parameters: complete blood count, ESR, C-reactive protein, serum creatinine, glucose, fasting lipid profile, C3 and C4 levels, antinuclear antibodies, anti-dsDNA antibodies, and antiphospholipid antibodies, including anti-cardiolipin antibodies (IgG or IgM isotype), anti-β2GPI antibodies (IgG or IgM isotype) and LA). Positivity for antiphospholipid antibodies was defined according to the updated Sapporo criteria for antiphospholipid syndrome [22]; medications: corticosteroids (cumulative dose prior to the baseline examination and during the follow-up period), hydroxycloroquine, immunosuppressants (methotrexate, leflunomide, azathioprine, cyclophosphamide, ciclosporin, mycophenolate mofetil), biologic agents (rituximab, infliximab, etanercept, adalimumab, abatacept, certolizumab, golimumab, anakinra, belimumab), and the use of antihypertensives, antiplatelets, anticoagulants, and statins; and traditional CVD risk factors: systolic and diastolic blood pressure after at least 10 min of rest, as the average of three sequential readings taken 1 min apart, (Microlife WatchBP Office, Microlife AG, Widnau, Switzerland), fasting total and high-density lipoprotein cholesterol levels, smoking status (measured in pack years), physical activity level (measured in min of exercise per week), family history of CVD, BMI calculated as weight/height2. For CVD risk stratification, we used the Systemic Coronary Risk Evaluation (SCORE) prediction of 10-year risk of fatal CVD according to the low-risk country charts, a validated instrument recommended by the European Society of Cardiology that includes age, gender, office measurements of systolic blood pressure, and fasting total and high-density lipoprotein cholesterol [23].
Disease activity indices were measured semi-annually at routine clinic visits, using the SLE disease activity index 2000 (SLEDAI-2K) [24] and physician global assessment [24] for SLE, and 28-joint-ESR DAS (DAS28-ESR) for RA [25]. The LLDAS was assessed according to the previously validated definition [26, 27] as SLEDAI-2K ⩽ 4 without major organ activity, no new disease activity, physician global assessment (0-3) ⩽ 1, prednisone ⩽7.5 mg/day and well-tolerated immunosuppressant dosages. Based on this definition, we identified patients in LLDAS for 100%, >75%, and >50% of their follow-up time (LLDAS-100, LLDAS-75 and LLDAS-50, respectively) [28]. We also identified SLE patients who maintained clinical remission throughout the follow-up period, defined as SLEDAI-2K = 0 excluding points for persistent anti-DNA antibodies or low complement, and allowing hydroxycloroquine, maintenance immunosuppressives, and low dose corticosteroids [29]. In patients with RA, low disease activity was defined as DAS28-ESR ⩽ 3.2 and remission as DAS28-ESR ⩽ 2.8 [25]. SLICC/ACR damage index score [30] was also recorded at baseline and the 3-year follow-up ultrasound examination.
Vascular ultrasound and outcome measures
The same experienced operator (G.K.), blinded to the participants’ disease status, performed all baseline and follow-up assessments using a high-resolution B-mode ultrasound device (Vivid 7 Pro, GE Healthcare) with a 14-MHz multi-frequency linear transducer. Detailed ultrasound methodology has been previously published [3, 12]. Atherosclerotic plaques were detected in the near and far walls of the common carotid arteries, carotid bulbs, internal carotid arteries and common femoral arteries, bilaterally. Plaques were defined as focal areas where intima-media thickness (IMT) was ⩾1.5 mm, or increased by either 0.5 mm or 50% compared with the IMT of the adjacent vascular wall. Atherosclerotic plaque progression was defined as the detection of an increased number of either carotid or femoral atherosclerotic plaques compared with the baseline examination.
Statistical analysis
We assessed correlations between quantitative parameters with Spearman correlation coefficient, due to deviations from normality. To assess differences between participant groups, we applied Kruskal-Wallis tests for quantitative variables, and chi-squared tests for qualitative characteristics.
We applied multiple logistic regression models using atherosclerotic plaque progression as the outcome variable, which was positive if new plaques were detected at the follow-up ultrasound examination compared with baseline. In order to derive a disease-specific odds ratio (OR) for progression vs controls, the models included an indicator variable with three levels denoting the participant group (0 = controls, 1 = SLE, 2 = RA), and were further adjusted for the SCORE prediction (continuously), and use of antihypertensives, antiplatelet agents and statins (categorically). We did not adapt the SCORE prediction by a 1.5 multiplication factor for RA patients, as the disease status was already included in the model. We then applied the same models restricting our sample to SLE and RA patients achieving low disease activity for >75% of the follow-up time, and the included SLE patients’ control matches.
To further investigate SLE-related determinants of atherosclerosis progression, we performed logistic regression models in the group of SLE patients only. Initially, we applied multiple logistic regression including baseline treatment with hydroxycloroquine, immunosuppressants, antihypertensives, antiplatelet agents, statins and presence of antiphospholipid antibodies at baseline as categorical variables, SCORE prediction, disease duration, and SLICC-damage index score at baseline as continuous variables, cumulative prednisone prior to baseline and cumulative prednisone during follow-up as continuous variables, and LLDAS-100 during follow-up as a categorical variable. We allowed the backward elimination algorithm (P > 0.1) to determine the variables included in the final model, except the SCORE prediction, which was included regardless of P-values to control for traditional risk factor confounding effects. We also applied logistic regression to assess the significance of an interaction between LLDAS-100 and the cumulative corticosteroid dose during follow-up, to allow for different corticosteroid effects on atherosclerotic plaque progression depending on disease activity status. Finally, because the interaction term was statistically significant, we performed separate stepwise multiple logistic regression analyses in the LLDAS-100, LLDAS-75, LLDAS-50, and clinical remission subgroups, in order to assess predictors of atherosclerosis in low activity patients. All analyses were performed using the STATA software (version 12.0, College Station, Texas, USA).
