-
PDF
- Split View
-
Views
-
Cite
Cite
T McDonnell, Matthew Thornber, T Cooksley, S Jain, S McGlynn, Left inferior ophthalmic vein thrombosis due to VITT: a case report, QJM: An International Journal of Medicine, Volume 114, Issue 11, November 2021, Pages 810–811, https://doi.org/10.1093/qjmed/hcab124
Close - Share Icon Share
Vaccine-induced thrombotic thrombocytopenia (VITT) is a recently described complication of SARS-CoV-2 adenovirus vector vaccines with patients presenting 1–4 weeks post-vaccine with thrombocytopenia and thrombosis in a broad spectrum of sites. This case further highlights the wide spectrum of potential thrombotic sites in VITT and rapid progression of this condition.
A 58-year-old woman with no prior medical history, presented with a 2 days history of lethargy, mild headache and a sense of fullness at the skull base 9 days post-ChAdOx1 nCoV-19 (AstraZeneca) SARS-CoV-2 vaccine. Her initial clinical examination was normal. Investigations revealed a thrombocytopenia of 31 × 109/l, fibrinogen 0.83 g/l (normal range 1.50–4.00 g/l), a D-Dimer of 119 000 ng/ml (normal range 70–500 ng/ml) and a blood film which showed the occasional red cell fragment. A presumed diagnosis of vaccine-induced thrombotic thrombocytopenia (VITT) was made.
An urgent CT brain and cerebral sinus venogram (CTV) showed a patent cerebral venous system and no other abnormalities. A single dose of 1 mg/kg intravenous immunoglobulin (IVIg) was administered but in the absence of proven thrombus and severe thrombocytopenia, non-heparinoid anticoagulation was withheld and she was closely observed.
Nineteen hours after her initial CTV, she gradually developed a sensation of fullness behind her left eye, mild peri-orbital swelling, visual blurring, rhinorrhoea and features in keeping with a high-pressure headache. Other than the observed swelling, physiological parameters, neurological examination and corrected vision were all normal. Twenty-one hours post-index scan, repeat CTV was obtained demonstrating acute venous thrombus in left inferior ophthalmic vein (Figure 1). Non-heparinoid anticoagulation (Fondaparinux) was started and 1 mg/kg of prednisolone was commenced. Ophthalmological examination of the eye revealed no central retinal vein occlusion and normal intraocular pressure.
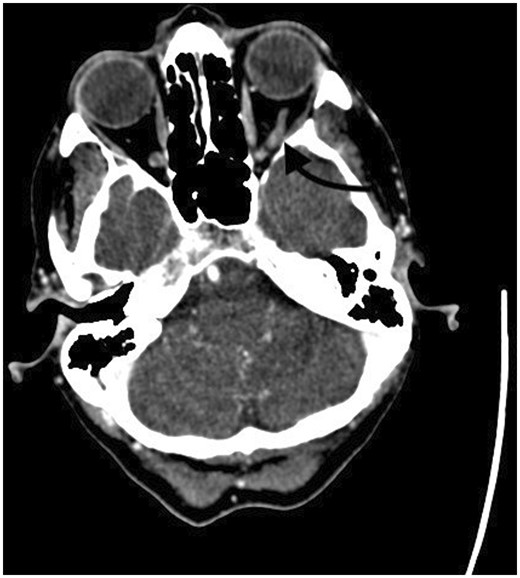
(a) Axial pre-contrast study shows left inferior ophthalmic vein hyperdensity and expansion and (b) post-contrast study confirming lack of contrast filling in left inferior ophthalmic vein.
With the rapid advent and rollout of SARS-CoV-2 vaccines, a new disease has been documented, in association with the ChAdO×1 nCoV-19 AstraZeneca vaccine, known as VITT. A constellation of thrombocytopenia and thrombosis, with a predilection for the cerebral venous and splanchnic circulation as well as pulmonary embolism has been documented. These patients possess platelet activating antibodies and test positive for antibodies against PF4-heparin, similar to heparin induced thrombocytopenia. This collection of symptoms typically occurs 6–24 days post-vaccine, carries high mortality.1
High-dose IVIg has been recommended with the aim of improving platelet number and thrombosis through inhibition of Fcγ receptor–mediated platelet activation.1,2 Accepting hesitancy of starting anticoagulation in the presence of significant thrombocytopenia, the British Society of Haematology currently recommends starting anticoagulation when fibrinogen is >1.5 g/l and platelets >30 × 109/l. This case also demonstrates the rapid clinical trajectory of VITT: our patient developed a left optic vein thrombosis within 21 h of a normal CT venogram, despite the administration of IVIg. This case further suggests the importance of anticoagulation in VITT, particularly in the absence of documented haemorrhage.
Conflict of interest. None declared.
References
British Society for Haematology. Guidance Produced from the Expert Haematology Panel (EHP) Focussed on Covid-19 Vaccine Induced Thrombosis and Thrombocytopenia (VITT). [Updated Guidance on Management. Version 1.3],




