-
PDF
- Split View
-
Views
-
Cite
Cite
Cheney J G Drew, Lori Quinn, Katy Hamana, Rhys Williams-Thomas, Lucy Marsh, Polyxeni Dimitropoulou, Rebecca Playle, Beth Ann Griffin, Mark Kelson, Robin Schubert, Lisa Muratori, Ralf Reilmann, Anne Rosser, Monica Busse, Physical Activity and Exercise Outcomes in Huntington Disease (PACE-HD): Protocol for a 12-Month Trial Within Cohort Evaluation of a Physical Activity Intervention in People With Huntington Disease, Physical Therapy, Volume 99, Issue 9, September 2019, Pages 1201–1210, https://doi.org/10.1093/ptj/pzz075
Close - Share Icon Share
Abstract
Exercise is emerging as an important aspect in the management of disease-related symptoms and functional decline in people with Huntington disease (HD). Long-term evaluation of physical activity and exercise participation in HD has yet to be undertaken.
The objective is to investigate the feasibility of a nested randomized controlled trial (RCT) alongside a longitudinal observational study of physical activity and exercise outcomes in people with HD.
This will be a 12-month longitudinal observational study (n = 120) with a nested evaluation of a physical activity intervention (n = 30) compared with usual activity (n = 30) using a “trial within a cohort” design.
The study will take place in HD specialist clinics in Germany, Spain, and the United States, with intervention delivery in community settings.
The participants will have early-mid–stage HD and be participating in the Enroll-HD study.
This will be a 12-month physical activity behavioral change intervention, delivered by physical therapists in 18 sessions, targeting uptake of aerobic exercise and increased physical activity.
All participants (n = 120) will complete Enroll-HD assessments (motor, cognitive, behavioral, and quality of life) at baseline and at 12 months. Additional Physical ACtivity and Exercise Outcomes in Huntington Disease (PACE-HD) assessments include fitness (predicted maximal oxygen uptake [V o2max]), self-reported and quantitative measures of physical activity, disease-specific symptoms, and walking endurance. RCT participants (n = 60) will complete an additional battery of quantitative motor assessments and a 6-month interim assessment. Enroll-HD data will be linked to PACE-HD physical activity and fitness data.
The limitations include that the embedded RCT is open, and assessors at RCT sites are not blinded to participant allocation.
PACE-HD will enable determination of the feasibility of long-term physical activity interventions in people with HD. The novel “trial within a cohort” design and incorporation of data linkage have potential to reduce participant burden. This design could be applied to other neurological diseases and movement disorders where recruitment and retention are challenging.
Huntington Disease (HD) is an inherited neurodegenerative disease resulting in the loss of striatal neurons leading to disruption of corticostriatal pathways. HD is characterized by progressive deficits in cognition, behavior, and movement1 leading to decline in function, performance of living daily activities, and quality of life,2 with associated caregiver and socioeconomic burdens.3,4 There is evidence that modifiable lifestyle factors such as education, activity levels, and specific motor training can minimize the functional impact of the disease and that targeting motor impairments can play a role in delaying disease progression.5,6
Clinical studies of short-term exercise interventions have demonstrated improvements in fitness, motor impairment, and quality of life in people with HD.7–12 Longer-term pragmatic evaluations of well-defined exercise interventions are required, although such studies are challenging to set up and deliver, particularly in rare diseases. Such studies are limited by poor recruitment, retention, and generalizability. Embedded studies, or trials within cohorts (TWiCs),13 provide an efficient method for recruitment and can reduce burden through the use of routinely collected prospective outcome data. Generalizability can be better achieved through access to cohort natural history data and information on routine care as well as relevant lifestyle factors. TWiC designs are most suited to open trials and comparisons with treatment as usual, which can introduce elements of assessor bias. The inclusion of measures less subject to investigator bias, including wearable technologies for the assessment of physical activity and using quantitative motor and cognitive assessments, is therefore crucial.
Trial Design
PACE-HD uses a TWiC design13 in which we are conducting a 12-month, observational cohort study in people with early and mid-stage HD (n = 120) with a nested randomized controlled trial (RCT) of a 12-month physical activity intervention (n = 30) compared with the pragmatic choice of activity as usual (n = 30) (Fig. 1). The intervention will be delivered by physical therapists, targeting uptake of aerobic exercise and increased physical activity. PACE-HD will use Enroll-HD,14 a global clinical research platform designed to facilitate clinical research in Huntington disease. Core datasets are collected annually on all research participants as part of this multicenter longitudinal observational study of HD. Data are monitored for quality and accuracy using a risk-based monitoring approach. All sites are required to obtain and maintain local ethics committee approvals. Researchers can apply for access to anonymized Enroll-HD data via a specific data request15 subject to approval from the Enroll-HD Scientific Review Committee. PACE-HD study data will be supplemented by linking data from participants’ annual Enroll-HD assessments. At the conclusion of the study the fully integrated dataset will be shared with Enroll-HD and made available to other researchers.