Results
Cohort characteristics
A follow-up carotid and femoral ultrasound examination was performed in 271 subjects: 101 patients with SLE, 85 patients with RA and 85 healthy controls. All participants were Caucasians and have no previous CVD history. Characteristics of three groups are shown in Table 1. There were no statistically significant differences in age, gender, time to follow-up or baseline SCORE prediction between the groups. SLE patients had a higher number of smoking pack years at baseline compared with the other two groups, and a higher frequency of antiplatelet, anticoagulant and antihypertensive medication use. Patients with RA had the highest mean blood pressure and the lowest exercise level at baseline; in addition, they had the highest rate of statin treatment initiation during follow-up (Table 1).
| . | SLE (n = 101) . | RA (n = 85) . | Controls (n = 85) . | |||
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age, years | 44 (12) | 48 (10) | 44 (13) | |||
| Female gender, % | 92 | 92 | 89 | |||
| Disease duration, years | 8.6 (7.5) | 9.9 (7.5) | NA | |||
| Follow-up time, years | 3.4 (0.5) | 3.2 (0.3) | 3.3 (0.8) | |||
| SCORE prediction | 0.3 (0.8) | 0.4 (1.0) | 0.3 (0.8) | |||
| Family history of CAD, % | 13 | 17 | 13 | |||
| Cumulative prednisone from disease diagnosis to baseline, g | 9.6 (12.3) | 7.6 (16.1) | NA | |||
| Data monitored during the entire follow-up period | ||||||
| Disease activity indices | ||||||
| Average SLEDAI | 1.4 (1.8) | NA | ||||
| Average DAS-28-ESR | NA | 2.9 (1.0) | NA | |||
| Low activity for >75% of the follow-up time, % | 88 | 74 | NA | |||
| Clinical remission, % | 37 | 51 | NA | |||
| Corticosteroid use, % | 65 | 78 | 0 | |||
| Prednisone daily dose, mg | 6.9 (10.5) | 4.1 (3.4) | 0 | |||
| Cumulative prednisone, g | 4.4 (5.6) | 4.1 (3.1) | 0 | |||
| Data at baseline and end of follow-up | Baseline | End of follow-up | Baseline | End of follow-up | Baseline | End of follow-up |
| Disease activity indices | ||||||
| SLEDAI | 2.3 (3.8) | 1.0 (1.8) | NA | NA | NA | NA |
| DAS-28-ESR | NA | NA | 3.4 (1.2 ) | 2.8 (1.2) | ||
| Systolic blood pressure, mmHg | 120 (18) | 116 (14 | 128 (17 ) | 121 (15) | 122 (17) | 120 (20) |
| Smoking pack, years | 13 (17) | 14 (18) | 7 (12) | 8 (13) | 7 (11) | 7 (12) |
| Total cholesterol, mg/dl | 201 (43) | 189 (31) | 215 (42) | 203 (38) | 201 (34) | 200 (35) |
| HDL, mg/dl | 63 (23) | 60 (17) | 67 (18) | 64 (15) | 60 (16) | 61 (14) |
| BMI, kg/m2 | 26 (6) | 27 (6) | 27 (5) | 28 (6) | 25 (5) | 26 (6) |
| Exercise level, min/week | 131 (151) | 113 (163) | 100 (163) | 124 (178) | 148 (187) | 122 (152) |
| Antihypertensives, % | 37 | 30 | 21 | 28 | 18 | 26 |
| Antiplatelet agents, % | 42 | 28 | 4 | 8 | 2 | 4 |
| Anticoagulants, % | 17 | 12 | 0 | 0 | 2 | 2 |
| Statins, % | 9 | 16 | 7 | 25 | 6 | 11 |
| Corticosteroids, % | 58 | 38 | 65 | 60 | 0 | 0 |
| HCQ, % | 65 | 81 | 5 | 11 | 0 | 0 |
| Immune suppressants | ||||||
| Biologics, % | 0 | 3 | 47 | 48 | NA | NA |
| Other, % | 40 | 34 | 59 | 41 | ||
| . | SLE (n = 101) . | RA (n = 85) . | Controls (n = 85) . | |||
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age, years | 44 (12) | 48 (10) | 44 (13) | |||
| Female gender, % | 92 | 92 | 89 | |||
| Disease duration, years | 8.6 (7.5) | 9.9 (7.5) | NA | |||
| Follow-up time, years | 3.4 (0.5) | 3.2 (0.3) | 3.3 (0.8) | |||
| SCORE prediction | 0.3 (0.8) | 0.4 (1.0) | 0.3 (0.8) | |||
| Family history of CAD, % | 13 | 17 | 13 | |||
| Cumulative prednisone from disease diagnosis to baseline, g | 9.6 (12.3) | 7.6 (16.1) | NA | |||
| Data monitored during the entire follow-up period | ||||||
| Disease activity indices | ||||||
| Average SLEDAI | 1.4 (1.8) | NA | ||||
| Average DAS-28-ESR | NA | 2.9 (1.0) | NA | |||
| Low activity for >75% of the follow-up time, % | 88 | 74 | NA | |||
| Clinical remission, % | 37 | 51 | NA | |||
| Corticosteroid use, % | 65 | 78 | 0 | |||
| Prednisone daily dose, mg | 6.9 (10.5) | 4.1 (3.4) | 0 | |||
| Cumulative prednisone, g | 4.4 (5.6) | 4.1 (3.1) | 0 | |||
| Data at baseline and end of follow-up | Baseline | End of follow-up | Baseline | End of follow-up | Baseline | End of follow-up |
| Disease activity indices | ||||||
| SLEDAI | 2.3 (3.8) | 1.0 (1.8) | NA | NA | NA | NA |
| DAS-28-ESR | NA | NA | 3.4 (1.2 ) | 2.8 (1.2) | ||
| Systolic blood pressure, mmHg | 120 (18) | 116 (14 | 128 (17 ) | 121 (15) | 122 (17) | 120 (20) |
| Smoking pack, years | 13 (17) | 14 (18) | 7 (12) | 8 (13) | 7 (11) | 7 (12) |
| Total cholesterol, mg/dl | 201 (43) | 189 (31) | 215 (42) | 203 (38) | 201 (34) | 200 (35) |
| HDL, mg/dl | 63 (23) | 60 (17) | 67 (18) | 64 (15) | 60 (16) | 61 (14) |
| BMI, kg/m2 | 26 (6) | 27 (6) | 27 (5) | 28 (6) | 25 (5) | 26 (6) |
| Exercise level, min/week | 131 (151) | 113 (163) | 100 (163) | 124 (178) | 148 (187) | 122 (152) |
| Antihypertensives, % | 37 | 30 | 21 | 28 | 18 | 26 |
| Antiplatelet agents, % | 42 | 28 | 4 | 8 | 2 | 4 |
| Anticoagulants, % | 17 | 12 | 0 | 0 | 2 | 2 |
| Statins, % | 9 | 16 | 7 | 25 | 6 | 11 |
| Corticosteroids, % | 58 | 38 | 65 | 60 | 0 | 0 |
| HCQ, % | 65 | 81 | 5 | 11 | 0 | 0 |
| Immune suppressants | ||||||
| Biologics, % | 0 | 3 | 47 | 48 | NA | NA |
| Other, % | 40 | 34 | 59 | 41 | ||
Values represent mean (s.d.) unless otherwise stated.
