-
PDF
- Split View
-
Views
-
Cite
Cite
Shafay Raheel, Eric L. Matteson, Cynthia S. Crowson, Elena Myasoedova, Improved flare and remission pattern in rheumatoid arthritis over recent decades: a population-based study, Rheumatology, Volume 56, Issue 12, December 2017, Pages 2154–2161, https://doi.org/10.1093/rheumatology/kex352
Close - Share Icon Share
Abstract
To assess trends in the occurrence of flares and remission in RA over recent decades.
A retrospective medical records review of each clinical visit was performed in a population-based cohort of patients with RA (age ⩾30 years; 1987 ACR criteria met in 1988–2007) to estimate flare and remission status. RA flare was defined as any worsening of RA activity leading to an initiation, change or increase of therapy (OMERACT 9). The primary definition for remission required the absence of RA disease activity (i.e. tender joint count 0, swollen joint count 0 and ESR ⩽10 mm/h) (OMERACT 7). All subjects were followed until death, migration or 1 July 2012.
The study included 650 RA patients (mean age 55.8 years; 69% female) with a mean follow up of 10.3 years. Patients were flaring at 2887 (17%) visits. There was a significant decline in the RA flare rate across disease duration (P < 0.001), predominantly in the first 5 years after diagnosis of RA. Patients diagnosed with RA in more recent years experienced fewer flares during first few years of RA (P < 0.001). There was no difference between the sexes in trends of flare rates over time (P = 0.42) Current smokers had higher flare rates than non-smokers (P = 0.047) and former smokers were not different from non-smokers (P = 0.87).
Patients diagnosed in more recent years have lower flare rates than those diagnosed in prior decades. Flare rates declined fastest in the first 5 years of disease and tended to be stable thereafter. Current smoking was associated with an adverse flare profile.
Rheumatology key messages
Patients diagnosed with RA in more recent years experienced lower flare rates.
Current smokers with RA had higher flare and lower remission rates than non-smokers.
RA patients who are RF positive had higher flare rates than RF-negative patients.
Introduction
RA is a systemic autoimmune disease characterized by inflammation and joint destruction that causes significant morbidity and mortality. Earlier diagnosis and earlier initiation of DMARDs, as well as the use of biologic response modifiers in recent decades, have revolutionized the treatment of RA, resulting in achievement of clinical remission or low disease activity state in a large proportion of patients with RA [1]. Furthermore, structural and functional remission is now a realistic target for these patients.
Despite these dramatic advancements in the management of RA disease, flare of RA disease activity is not uncommon and remains an inherent feature of RA and is associated with a significant impact on quality of life, joint damage and disability [2, 3].
It has been suggested that there are improving trends towards lower RA disease activity in recent years as compared with previous decades [4]. However, long-term data on flare and remission rates in RA over calendar time are lacking. The aims of this study were to assess trends in the occurrence of flares and remission in RA over recent decades and establish whether the pattern of flares varies depending on age, sex, smoking status, obesity and RF positivity.
Methods
A population-based inception cohort of Olmsted County, Minnesota (USA), residents ≥18 years of age (1987 ACR criteria for RA met between 1 January 1988 and 31 December 2007) was previously identified and assembled using the resources of the Rochester Epidemiology Project (REP). The REP is a population-based medical records linkage system that allows ready access to the complete (inpatient and outpatient) medical records from all community medical providers [5–7]. For each patient, the earliest date of fulfilment of four or more ACR criteria was considered the RA incidence date. Patients were followed up through medical records review until death, migration or 1 July 2012. Flare and remission rates were calculated as the percentage of visits in flare or remission in each calendar year. The study was approved by the Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center. The need for informed consent was waived.
Flare of RA disease activity was defined at the medical provider visit as any worsening of disease activity leading to an initiation, change or increase of therapy or mention in medical record of flare up, ongoing or active disease [8–10]. Remission was defined as the absence of disease activity based on a tender joint count of 0, a swollen joint count of 0 and an ESR ⩽10 mm/h (primary definition, OMERACT 7) or a tender joint count ⩽1, swollen joint count ⩽1 and CRP ⩽10 mg/l (ACR/EULAR provisional definition of remission) when ESR was not available [11, 12] and/or mention in medical record of remission, inactive, quiet or quiescent disease. Visits not classified as flare/remission were considered as intermediate activity.
