-
PDF
- Split View
-
Views
-
Cite
Cite
A Kono, R Yoshioka, P Hawk, K Iwashina, D Inoue, M Suzuki, C Narita, K Haruta, A Miyake, H Yoshida, N Tosaka, A case of severe interstitial lung disease after COVID-19 vaccination, QJM: An International Journal of Medicine, Volume 114, Issue 11, November 2021, Pages 805–806, https://doi.org/10.1093/qjmed/hcab263
Close - Share Icon Share
Many drugs can cause interstitial lung disease (ILD), and influenza vaccine-induced ILD has previously been reported.
As the coronavirus disease 2019 (COVID-19) vaccines are the first mRNA vaccines to be widely used, the likelihood of adverse events is unknown.
Here we describe a case of ILD after COVID-19 vaccination.
Introduction
In early August 2021, a 66-year-old man presented to the emergency department of our hospital with respiratory failure 13 days after receiving his second dose of the coronavirus disease 2019 (COVID-19) vaccination (BNT162b2). He had developed a fever on the second day after the second vaccination, but it decreased to 37°C without medication. On the fifth day, he had a fever of 39°C, cough and malaise, and his symptoms worsened. On the 13th day, he visited our hospital with marked hypoxemia.
Vital signs on arrival were body temperature of 39.3°C, respiratory rate of 42 breaths per minute, blood pressure of 156/82 mmHg and oxygen saturation of peripheral artery (SpO2) of 40% on room air. The initial nasopharyngeal swab test for the SARS-CoV-2 nucleic acid (TRC Ready SARS-CoV-2 provided by Tosoh Corp., Yamaguchi, Japan) was negative. The results of blood tests were as follows: white blood cells 18 300/μl, hematocrit 42.1%, platelets 421 000/μl, D-dimer 1.2 μg/ml, creatinine 0.90 mg/dl, creatinine kinase 92 IU/l, KL-6 401U/ml, pulmonary surfactant protein-D 145 ng/ml, C-reactive protein 8.7 mg/dl, and procalcitonin 0.95 ng/ml. Chest computed tomography showed diffuse ground-glass opacity bilaterally. The radiologist diagnosed interstitial lung disease (ILD) with a diffuse alveolar damage pattern.
After admission, SpO2 could not be maintained above 85% even with high flow nasal oxygen therapy. Due to hypoxemia, the patient was intubated 6 h after admission and started on intravenous methylprednisolone 1000 mg/day with ventilator management. Respiratory failure was markedly improved after steroid administration. Two days later, the patient was successfully extubated. On the fourth day of hospitalization, the steroid dose was reduced to prednisolone 30 mg/day orally. The patient was discharged on the seventh day of hospitalization. After discharge, steroid dose was gradually reduced without any relapse. Later blood test results revealed that other diseases were negative. Based on the course of the disease, we diagnosed the case as drug-induced interstitial lung disease (DIILD) caused by the COVID-19 vaccine.
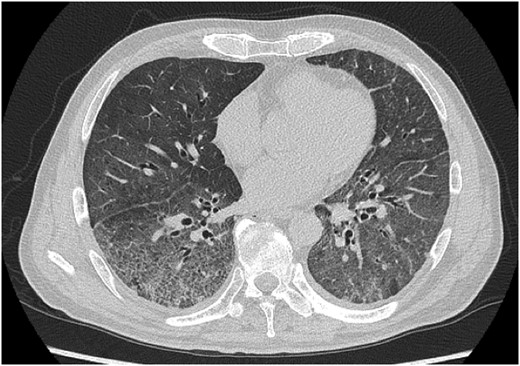
Chest CT on the day of admission showing diffuse ground glass opacity bilaterally.
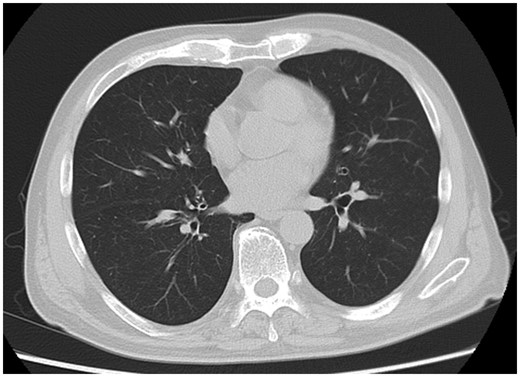
Chest CT 39 days after admission showing improvement in lung shadow.
Discussion
DIILD is characterized by a wide variety of symptoms. DIILD diagnosis is based on clinical, physiological and radiological findings. Camus et al.1 have proposed the following diagnostic criteria for drug-induced infiltrative lung disease: identification, specificity, temporal eligibility, features and exclusion of other causes. In the present case, all of these criteria were met, as the patient was not taking any medication other than the COVID-19 vaccine, had no symptoms prior to vaccination and was negative for infectious, collagen and allergic diseases based on blood, sputum and nasopharyngeal swab tests.
In Japan, there are three licensed COVID-19 vaccines, BNT162b2, mRNA-1273 and ChAdOx1, among which BNT162b2 is the most commonly used. According to VigiAccess™, there have been 24 respirator-dependent cases among COVID-19 vaccine-related adverse events. To the best of our knowledge, this is the second case report of ILD caused by the BNT162b2 vaccine. Park et al.2 reported the first case, but their case was not severe enough to require intubation. Our case is the first to require ventilator management. Vaccine-induced DIILD has been reported for the influenza vaccine. Watanabe et al.3 reported that five out of seven cases of influenza vaccine-associated ILD were Asians. In relation to the COVID-19 vaccines, both the first and second cases have been Asians.
In conclusion, the COVID-19 vaccine is playing a key role in the fight against the global COVID-19 pandemic, but careful follow-up to detect potentially severe adverse events is required.
Conflict of interest. The authors declare no conflicts of interest associated with this manuscript.