SCORE: Systemic Coronary Risk Evaluation prediction of 10-year fatal cardiovascular disease risk according to the low-risk country charts published by the European Society of Cardiology; CAD: coronary artery disease; DAS-28-ESR: 28-joint ESR DAS; HDL: high-density lipoprotein; NA: not applicable.
| . | SLE (n = 101) . | RA (n = 85) . | Controls (n = 85) . | |||
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age, years | 44 (12) | 48 (10) | 44 (13) | |||
| Female gender, % | 92 | 92 | 89 | |||
| Disease duration, years | 8.6 (7.5) | 9.9 (7.5) | NA | |||
| Follow-up time, years | 3.4 (0.5) | 3.2 (0.3) | 3.3 (0.8) | |||
| SCORE prediction | 0.3 (0.8) | 0.4 (1.0) | 0.3 (0.8) | |||
| Family history of CAD, % | 13 | 17 | 13 | |||
| Cumulative prednisone from disease diagnosis to baseline, g | 9.6 (12.3) | 7.6 (16.1) | NA | |||
| Data monitored during the entire follow-up period | ||||||
| Disease activity indices | ||||||
| Average SLEDAI | 1.4 (1.8) | NA | ||||
| Average DAS-28-ESR | NA | 2.9 (1.0) | NA | |||
| Low activity for >75% of the follow-up time, % | 88 | 74 | NA | |||
| Clinical remission, % | 37 | 51 | NA | |||
| Corticosteroid use, % | 65 | 78 | 0 | |||
| Prednisone daily dose, mg | 6.9 (10.5) | 4.1 (3.4) | 0 | |||
| Cumulative prednisone, g | 4.4 (5.6) | 4.1 (3.1) | 0 | |||
| Data at baseline and end of follow-up | Baseline | End of follow-up | Baseline | End of follow-up | Baseline | End of follow-up |
| Disease activity indices | ||||||
| SLEDAI | 2.3 (3.8) | 1.0 (1.8) | NA | NA | NA | NA |
| DAS-28-ESR | NA | NA | 3.4 (1.2 ) | 2.8 (1.2) | ||
| Systolic blood pressure, mmHg | 120 (18) | 116 (14 | 128 (17 ) | 121 (15) | 122 (17) | 120 (20) |
| Smoking pack, years | 13 (17) | 14 (18) | 7 (12) | 8 (13) | 7 (11) | 7 (12) |
| Total cholesterol, mg/dl | 201 (43) | 189 (31) | 215 (42) | 203 (38) | 201 (34) | 200 (35) |
| HDL, mg/dl | 63 (23) | 60 (17) | 67 (18) | 64 (15) | 60 (16) | 61 (14) |
| BMI, kg/m2 | 26 (6) | 27 (6) | 27 (5) | 28 (6) | 25 (5) | 26 (6) |
| Exercise level, min/week | 131 (151) | 113 (163) | 100 (163) | 124 (178) | 148 (187) | 122 (152) |
| Antihypertensives, % | 37 | 30 | 21 | 28 | 18 | 26 |
| Antiplatelet agents, % | 42 | 28 | 4 | 8 | 2 | 4 |
| Anticoagulants, % | 17 | 12 | 0 | 0 | 2 | 2 |
| Statins, % | 9 | 16 | 7 | 25 | 6 | 11 |
| Corticosteroids, % | 58 | 38 | 65 | 60 | 0 | 0 |
| HCQ, % | 65 | 81 | 5 | 11 | 0 | 0 |
| Immune suppressants | ||||||
| Biologics, % | 0 | 3 | 47 | 48 | NA | NA |
| Other, % | 40 | 34 | 59 | 41 | ||
| . | SLE (n = 101) . | RA (n = 85) . | Controls (n = 85) . | |||
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age, years | 44 (12) | 48 (10) | 44 (13) | |||
| Female gender, % | 92 | 92 | 89 | |||
| Disease duration, years | 8.6 (7.5) | 9.9 (7.5) | NA | |||
| Follow-up time, years | 3.4 (0.5) | 3.2 (0.3) | 3.3 (0.8) | |||
| SCORE prediction | 0.3 (0.8) | 0.4 (1.0) | 0.3 (0.8) | |||
| Family history of CAD, % | 13 | 17 | 13 | |||
| Cumulative prednisone from disease diagnosis to baseline, g | 9.6 (12.3) | 7.6 (16.1) | NA | |||
| Data monitored during the entire follow-up period | ||||||
| Disease activity indices | ||||||
| Average SLEDAI | 1.4 (1.8) | NA | ||||
| Average DAS-28-ESR | NA | 2.9 (1.0) | NA | |||
| Low activity for >75% of the follow-up time, % | 88 | 74 | NA | |||
| Clinical remission, % | 37 | 51 | NA | |||
| Corticosteroid use, % | 65 | 78 | 0 | |||
| Prednisone daily dose, mg | 6.9 (10.5) | 4.1 (3.4) | 0 | |||
| Cumulative prednisone, g | 4.4 (5.6) | 4.1 (3.1) | 0 | |||
| Data at baseline and end of follow-up | Baseline | End of follow-up | Baseline | End of follow-up | Baseline | End of follow-up |
| Disease activity indices | ||||||
| SLEDAI | 2.3 (3.8) | 1.0 (1.8) | NA | NA | NA | NA |
| DAS-28-ESR | NA | NA | 3.4 (1.2 ) | 2.8 (1.2) | ||
| Systolic blood pressure, mmHg | 120 (18) | 116 (14 | 128 (17 ) | 121 (15) | 122 (17) | 120 (20) |
| Smoking pack, years | 13 (17) | 14 (18) | 7 (12) | 8 (13) | 7 (11) | 7 (12) |
| Total cholesterol, mg/dl | 201 (43) | 189 (31) | 215 (42) | 203 (38) | 201 (34) | 200 (35) |
| HDL, mg/dl | 63 (23) | 60 (17) | 67 (18) | 64 (15) | 60 (16) | 61 (14) |
| BMI, kg/m2 | 26 (6) | 27 (6) | 27 (5) | 28 (6) | 25 (5) | 26 (6) |
| Exercise level, min/week | 131 (151) | 113 (163) | 100 (163) | 124 (178) | 148 (187) | 122 (152) |
| Antihypertensives, % | 37 | 30 | 21 | 28 | 18 | 26 |
| Antiplatelet agents, % | 42 | 28 | 4 | 8 | 2 | 4 |
| Anticoagulants, % | 17 | 12 | 0 | 0 | 2 | 2 |
| Statins, % | 9 | 16 | 7 | 25 | 6 | 11 |
| Corticosteroids, % | 58 | 38 | 65 | 60 | 0 | 0 |
| HCQ, % | 65 | 81 | 5 | 11 | 0 | 0 |
| Immune suppressants | ||||||
| Biologics, % | 0 | 3 | 47 | 48 | NA | NA |
| Other, % | 40 | 34 | 59 | 41 | ||
Values represent mean (s.d.) unless otherwise stated.