Information on RF positivity, joint erosions/destructive changes and severe extra-articular RA manifestations, including pericarditis, pleuritis, Felty’s syndrome, polyneuropathy, mononeuropathy, scleritis, episcleritis, glomerulonephritis, major cutaneous vasculitis and vasculitis involving other organs, during the follow-up was collected from the medical records of patients with RA [13]. Data on anti-rheumatic medication use, including MTX, HCQ, other DMARDs, as well as biologic response modifiers and glucocorticoids, at any time during the follow-up were also gathered for these patients. Smoking status was collected at baseline and individuals were classified as current, former or never-smokers. Obesity was defined as a BMI ⩾30 kg/m2.
Statistical methods
Two multivariable generalized linear models were examined with a binomial outcome of either flare or remission including age, sex, calendar year of each visit, disease duration at each visit, RF positivity, smoking status and obesity. These models included random subject effects to account for multiple visits per patient. Smoothing splines were used to examine non-linear effects. Two-way interactions between variables of interest were examined. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R version 3.0.2 (R Project for Statistical Computing, Vienna, Austria).
Results
The study included 650 patients with RA. The mean age at incidence was 55.8 years (s.d. 14.6), 69% were women and 67% were RF positive (Table 1). The patients were followed for a mean of 10.3 years (s.d. 6.0). Disease status was collected for a total of 17 323 clinical visits (mean 26.7 visits per patient, rate 2.6 visits/person-year). Of these, 9733 (56%) visits were with a rheumatologist, 6% with family medicine, 6% with primary care, 23% with internal medicine and 9% with other providers. Patients were flaring during 2887 (17%) visits and were in remission during 1747 (10%) visits. The visit rates were 12% higher in women vs men (P = 0.002). Visit rates were 21% higher among patients positive for RF compared with RF-negative patients (P < 0.001). The overall visit rates decreased by 3% per year of disease duration (P < 0.001). No statistically significant difference was found in visit rates based on age, smoking status or obesity.
| Characteristic . | Value . |
|---|---|
| Age, mean (s.d.), years | 55.8 (15.7) |
| Female sex, n (%) | 448 (68.9) |
| Length of follow-up, mean (s.d.), years | 10.3 (6.0) |
| RF positivity, n (%) | 434 (66.8) |
| Smoking status at RA incidence, n (%) | |
| Never | 301 (46.3) |
| Current | 119 (18.3) |
| Former | 230 (35.4) |
| Obese at incidence, n (%) | 435 (66.9) |
| Severe exRA, ever during the follow-up, n (%) | 64 (9.8) |
| Erosive RA, ever during the follow-up, n (%) | 357 (54.9) |
| Medication use, ever during follow-up, n (%) | |
| MTX | 425 (65.4) |
| HCQ | 416 (64.0) |
| Other DMARDs | 205 (31.5) |
| Biologics | 159 (24.5) |
| Corticosteroids | 540 (83.1) |
| Characteristic . | Value . |
|---|---|
| Age, mean (s.d.), years | 55.8 (15.7) |
| Female sex, n (%) | 448 (68.9) |
| Length of follow-up, mean (s.d.), years | 10.3 (6.0) |
| RF positivity, n (%) | 434 (66.8) |
| Smoking status at RA incidence, n (%) | |
| Never | 301 (46.3) |
| Current | 119 (18.3) |
| Former | 230 (35.4) |
| Obese at incidence, n (%) | 435 (66.9) |
| Severe exRA, ever during the follow-up, n (%) | 64 (9.8) |
| Erosive RA, ever during the follow-up, n (%) | 357 (54.9) |
| Medication use, ever during follow-up, n (%) | |
| MTX | 425 (65.4) |
| HCQ | 416 (64.0) |
| Other DMARDs | 205 (31.5) |
| Biologics | 159 (24.5) |
| Corticosteroids | 540 (83.1) |
exRA: extra-articular manifestations of RA.
