-
PDF
- Split View
-
Views
-
Cite
Cite
C Chan-Chung, C S Ong, L L Chan, E K Tan, Eosinophilic granulomatosis with polyangiitis after COVID-19 vaccination, QJM: An International Journal of Medicine, Volume 114, Issue 11, November 2021, Pages 807–809, https://doi.org/10.1093/qjmed/hcab273
Close - Share Icon Share
In our current fight against the SARS-CoV-2 (COVID-19) pandemic, we should remain vigilant in identifying the possible complications of COVID-19 vaccination. Our case illustrates a possible link between eosinophilic granulomatosis with polyangiitis and the COVID-19 vaccine.
Introduction
Vaccination against SARS-CoV-2 (COVID-19) is crucial in the global fight against the current pandemic. Despite concerns about the possible side effects of messenger ribonucleic acid vaccines, serious adverse effects are generally rare.1 Nonetheless, we should remain vigilant in identifying possible complications linked to the new vaccines. To the best of our knowledge, we believe our case is the first report of an acute neurological presentation of Churg–Strauss syndrome following COVID-19 vaccination.
Case
A previously healthy 62-year-old woman with a history of mild asthma presented with low-grade fever and periorbital edema a few days after receiving the second dose of Pfizer COVID-19 vaccine. She subsequently experienced pain and numbness in both feet and palms, associated with poor balance. She was initially treated with anti-histamines and a short course of prednisolone. However, despite the rapid resolution of her periorbital edema and fever, her neurological symptoms continued to progress to an extent that she was unable to walk 3 weeks after her last vaccination.
Neurologic examination revealed an asymmetric lower motor neuron pattern of weakness affecting the lower limbs (with absent deep tendon reflexes in the lower limbs). There was loss of sensation to all modalities distal to the ankles. She also had truncal ataxia and Romberg’s test was positive, with no other cerebellar signs. She needed assistance to ambulate, with flapping of both her feet as she could not feel the ground. There was a relative afferent pupillary defect in the right eye with cilioretinal-sparing retinal artery occlusion. One day after her admission to Neurology, she developed non-pruritic purpuric macules in her palms (Figure 1A).
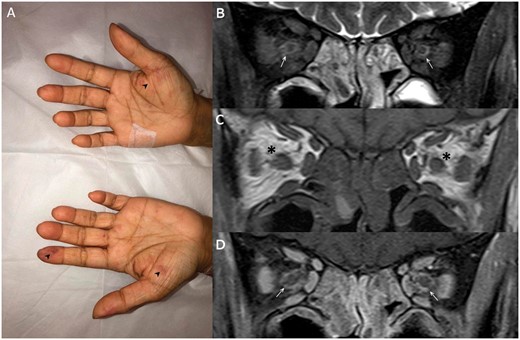
Purpuric macules in hands (A) (arrowheads) and, MRI orbital coronal T2 (B), pre (C) and post (D) contrast-enhanced T1-weighted images demonstrating extensive naso-ethmoidal sinusitis and retrobulbar inflammatory stranding (asterisks) centered around the peri-optic neuritis (arrows) compared to normal fat signal in the superomedial extraconal spaces.
Baseline investigations and autoimmune tests revealed eosinophilia (52%, absolute count 13.40 × 109/L) and a systemic inflammatory process (white cell count 25.67 × 109/L, erythrocyte sedimentation rate 47/h). Her anti-myeloperoxidase was >200 RU/ml. Renal and liver function tests and lumbar puncture findings were unremarkable. Magnetic resonance imaging (MRI) of the brain and spine were also unremarkable. However, MRI orbits showed avid enhancement of and around both optic nerve sheaths (Figure 1B–D) and extensive naso-ethmoidal inflammatory mucosal disease. Nerve conduction study showed a severe sensorimotor polyneuropathy predominantly affecting the lower limbs, with absent sural and near-absent peroneal conduction.
Her chest radiograph was unremarkable. A transthoracic echocardiography revealed a severely impaired systolic ejection fraction (29%), albeit diagnostic coronary angiogram was normal. An endomyocardial biopsy showed an eosinophilic myocarditis, without granulomas or giant cells. A skin biopsy of the left calf showed features of small vessel leukocytoclastic vasculitis.
She was diagnosed with new-onset eosinophilic granulomatosis with polyangiitis (EGPA) or Churg–Strauss syndrome (based on diagnostic criteria of the American College of Rheumatology 1990). She was treated with intravenous methylprednisolone 1 g daily for 3 days and an intravenous infusion of rituximab 1000 mg. Her neuropathic symptoms were treated with Gabapentin. She responded well symptomatically and had a significant improvement in her eosinophilic count.
Discussion
One of the biggest challenges is to identify healthy subjects or patients with specific allergies or autoimmune diseases who may be at higher risk of developing vaccine complications. Non-specific numbness or aches are common mild vaccination adverse effects.2 Systemic vasculitis developing after vaccination is rare. There was a previous case report of a middle age woman who developed EGPA following Influenza A (H1N1) vaccination.4 Previous reports have postulated the concepts of ‘molecular mimicry’ or ‘bystander activation’ as possible triggering mechanisms for autoimmune manifestations.3,5 Our patient is unusual in her predominantly neuropathic manifestations preceding systemic signs. While the cause-and-effect association with the vaccine cannot be proven with certainty, clinicians should have a high index of suspicion for autoimmune-related polyneuritis in asthmatic patients presenting with sensorimotor symptoms and poor balance after COVID-19 vaccination.
Acknowledgements
We would like to thank our colleagues from various specialties (Radiology, Rheumatology, Cardiology, Pathology, Dermatology, Ophthalmology) who contributed in the management of this patient.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. E.-K.T. is supported by the National Medical Research Council (STaR and PD LCG 0002).
Conflict of interest. None declared.
Patient consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine 2005; 23:3876–86