SCORE: Systemic Coronary Risk Evaluation prediction of 10-year fatal cardiovascular disease risk according to the low-risk country charts published by the European Society of Cardiology; CAD: coronary artery disease; DAS-28-ESR: 28-joint ESR DAS; HDL: high-density lipoprotein; NA: not applicable.
Among patients with SLE, antiphospholipid antibodies were prevalent in 38% both at baseline and follow-up ultrasound examinations, and 17% also met criteria for the antiphospholipid syndrome. A history of major SLE manifestations was present in 47% of patients at baseline and was most commonly lupus nephritis (27%), followed by serositis (25%), CNS involvement (15%), alopecia (13%), angiitis (6%), severe cytopenia (4%) and pneumonitis (1%). Average SLEDAI was 2.3 (3.8) at baseline and 1.0 (1.8) at the follow-up ultrasound examination. SLICC was 0.4 (0.5) at baseline and increased to 0.6 (0.9) at the follow-up examination. Other disease-related characteristics and rheumatologic medication use for patients with SLE and RA are presented in Tables 1 and 2.
Baseline characteristics and atherosclerotic plaque prevalence in SLE patients, according to LDA status
| Parameter . | SLE . | P-value . | RA . | . | ||
|---|---|---|---|---|---|---|
| LDA (n = 89) . | Active (n = 12) . | LDA (n = 63) . | Active (n = 22) . | P-value . | ||
| Age, mean (s.d.), years | 45 (12) | 38 (12) | 0.069 | 48 (11) | 48 (9) | 0.865 |
| Female, % | 92 | 92 | 0.955 | 92 | 82 | 0.179 |
| Disease duration | 8.7 (7.5) | 8.5 (7.9) | 0.724 | 9.2 (10.6) | 11.7 (8.5) | 0.026* |
| SCORE, mean (s.d.) | 0.4 (0.8) | 0.2 (0.4) | 0.528* | 0.5 (1.1) | 0.4 (0.7) | 0.990 |
| HCQ at baseline, % | 70 | 33 | 0.013 | 3 | 9 | 0.259 |
| HCQ at follow-up, % | 80 | 92 | 0.322 | 11 | 9 | 0.791 |
| Corticosteroids at baseline, % | 58 | 83 | 0.096 | 64 | 68 | 0.692 |
| Prednisone daily dose (mg) at baseline, mean (s.d.) | 4.7 (6.4) | 22.7 (19.1) | <0.001* | 4.0 (3.5) | 4.5 (3.4) | 0.533* |
| Cumulative prednisone dose (g) from diagnosis to baseline, mean (s.d.) | 8.8 (11.6) | 15.5 (15.6) | 0.143* | 8.8 (19.4) | 5.3 (6.3) | 0.910* |
| Cumulative prednisone during follow-up, mean (s.d.) | 3.2 (3.9) | 13.3 (7.9) | <0.001* | 3.6 (3.5) | 5.4 (4.1) | 0.052* |
| Immunosuppressants at baseline, % | 36 | 67 | 0.041 | 65 | 41 | 0.047 |
| Immunosuppressants at follow-up, % | 30 | 67 | 0.013 | 46 | 27 | 0.124 |
| Parameter . | SLE . | P-value . | RA . | . | ||
|---|---|---|---|---|---|---|
| LDA (n = 89) . | Active (n = 12) . | LDA (n = 63) . | Active (n = 22) . | P-value . | ||
| Age, mean (s.d.), years | 45 (12) | 38 (12) | 0.069 | 48 (11) | 48 (9) | 0.865 |
| Female, % | 92 | 92 | 0.955 | 92 | 82 | 0.179 |
| Disease duration | 8.7 (7.5) | 8.5 (7.9) | 0.724 | 9.2 (10.6) | 11.7 (8.5) | 0.026* |
| SCORE, mean (s.d.) | 0.4 (0.8) | 0.2 (0.4) | 0.528* | 0.5 (1.1) | 0.4 (0.7) | 0.990 |
| HCQ at baseline, % | 70 | 33 | 0.013 | 3 | 9 | 0.259 |
| HCQ at follow-up, % | 80 | 92 | 0.322 | 11 | 9 | 0.791 |
| Corticosteroids at baseline, % | 58 | 83 | 0.096 | 64 | 68 | 0.692 |
| Prednisone daily dose (mg) at baseline, mean (s.d.) | 4.7 (6.4) | 22.7 (19.1) | <0.001* | 4.0 (3.5) | 4.5 (3.4) | 0.533* |
| Cumulative prednisone dose (g) from diagnosis to baseline, mean (s.d.) | 8.8 (11.6) | 15.5 (15.6) | 0.143* | 8.8 (19.4) | 5.3 (6.3) | 0.910* |
| Cumulative prednisone during follow-up, mean (s.d.) | 3.2 (3.9) | 13.3 (7.9) | <0.001* | 3.6 (3.5) | 5.4 (4.1) | 0.052* |
| Immunosuppressants at baseline, % | 36 | 67 | 0.041 | 65 | 41 | 0.047 |
| Immunosuppressants at follow-up, % | 30 | 67 | 0.013 | 46 | 27 | 0.124 |
P-values derived from chi-squared tests for qualitative and Student’s test for quantitative variables, except when marked with *, in which case the Mann–Whitney test was used due to deviation from normality. LDA was defined as fulfilment of the following criteria for at least 75% of the follow-up time: DAS28-ESR ≤ 2.8 in RA, and as SLEDAI-2K ≤ 4 and PGA (0-3) ≤1 without new disease activity or major organ activity, prednisone ≤7.5 mg/day and/or well-tolerated immunosuppressant dosages in SLE.
SCORE: Systemic Coronary Risk Evaluation; LDA: low disease activity; PGA: physician global assessment.