| Characteristic . | Value . |
|---|---|
| Age, mean (s.d.), years | 55.8 (15.7) |
| Female sex, n (%) | 448 (68.9) |
| Length of follow-up, mean (s.d.), years | 10.3 (6.0) |
| RF positivity, n (%) | 434 (66.8) |
| Smoking status at RA incidence, n (%) | |
| Never | 301 (46.3) |
| Current | 119 (18.3) |
| Former | 230 (35.4) |
| Obese at incidence, n (%) | 435 (66.9) |
| Severe exRA, ever during the follow-up, n (%) | 64 (9.8) |
| Erosive RA, ever during the follow-up, n (%) | 357 (54.9) |
| Medication use, ever during follow-up, n (%) | |
| MTX | 425 (65.4) |
| HCQ | 416 (64.0) |
| Other DMARDs | 205 (31.5) |
| Biologics | 159 (24.5) |
| Corticosteroids | 540 (83.1) |
| Characteristic . | Value . |
|---|---|
| Age, mean (s.d.), years | 55.8 (15.7) |
| Female sex, n (%) | 448 (68.9) |
| Length of follow-up, mean (s.d.), years | 10.3 (6.0) |
| RF positivity, n (%) | 434 (66.8) |
| Smoking status at RA incidence, n (%) | |
| Never | 301 (46.3) |
| Current | 119 (18.3) |
| Former | 230 (35.4) |
| Obese at incidence, n (%) | 435 (66.9) |
| Severe exRA, ever during the follow-up, n (%) | 64 (9.8) |
| Erosive RA, ever during the follow-up, n (%) | 357 (54.9) |
| Medication use, ever during follow-up, n (%) | |
| MTX | 425 (65.4) |
| HCQ | 416 (64.0) |
| Other DMARDs | 205 (31.5) |
| Biologics | 159 (24.5) |
| Corticosteroids | 540 (83.1) |
exRA: extra-articular manifestations of RA.
Time from RA incidence to initiation of the first DMARD was lower in the patients with RA onset in 1998–2007 as compared with those with RA onset in 1988–1997: 0.5 months (0.0, 1.6) vs 1.2 (0.2, 7.8), respectively.
Trends in flare rates
Figure 1 shows trends in flare rates after RA onset by RA disease characteristics, age, sex, obesity and smoking. There was a statistically significant decline in the RA flare rate across disease duration (P < 0.001), predominantly in the first 5 years after diagnosis of RA (Fig. 1A). Beyond the 5 years of RA disease onset, flares continued to occur at a constant rate. The flare rate declined marginally across the calendar year of RA diagnosis (P = 0.055). Patients diagnosed with RA in more recent years experienced lower flare rates, even in the first few years of RA. The flare rate declined significantly across the calendar year of visit date (P = 0.047; Fig. 1B). Patients seen in more recent years experienced fewer flares.
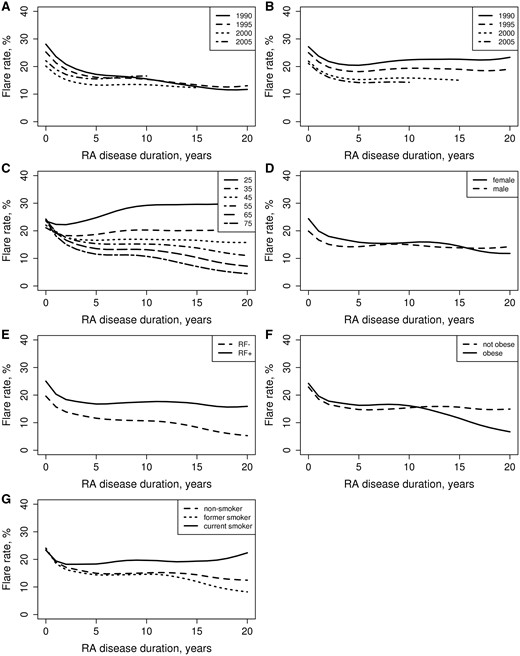
Flare rates by patient characteristics
(A) Calendar year of diagnosis and RA disease duration, (B) calendar year of visit date, (C) age at RA diagnosis, (D) sex, (E) RF status, (F) obesity at RA diagnosis, (G) smoking status at RA diagnosis.
There was a significant interaction between age and RA disease duration (P < 0.001), indicating that all patients had similar flare rates in the first few years after RA diagnosis. In subsequent years, patients who were diagnosed with RA at a younger age had more persistent flare rates and those diagnosed with RA at an older age had declining flare rates over time (Fig. 1C). Flare rates were not different between women and men (P = 0.16; Fig. 1D). There were no differences between the sexes in trends of flare rates over time (P = 0.42).
Patients with positive RF had higher flare rates than those with negative RF (P < 0.001; Fig. 1E). Flare rates declined at a faster rate in patients with negative RF than those with positive RF (interaction P = 0.047). There was no significant interaction of age and RF positivity on flare rates (P = 0.15).
Overall, current smokers had higher flare rates than non-smokers (P = 0.047), but there were no differences in flare rate between former smokers and non-smokers (P = 0.87; Fig. 1G). An interaction of borderline significance (P = 0.072) indicated there were no differences in flare rates by smoking status during the first few years of RA disease duration, but subsequently current smokers had higher flare rates over time than non-smokers. The pattern of RA disease flares over the follow-up time was similar among former smokers and non-smokers (P = 0.16). There was no evidence that the association between smoking and flare rate differed by sex (interaction P = 0.60).
Overall there was no difference in flare rates between patients who were obese and those who were not obese at the diagnosis of RA (P = 0.69; Fig. 1F). However, there was a significant interaction between obesity and RA disease duration (P = 0.038), indicating that patients who were obese at the diagnosis of RA had greater declines in flare rates over the disease course than their non-obese counterparts.
Trends in occurrence of remission
Changes in remission rates mirrored the dynamics of the flares. The remission rate increased significantly across disease duration (P < 0.001; Fig. 2A), with most of the increase occurring in the first 5 years after the diagnosis of RA. The occurrence of remission increased significantly across the calendar year of RA diagnosis (P = 0.013).
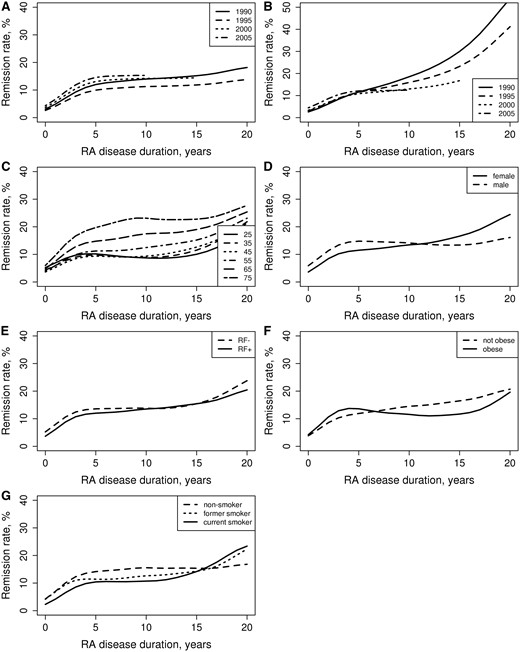
Remission rates by patient characteristics
(A) Calendar year of diagnosis and RA disease duration, (B) calendar year of visit date, (C) age at RA diagnosis, (D) sex, (E) RF status, (F) obesity at RA diagnosis, (G) smoking status at RA diagnosis.
The remission rate increased significantly across the calendar year of the visit date (P = 0.010; Fig. 2B). Patients diagnosed with RA in more recent years were more likely to achieve remission in the first 5 years but less likely to achieve remission subsequently (interaction P < 0.001).
There was a significant age effect, indicating that patients diagnosed with RA at an older age achieved higher remission rates (Fig. 2C). There was no indication that remission rates differed over the disease course for patients diagnosed at different ages (interaction P = 0.25). Overall there was no difference in remission rates for women vs men (P = 0.29; Fig. 2D). However, there was a significant interaction between sex and RA disease duration (P = 0.025), indicating that remission rates plateaued after 5 years of disease duration among men but continued to increase over the disease course among women.