Baseline characteristics and atherosclerotic plaque prevalence in SLE patients, according to LDA status
| Parameter . | SLE . | P-value . | RA . | . | ||
|---|---|---|---|---|---|---|
| LDA (n = 89) . | Active (n = 12) . | LDA (n = 63) . | Active (n = 22) . | P-value . | ||
| Age, mean (s.d.), years | 45 (12) | 38 (12) | 0.069 | 48 (11) | 48 (9) | 0.865 |
| Female, % | 92 | 92 | 0.955 | 92 | 82 | 0.179 |
| Disease duration | 8.7 (7.5) | 8.5 (7.9) | 0.724 | 9.2 (10.6) | 11.7 (8.5) | 0.026* |
| SCORE, mean (s.d.) | 0.4 (0.8) | 0.2 (0.4) | 0.528* | 0.5 (1.1) | 0.4 (0.7) | 0.990 |
| HCQ at baseline, % | 70 | 33 | 0.013 | 3 | 9 | 0.259 |
| HCQ at follow-up, % | 80 | 92 | 0.322 | 11 | 9 | 0.791 |
| Corticosteroids at baseline, % | 58 | 83 | 0.096 | 64 | 68 | 0.692 |
| Prednisone daily dose (mg) at baseline, mean (s.d.) | 4.7 (6.4) | 22.7 (19.1) | <0.001* | 4.0 (3.5) | 4.5 (3.4) | 0.533* |
| Cumulative prednisone dose (g) from diagnosis to baseline, mean (s.d.) | 8.8 (11.6) | 15.5 (15.6) | 0.143* | 8.8 (19.4) | 5.3 (6.3) | 0.910* |
| Cumulative prednisone during follow-up, mean (s.d.) | 3.2 (3.9) | 13.3 (7.9) | <0.001* | 3.6 (3.5) | 5.4 (4.1) | 0.052* |
| Immunosuppressants at baseline, % | 36 | 67 | 0.041 | 65 | 41 | 0.047 |
| Immunosuppressants at follow-up, % | 30 | 67 | 0.013 | 46 | 27 | 0.124 |
| Parameter . | SLE . | P-value . | RA . | . | ||
|---|---|---|---|---|---|---|
| LDA (n = 89) . | Active (n = 12) . | LDA (n = 63) . | Active (n = 22) . | P-value . | ||
| Age, mean (s.d.), years | 45 (12) | 38 (12) | 0.069 | 48 (11) | 48 (9) | 0.865 |
| Female, % | 92 | 92 | 0.955 | 92 | 82 | 0.179 |
| Disease duration | 8.7 (7.5) | 8.5 (7.9) | 0.724 | 9.2 (10.6) | 11.7 (8.5) | 0.026* |
| SCORE, mean (s.d.) | 0.4 (0.8) | 0.2 (0.4) | 0.528* | 0.5 (1.1) | 0.4 (0.7) | 0.990 |
| HCQ at baseline, % | 70 | 33 | 0.013 | 3 | 9 | 0.259 |
| HCQ at follow-up, % | 80 | 92 | 0.322 | 11 | 9 | 0.791 |
| Corticosteroids at baseline, % | 58 | 83 | 0.096 | 64 | 68 | 0.692 |
| Prednisone daily dose (mg) at baseline, mean (s.d.) | 4.7 (6.4) | 22.7 (19.1) | <0.001* | 4.0 (3.5) | 4.5 (3.4) | 0.533* |
| Cumulative prednisone dose (g) from diagnosis to baseline, mean (s.d.) | 8.8 (11.6) | 15.5 (15.6) | 0.143* | 8.8 (19.4) | 5.3 (6.3) | 0.910* |
| Cumulative prednisone during follow-up, mean (s.d.) | 3.2 (3.9) | 13.3 (7.9) | <0.001* | 3.6 (3.5) | 5.4 (4.1) | 0.052* |
| Immunosuppressants at baseline, % | 36 | 67 | 0.041 | 65 | 41 | 0.047 |
| Immunosuppressants at follow-up, % | 30 | 67 | 0.013 | 46 | 27 | 0.124 |
P-values derived from chi-squared tests for qualitative and Student’s test for quantitative variables, except when marked with *, in which case the Mann–Whitney test was used due to deviation from normality. LDA was defined as fulfilment of the following criteria for at least 75% of the follow-up time: DAS28-ESR ≤ 2.8 in RA, and as SLEDAI-2K ≤ 4 and PGA (0-3) ≤1 without new disease activity or major organ activity, prednisone ≤7.5 mg/day and/or well-tolerated immunosuppressant dosages in SLE.
SCORE: Systemic Coronary Risk Evaluation; LDA: low disease activity; PGA: physician global assessment.
Atherosclerosis progression between subgroups
Atherosclerosis progression, as measured by new atherosclerotic plaques compared with baseline, was detected in 21% of SLE patients, 18% of RA patients, and 8% of healthy controls (P = 0.057). In patients with SLE and RA, new plaques were found both in patients with and without plaques at baseline. In healthy controls, new plaques developed only in those without plaques at baseline (Fig. 2). Plaque regression was not detected in any of the patient or control groups.

Three-year progression of atherosclerotic plaques in each participant group, according to atherosclerosis plaque status at baseline
In multiple regression analysis, SLE patients had a statistically significant, 3-fold increased risk of subclinical atherosclerosis progression vs controls (OR = 2.81, 95% CI 1.04, 7.64) after adjusting for the risk conferred by traditional factors using SCORE, and treatment with antihypertensives, antiplatelet medications and statins. Patients with RA had a 2-fold risk vs controls, which was not statistically significant (OR = 2.22, 95% CI 0.84, 5.90, Table 3).
Disease-conferred risk of plaque progression over 3 years in patients with SLE and RA
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| All participants (n = 271) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.81 | 1.04, 7.64 | 0.043 |
| RA | 2.22 | 0.84, 5.90 | 0.109 |
| SCORE prediction (per point) | 1.69 | 1.17, 2.46 | 0.006 |
| SLE and RA patients of low disease activity and matched controls (n = 224) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.96 | 1.02, 8.56 | 0.045 |
| RA | 2.12 | 0.72, 6.29 | 0.175 |
| SCORE prediction (per point) | 1.61 | 1.10, 2.37 | 0.015 |
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| All participants (n = 271) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.81 | 1.04, 7.64 | 0.043 |
| RA | 2.22 | 0.84, 5.90 | 0.109 |
| SCORE prediction (per point) | 1.69 | 1.17, 2.46 | 0.006 |
| SLE and RA patients of low disease activity and matched controls (n = 224) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.96 | 1.02, 8.56 | 0.045 |
| RA | 2.12 | 0.72, 6.29 | 0.175 |
| SCORE prediction (per point) | 1.61 | 1.10, 2.37 | 0.015 |
Use of antihypertensives, antiplatelet agents and statins included in multivariate logistic regression models (estimates not shown, P > 0.2 for all three).
SCORE: Systemic Coronary Risk Evaluation prediction of 10-year risk of fatal cardiovascular disease according to the low-risk country charts published by the European Society of Cardiology.
Disease-conferred risk of plaque progression over 3 years in patients with SLE and RA
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| All participants (n = 271) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.81 | 1.04, 7.64 | 0.043 |
| RA | 2.22 | 0.84, 5.90 | 0.109 |
| SCORE prediction (per point) | 1.69 | 1.17, 2.46 | 0.006 |
| SLE and RA patients of low disease activity and matched controls (n = 224) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.96 | 1.02, 8.56 | 0.045 |
| RA | 2.12 | 0.72, 6.29 | 0.175 |
| SCORE prediction (per point) | 1.61 | 1.10, 2.37 | 0.015 |
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| All participants (n = 271) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.81 | 1.04, 7.64 | 0.043 |
| RA | 2.22 | 0.84, 5.90 | 0.109 |
| SCORE prediction (per point) | 1.69 | 1.17, 2.46 | 0.006 |
| SLE and RA patients of low disease activity and matched controls (n = 224) | |||
| Participant group | |||
| Controls | Baseline | ||
| SLE | 2.96 | 1.02, 8.56 | 0.045 |
| RA | 2.12 | 0.72, 6.29 | 0.175 |
| SCORE prediction (per point) | 1.61 | 1.10, 2.37 | 0.015 |
Use of antihypertensives, antiplatelet agents and statins included in multivariate logistic regression models (estimates not shown, P > 0.2 for all three).
SCORE: Systemic Coronary Risk Evaluation prediction of 10-year risk of fatal cardiovascular disease according to the low-risk country charts published by the European Society of Cardiology.
Eighty-eight percent of patients with SLE and 74% of patients with RA had low disease activity for >75% of their follow-up time (LLDAS-75). When the same multiple regression model was applied to the subset of patients with LLDAS-75 vs their control matches, the results were similar (SLE: OR = 2.96, 95% CI 1.02, 8.56, and RA: OR = 2.12, 95% CI 0.72, 6.29).
Atherosclerotic progression determinants in patients with SLE
In multivariate analysis, the presence of antiphospholipid antibodies (either anti-cardiolipin antibodies, anti-beta2-glycoprotein I antibodies, or LA) and the SCORE prediction were identified as independent predictors of atherosclerosis progression in SLE. The cumulative prednisone dose during follow-up was associated with plaque progression only in low disease activity patients (P for interaction with LLDAS-100 = 0.035). This result was further confirmed in separate multivariate analysis models applied to subgroups of SLE patients with different duration of low disease activity status, while controlling for SCORE, antihypertensives, antiplatelet agents and statins. Despite relatively low prednisone doses (median of 2.5 mg/d), the OR for plaque progression with each gram of cumulative prednisone was 1.16 (95% CI 1.02, 1.31) in the LLDAS-50 subgroup, 1.16 (95% CI 0.99, 1.35) in the LLDAS-75 subgroup, 1.38 (1.07–1.78) in the LLDAS-100 subgroup and 1.56 (95% CI 1.05, 2.31) in the clinical remission subgroup. Other multivariate disease-related plaque progression determinants in all LLDAS subgroups included disease duration and antiphospholipid antibodies (Table 4).
Multivariate determinants of atherosclerotic plaque progression in patients with different LLDAS durations
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model for all SLE patients (n = 101) | |||
| Antiphospholipid antibodies | 2.00 | 1.03, 7.95 | 0.043 |
| SCORE prediction | 2.87 | 1.12, 3.57 | 0.019 |
| Model for SLE patients with LLDAS >50% of follow-up time (n = 96) | |||
| Disease duration, per year | 1.09 | 1.01, 1.18 | 0.036 |
| Antiphospholipid antibodies | 5.78 | 1.47, 22.73 | 0.012 |
| SCORE prediction | 1.70 | 0.94, 3.07 | 0.082 |
| Antiplatelet agents | 0.28 | 0.71, 1.13 | 0.073 |
| Cumulative prednisone during follow-up, g | 1.16 | 1.02, 1.31 | 0.019 |
| Model for patients with LLDAS >75% of follow-up time (n = 89) | |||
| Disease duration (per year) | 1.11 | 1.02, 1.21 | 0.015 |
| Antiphospholipid antibodies | 7.04 | 1.57, 31.58 | 0.011 |
| SCORE prediction | 1.67 | 0.91, 3.08 | 0.099 |
| Antiplatelet agents at baseline | 0.21 | 0.05, 0.99 | 0.049 |
| Cumulative prednisone during follow-up, g | 1.16 | 0.99 , 1.35 | 0.069 |
| Model for SLE patients with LLDAS 100% of follow-up time (n = 66) | |||
| Disease duration (per year) | 1.20 | 1.03, 1.39 | 0.022 |
| Antiphospholipid antibodies | 5.83 | 0.99, 34.38 | 0.052 |
| SCORE prediction | 1.88 | 0.82, 4.30 | 0.136 |
| Antihypertensives at baseline | 6.42 | 0.93, 44.4 | 0.060 |
| Cumulative prednisone during follow-up, g | 1.38 | 1.07, 1.78 | 0.013 |
| Cumulative prednisone at baseline, g | 0.93 | 0.86, 1.01 | 0.091 |
| Model for SLE patients in clinical remision during follow-up (n = 52) | |||
| Disease duration | 1.15 | 1.03, 1.30 | 0.016 |
| SCORE prediction | 1.10 | 0.43, 2.80 | 0.837 |
| Cumulative prednisone during follow-up, g | 1.56 | 1.05, 2.31 | 0.026 |
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model for all SLE patients (n = 101) | |||
| Antiphospholipid antibodies | 2.00 | 1.03, 7.95 | 0.043 |
| SCORE prediction | 2.87 | 1.12, 3.57 | 0.019 |
| Model for SLE patients with LLDAS >50% of follow-up time (n = 96) | |||
| Disease duration, per year | 1.09 | 1.01, 1.18 | 0.036 |
| Antiphospholipid antibodies | 5.78 | 1.47, 22.73 | 0.012 |
| SCORE prediction | 1.70 | 0.94, 3.07 | 0.082 |
| Antiplatelet agents | 0.28 | 0.71, 1.13 | 0.073 |
| Cumulative prednisone during follow-up, g | 1.16 | 1.02, 1.31 | 0.019 |
| Model for patients with LLDAS >75% of follow-up time (n = 89) | |||
| Disease duration (per year) | 1.11 | 1.02, 1.21 | 0.015 |
| Antiphospholipid antibodies | 7.04 | 1.57, 31.58 | 0.011 |
| SCORE prediction | 1.67 | 0.91, 3.08 | 0.099 |
| Antiplatelet agents at baseline | 0.21 | 0.05, 0.99 | 0.049 |
| Cumulative prednisone during follow-up, g | 1.16 | 0.99 , 1.35 | 0.069 |
| Model for SLE patients with LLDAS 100% of follow-up time (n = 66) | |||
| Disease duration (per year) | 1.20 | 1.03, 1.39 | 0.022 |
| Antiphospholipid antibodies | 5.83 | 0.99, 34.38 | 0.052 |
| SCORE prediction | 1.88 | 0.82, 4.30 | 0.136 |
| Antihypertensives at baseline | 6.42 | 0.93, 44.4 | 0.060 |
| Cumulative prednisone during follow-up, g | 1.38 | 1.07, 1.78 | 0.013 |
| Cumulative prednisone at baseline, g | 0.93 | 0.86, 1.01 | 0.091 |
| Model for SLE patients in clinical remision during follow-up (n = 52) | |||
| Disease duration | 1.15 | 1.03, 1.30 | 0.016 |
| SCORE prediction | 1.10 | 0.43, 2.80 | 0.837 |
| Cumulative prednisone during follow-up, g | 1.56 | 1.05, 2.31 | 0.026 |
All models adjusted for the Coronary Risk Evaluation (SCORE) prediction of 10-year risk of fatal cardiovascular event (low risk country), and derived via stepwise backward elimination from initial models including baseline disease duration, antiphospholipid antibody presence, SLICC-SDI, use of hydroxycloroquine, immunosuppressants, antiplatelets, antihypertensives, statins and cumulative prednisone at baseline and during follow-up.