Overall there were no differences in remission rates between patients with negative and positive RF (P = 0.43; Fig. 2E). There was also no evidence of an interaction between RF positivity and disease duration (P = 0.71) and between RF positivity and age on remission rates (P = 0.52).
Current smokers had significantly lower remission rates than non-smokers (P = 0.034; Fig. 2G). There was no difference in remission rates among former smokers vs non-smokers (P = 0.24). There was no indication that remission rates differed over the disease course for current smokers (interaction P = 0.42) or former smokers (interaction P = 0.93) vs non-smokers. There was no evidence that the association between smoking and remission rate differed by sex (interaction P = 0.97).
There was no difference in remission rates for obese vs non-obese patients (P = 0.74; Fig. 2F). Remission rates were not different between obese and non-obese patients over the disease course (interaction P = 0.09).
Discussion
The literature on the long-term trends of flare and remission in RA disease is scarce. To the best of our knowledge this is the first study to show a significant decline in flare occurrence and a concurrent increase in remission rates in patients with RA over recent decades and across RA disease duration, particularly in the first 5 years of disease. This observation is important and can be attributed, at least in part, to improved management of RA disease in recent years resulting in early and effective control of RA disease activity. Indeed, the time from fulfilment of RA criteria to initiation of the first DMARD decreased in patients diagnosed with RA in 1998–2007 as compared with the previous decade. Some authors suggested a trend toward milder RA disease course in recent years [4, 14–16]. Our earlier studies in Olmsted County, Minnesota reported significantly lower ESRs and a marginally lower proportion of patients positive for RF in the 1995–2007 vs 1985–94 cohorts [17]. While milder RA disease at the onset could have contributed to the changing flare/remission pattern, coinciding changes in treatment strategy over time likely played a significant role in these findings. Further studies are needed to understand the relative impact of these two factors on flare and remission patterns in RA.
Following current remission-oriented RA treatment guidelines, it could be demonstrated that patients diagnosed with RA more recently have a higher likelihood of remission, particularly in the first 5 years of disease, but not after, as compared with those diagnosed in prior decades. The reasons for persistent flare and remission rates beyond the 5-year mark are unclear. There was a decline in the rate of provider visits over RA disease duration. This could be due to a decrease in the number of visits for patients who are in remission and do not need frequent follow-up, thus keeping the flare rate at a constant proportion of visits that take place. Correspondingly, a longer duration of disease has been previously linked to a lower likelihood of remission in RA [18]. Improved flare and remission rates across the calendar year of the visit date that includes patients with various dates of RA onset supports a hypothesis that changes in RA management practices have contributed to the improving trends.
Some prior studies have associated male sex with a higher likelihood of remission, including sustained remission [19, 20]. Our study did not find this association. In fact, in our study, sex did not appear to affect the flare trends, while remission rates after 5 years of RA disease were increasing in women but not men. This could potentially be due to the difference in population demographics and/or the different remission definition [OMERACT 7 in our study vs 28-joint DAS (DAS28) in the previous studies]. Higher adherence to health practices and well visits and potentially better medication compliance could play a role in the more beneficial trends in remission in women but not men. Indeed, women in this study were more likely to be seen for their RA than men.
Similarly, more beneficial flare/remission trends in patients who were diagnosed with RA at older ages as compared with those who were diagnosed as young adults could suggest better compliance and better adherence to health maintenance, as well as possibly a less stressful lifestyle in older age, as potential explanations for these findings. Other factors, including differences in RA disease severity and response to treatment in older vs younger individuals, may be considered as explanations for these differences but require further study. Older age at the onset of RA, particularly in the setting of negative RF, has been identified among predictors of remission [19, 20]. In our study, the interaction of age and RF positivity was not significant for flare or remission rates.
Patients with positive RF and those who were smokers tended to have higher flare rates and less favourable flare trends over time as compared with RF-negative patients and those who were non-smokers, respectively. This observation is in line with existing understanding of RF seropositivity and smoking as risk factors for more active and severe RA disease and lower treatment response rate [21]. Higher visit rates in patients who are RF positive as compared with patients who are RF negative may be reflective of a more active disease course requiring frequent medication adjustments.