LLDAS: lupus low disease activity state; SDI: damage index score.
Multivariate determinants of atherosclerotic plaque progression in patients with different LLDAS durations
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model for all SLE patients (n = 101) | |||
| Antiphospholipid antibodies | 2.00 | 1.03, 7.95 | 0.043 |
| SCORE prediction | 2.87 | 1.12, 3.57 | 0.019 |
| Model for SLE patients with LLDAS >50% of follow-up time (n = 96) | |||
| Disease duration, per year | 1.09 | 1.01, 1.18 | 0.036 |
| Antiphospholipid antibodies | 5.78 | 1.47, 22.73 | 0.012 |
| SCORE prediction | 1.70 | 0.94, 3.07 | 0.082 |
| Antiplatelet agents | 0.28 | 0.71, 1.13 | 0.073 |
| Cumulative prednisone during follow-up, g | 1.16 | 1.02, 1.31 | 0.019 |
| Model for patients with LLDAS >75% of follow-up time (n = 89) | |||
| Disease duration (per year) | 1.11 | 1.02, 1.21 | 0.015 |
| Antiphospholipid antibodies | 7.04 | 1.57, 31.58 | 0.011 |
| SCORE prediction | 1.67 | 0.91, 3.08 | 0.099 |
| Antiplatelet agents at baseline | 0.21 | 0.05, 0.99 | 0.049 |
| Cumulative prednisone during follow-up, g | 1.16 | 0.99 , 1.35 | 0.069 |
| Model for SLE patients with LLDAS 100% of follow-up time (n = 66) | |||
| Disease duration (per year) | 1.20 | 1.03, 1.39 | 0.022 |
| Antiphospholipid antibodies | 5.83 | 0.99, 34.38 | 0.052 |
| SCORE prediction | 1.88 | 0.82, 4.30 | 0.136 |
| Antihypertensives at baseline | 6.42 | 0.93, 44.4 | 0.060 |
| Cumulative prednisone during follow-up, g | 1.38 | 1.07, 1.78 | 0.013 |
| Cumulative prednisone at baseline, g | 0.93 | 0.86, 1.01 | 0.091 |
| Model for SLE patients in clinical remision during follow-up (n = 52) | |||
| Disease duration | 1.15 | 1.03, 1.30 | 0.016 |
| SCORE prediction | 1.10 | 0.43, 2.80 | 0.837 |
| Cumulative prednisone during follow-up, g | 1.56 | 1.05, 2.31 | 0.026 |
| . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model for all SLE patients (n = 101) | |||
| Antiphospholipid antibodies | 2.00 | 1.03, 7.95 | 0.043 |
| SCORE prediction | 2.87 | 1.12, 3.57 | 0.019 |
| Model for SLE patients with LLDAS >50% of follow-up time (n = 96) | |||
| Disease duration, per year | 1.09 | 1.01, 1.18 | 0.036 |
| Antiphospholipid antibodies | 5.78 | 1.47, 22.73 | 0.012 |
| SCORE prediction | 1.70 | 0.94, 3.07 | 0.082 |
| Antiplatelet agents | 0.28 | 0.71, 1.13 | 0.073 |
| Cumulative prednisone during follow-up, g | 1.16 | 1.02, 1.31 | 0.019 |
| Model for patients with LLDAS >75% of follow-up time (n = 89) | |||
| Disease duration (per year) | 1.11 | 1.02, 1.21 | 0.015 |
| Antiphospholipid antibodies | 7.04 | 1.57, 31.58 | 0.011 |
| SCORE prediction | 1.67 | 0.91, 3.08 | 0.099 |
| Antiplatelet agents at baseline | 0.21 | 0.05, 0.99 | 0.049 |
| Cumulative prednisone during follow-up, g | 1.16 | 0.99 , 1.35 | 0.069 |
| Model for SLE patients with LLDAS 100% of follow-up time (n = 66) | |||
| Disease duration (per year) | 1.20 | 1.03, 1.39 | 0.022 |
| Antiphospholipid antibodies | 5.83 | 0.99, 34.38 | 0.052 |
| SCORE prediction | 1.88 | 0.82, 4.30 | 0.136 |
| Antihypertensives at baseline | 6.42 | 0.93, 44.4 | 0.060 |
| Cumulative prednisone during follow-up, g | 1.38 | 1.07, 1.78 | 0.013 |
| Cumulative prednisone at baseline, g | 0.93 | 0.86, 1.01 | 0.091 |
| Model for SLE patients in clinical remision during follow-up (n = 52) | |||
| Disease duration | 1.15 | 1.03, 1.30 | 0.016 |
| SCORE prediction | 1.10 | 0.43, 2.80 | 0.837 |
| Cumulative prednisone during follow-up, g | 1.56 | 1.05, 2.31 | 0.026 |
All models adjusted for the Coronary Risk Evaluation (SCORE) prediction of 10-year risk of fatal cardiovascular event (low risk country), and derived via stepwise backward elimination from initial models including baseline disease duration, antiphospholipid antibody presence, SLICC-SDI, use of hydroxycloroquine, immunosuppressants, antiplatelets, antihypertensives, statins and cumulative prednisone at baseline and during follow-up.