In contrast, RF seronegative status and non-smoking have been previously identified among independent predictors of remission in RA [20]. In our study, despite improved control of flares, RF-negative patients were similar to RF-positive patients in their remission rates. One possible explanation is confounding by indication, where remission is targeted more aggressively in RF-positive patients due to a greater certainty in RA diagnosis and clear management guidelines as opposed to RF-negative patients, resulting in decreasing flare rates but not completely achieving the remission goals.
A recent study in Japanese patients with RA found an adverse association of current and former smoking in male patients, but not in females, with remission defined by DAS28-ESR [21]. While an association of current smoking was detected with lower remission rates in RA as compared with non-smoking, there was no interaction between smoking and sex on flare or remission rates in our study.
A recent meta-analysis suggested that obesity reduces the chances to achieve minimal disease activity in patients with rheumatic diseases receiving treatment with traditional or biologic DMARDs [22]. Likewise, a meta-analysis by Liu et al. [23] showed that obese patients with RA have a lower likelihood of achieving and sustaining remission than non-obese RA subjects. Our study did not show differences in flare or remission rates between obese and non-obese patients overall. The more pronounced decline in flare rates in patients who were obese at RA diagnosis than in non-obese patients is difficult to explain. Lower body mass as a marker of more severe RA disease could be a considered as a potential explanation, although the reasons for this trend are unclear and require further study.
In the era of improved therapeutics and treat-to-target management strategies, results of this large population-based study are important for understanding of long-term trends in RA disease activity and suggesting potential implications of these changes on outcomes in RA. Previous work from this group recently showed a deleterious effect of RA flares on cardiovascular disease, one of the major contributors to morbidity and mortality in patients with RA [24]. Increasing evidence suggests that improved flare management and early and sustained remission may provide significant survival benefits in RA [25–27]. Given the pivotal role of inflammation in shaping the comorbidity and outcome profile in patients with RA, the implications of an improved flare/remission profile may extend beyond optimization of RA disease management and include improved outcomes and longevity in RA.
A potential limitation of our study is its retrospective design, with only information coming to medical attention being used to define flare and remission. In addition, only 10 451 (60%) visits had joint counts. This could result in misclassification of visits into the intermediate activity category for patients who did not seek medical attention either during a flare or after the flare resolved. However, the comprehensive resources of the REP and the use of alternative definitions for flare and remission likely minimized shortcomings associated with these retrospective data. In addition, patients with flares would be expected to be more likely to seek medical attention as compared with patients in remission, while a decrease in flare rates and an increase in remission rates was observed over time, which somewhat mitigates the misclassification bias. An increased risk of type I error due to multiple comparisons is also possible. While only two outcomes and seven predictors were included, many two-way interactions were assessed.
In this study neither patients’ compliance to therapy nor whether remission was treatment induced or spontaneous was explored, outlining areas for further research. Smoking status was recorded at RA incidence and no records regarding changes in smoking status during the disease course were available. Whether and to what extent changes in smoking status could have affected the results remains to be investigated. The population of Olmsted County is predominantly white, and it is possible that the results may not be generalizable to non-white individuals. While treat-to-target is a goal in the management of patients with RA at the Mayo Clinic, the trends in flares and remissions described in this study may not be generalizable to other clinical settings where alternative therapeutic goals are used.
In conclusion, patients diagnosed more recently have lower flare rates than those diagnosed in prior decades. Flare rates declined in the first 5 years of disease and tended to be stable thereafter. These patterns may reflect improved control of RA activity in recent years resulting from more rigorous RA management with improved therapeutic strategies. However, flares continue to occur persistently at a constant rate after 5 years of RA, reflecting the need for improved long-term disease management. RA patients who are RF positive, diagnosed with RA at a younger age and who are smokers tended to have worse flare/remission profiles as compared with those without these risk factors. More studies are needed to better understand the reasons and implications of changing trends in flare and remission occurrence in RA.
Funding: This work was funded by a grant from Roche and by a grant from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR46849) and the National Institute on Aging of the National Institutes of Health (R01AG034676). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure statement: The authors have declared no conflicts of interest.
References




Comments