LLDAS: lupus low disease activity state; SDI: damage index score.
Discussion
Our results showed that atherosclerotic plaque progression is accelerated in SLE compared with RA and healthy controls after adjusting for traditional risk factors, and regardless of disease activity status. In all different subgroups of SLE patients maintaining LLDAS for >50%, >75% or 100% of the follow-up time, longer disease duration at baseline and higher cumulative prednisone dose during follow-up were independent predictors of plaque progression, in addition to antiphospholipid antibodies and SCORE risk. These findings are of high importance given the impact of subclinical atherosclerosis in the prediction of CVD events, and the increased prevalence of CVD-mediated mortality in both RA [31] and SLE patients [32, 33].
Patients with RA in our cohort had lower rates of plaque progression than SLE patients, and not significantly higher than controls (Fig. 2). Because the majority of patients in our cohort had well-controlled disease, our results are consistent with previous studies supporting that atherosclerosis is not accelerated in RA of low disease activity [4, 5]. In contrast, previous studies in patients with SLE yielded mixed results regarding the impact of disease activity measures on plaque progression. Thompson et al. [21] found an association with baseline SLAM, and Kiani et al. [16] with baseline physician global assessment, while others reported no association with baseline SLEDAI in multivariate analysis [14, 18, 19]. It should be noted that studies with positive associations had a mixed patient population in terms of disease activity [16, 21], while those without an association included more patients with low disease activity [14, 18], similar to our cohort.
No previous studies have investigated the effect of disease activity on atherosclerosis progression using the recent consensus definition of LLDAS, which reflects the disease status throughout the follow-up time. Our data indicate that LLDAS can modify the effects of other SLE-related risk factors. Specifically, our analysis highlights the detrimental atherogenic effects of longer disease duration, cumulative corticosteroid dose and antiphospholipid antibodies on atherosclerosis progression in patients with LLDAS. The significance of these factors was robust to sensitivity analyses applying LLDAS criteria to different percentages of follow-up time.
Our study supports an association between corticosteroid use during follow-up and atherosclerotic plaque progression in LLDAS and clinical remission, despite relatively low prednisone doses (median prednisone equivalents of 2.5 mg/day). Corticosteroids have been correlated with subclinical atherosclerosis progression in one prospective SLE cohort using electron beam computed tomography to measure coronary artery and aorta calcium [17], but not in carotid ultrasound studies. This could be because other prospective ultrasound studies assessed corticosteroid use as a binary variable at baseline [14, 21] or as mean daily dose [14, 16, 18, 19, 21] rather than cumulative dose, and did not stratify the analysis by disease activity.
Nevertheless, our findings are consistent with a meta-analysis of published cross-sectional data correlating plaque prevalence to past cumulative prednisone exposure in SLE [34], as well as our previous baseline analysis of this patient cohort [3]. The association between the progression of atherosclerotic plaques and cumulative prednisone dose during follow-up underlines the negative impact of corticosteroids on several aspects of SLE, supporting previous evidence about their role in the development of CVD events, SLE damage and lupus mortality [35, 36].
Antiphospholipid antibody positivity at baseline (either anti-cardiolipin antibodies, anti-beta2-glycoprotein I antibodies or LA) was consistently associated with plaque progression in the entire cohort of patients with SLE as well as in all different LLDAS subgroups. Although several studies in basic and translational research support the atherogenic potential of antiphospholipid antibodies [37–40], this is the first prospective ultrasonographic study to report this association. Five of six ultrasound studies examining progression of atherosclerosis in SLE failed to correlate baseline antiphospholipid antibodies with future IMT or plaque progression, possibly because none considered all three antiphospholipid antibodies. Kiani et al. [16] considered LA only, Roman et al. [19] and Rua-Figueroa et al. [20] LA and anti-cardiolipin antibodies [19, 20], while McMahon et al. [18] and Thompson et al. [21] anti-cardiolipin antibodies only.
Traditional CVD risk factors as expressed by the SCORE prediction were the most significant determinants of atherosclerotic plaque progression among all participants, and within SLE patients overall. The significance of traditional risk factors for atherosclerosis progression has been reported in all previous studies of progression of subclinical atherosclerosis in SLE, including hypertension [15, 20, 21, 41], dyslipidaemia [16, 18, 21], smoking [16, 21], homocysteine levels [18, 19] or the SCORE prediction [14]. This finding underscores the significance of proactive management of modifiable CVD risk factors in patients with SLE.
The main strength of our study is the direct comparison of ultrasonographic progression of subclinical atherosclerosis in a significant number of SLE patients and age- and sex-matched patients with RA and healthy controls, over a 3-year follow-up period. The same, blinded observer evaluated atherosclerosis by vascular ultrasound at baseline and follow-up, and progression was defined as an increase in atherosclerotic plaques rather than IMT, as plaque detection is a more relevant marker of atherosclerosis and a better predictor of future CVD events in patients with autoimmune diseases [42–44]. Furthermore, use of LLDAS-75, a marker analogous to low disease activity in >75% of follow-up time in both SLE and RA, allowed for a meaningful comparison between SLE and RA patients, and enabled us to adequately explore confounding effects of SLE treatments. In addition, we used the SCORE, a validated, clinically useful and straightforward CVD risk prediction tool, rather than examine each CVD risk factor separately. Moreover, this is the first prospective study in SLE to evaluate both the carotid and femoral arteries, which has been previously shown to increase sensitivity for atherosclerosis detection [3, 45].
Our study has some limitations. Due to the small number of SLE patients with chronic active or flaring disease, our data do not allow us to make safe conclusions regarding atherosclerosis determinants in this particular group. Furthermore, our cohort is exclusively comprised of Caucasian patients, and our findings may not apply to SLE patients of different race.
In conclusion, atherosclerosis is accelerated in SLE patients, regardless of disease activity status. Atherosclerotic plaque progression in SLE is spurred by accentuated effects of traditional CVD risk factors and the presence of antiphospholipid antibodies, and further aggravated by corticosteroid use and longer disease duration in patients with LLDAS. Physicians need to identify high-risk SLE patients using validated tools, implement CVD prevention strategies primarily aimed at traditional risk factor management, and minimize even low dose corticosteroid exposure in low disease activity patients.
Funding: This work was supported by the Special Account for Research Grants (Grant No. 11123) of the National and Kapodistrian University of Athens, and a Research Grant from the Greek Rheumatology Society and Professional Association of Rheumatologists.
Disclosure statement: The authors have declared no conflicts of interest.




Comments