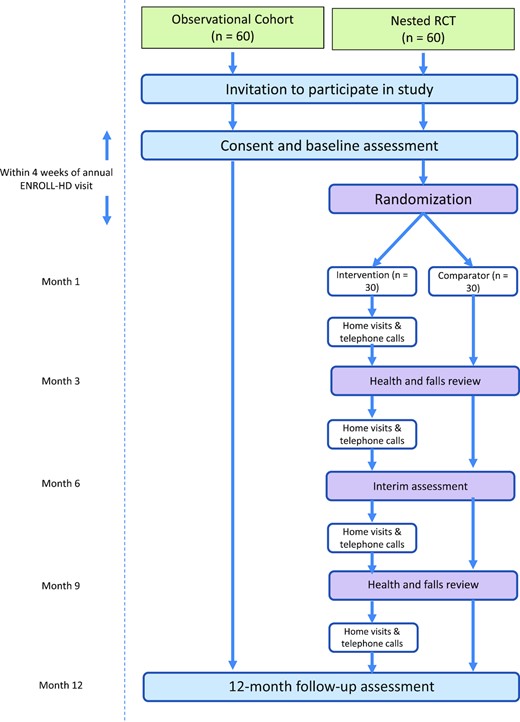
Schematic of participant flow through the study. RCT = randomized controlled trial.
Study Objectives and Outcomes
The primary objective of this study is to establish the feasibility of a within-cohort nested RCT of a 12-month physical activity intervention in people with HD. The primary outcome is feasibility of the nested RCT in terms of recruitment, retention, data completeness, adherence, fidelity, and acceptability. Secondary objectives include: (1) exploration of effect estimates for long-term exercise in HD compared with usual activity; (2) exploration of the influence of physical activity and function on cognitive, motor, and functional abilities over a 1-year period; and (3) exploration of the predictive validity of physical fitness at 6 months on motor and cognitive outcomes.
Methods
Study Population
Participants (n = 120) will be recruited at 6 sites. Three sites will serve as observational sites only, and 3 additional sites will conduct the nested RCT (see supplementary information for full site details). Sites have been selected as currently recruiting Enroll-HD centers with capability and potential participant capacity required to deliver the study as intended. Potentially eligible participants will be identified according to study inclusion criteria from local Enroll-HD records. Inclusion criteria are: (1) confirmed genetic diagnosis of HD, (2) over 18 years of age, (3) currently registered as a participant in Enroll-HD, and (4) up to and including stage 2 disease status (defined as having a total functional capacity of 7–13). Exclusion criteria are: (1) diagnosis of juvenile-onset HD, (2) history of comorbid neurological conditions such as multiple sclerosis or stroke, (3) an acute orthopedic condition, for example, a sprained ankle or fracture, and (4) inability or unwillingness to give written informed consent. Interested participants will be provided with written information about the study and invited to attend a baseline assessment. Written informed consent will be obtained by appropriately trained and delegated site staff.
Sample Size
Because the primary outcome of PACE-HD is feasibility of the nested RCT (in terms of recruitment, retention, data completeness, safety, fidelity, and acceptability) a formal sample size calculation for efficacy has not been performed and formal hypothesis testing will not be carried out. Effect sizes and 95% confidence intervals (CIs) will be presented to inform planning of future studies. For a total sample size of 120 participants recruited into the study we can determine a 95% CI for a 70% retention rate to within ± 8.2%.
Assessments and Secondary Outcome Measures
All study participants will complete the extended battery of Enroll-HD assessments (eAppendix A, available at https://dbpia.nl.go.kr/ptj)16 at baseline and 12 months at the participating site (typically hospital or rehabilitation center). Participants in the nested RCT will complete an additional battery of novel, quantitative motor assessments (Clinch Token Transfer Test,17 Geneactiv accelerometers18 for physical activity monitoring, and Q-Motor and Q-Cog assessments),19 as well as a 6-month interim assessment (Table). Both baseline and 12-month assessments must be conducted within ± 6 weeks of the participant's annual Enroll-HD visit.
PACE-HD (Physical ACtivity and Exercise Outcomes in Huntington Disease) Specific Assessmentsa
| Construct . | Measure . | Time Point (month) . | ||
|---|---|---|---|---|
| 0 . | 6 . | 12 . | ||
| All sites | ||||
| Fitness | Predicted V̇o2max will be measured during stepwise incremental exercise test.29 The test is performed on a cycle ergometer with participants seated in a standardized position. Participants will attempt to maintain a cadence of 50 revolutions per minute (rpm), starting at 50 W and increasing by 25 W every 2 min until test termination. The test will be terminated when the participant reaches volitional exhaustion or cadence drops by 10 rpm. At the end of each minute, work-rate (W), rating of perceived exertion (Borg RPE scale), and heart rate will be recorded for analysis and conversion to predicted V̇o2max score | ✓ | ✓ (RCT only) | ✓ |
| Walking endurance | The 6-minute walk test will be used as a measure of walking endurance. This test evaluates the distance walked over a 6-min period, and has been validated for use in HD30 | ✓ | ✓ (RCT only) | ✓ |
| Participant-reported clinical symptoms | HD Pro-Triad,31 a quality-of-life measure specific to HD, will be used to assess disease-specific symptoms including cognitive decline, emotional/behavioral disturbance, and motor dysfunction | ✓ | ✓ | |
| Physical activity monitoring | Brunel Lifestyle Physical Activity Questionnaire32 is a validated self-report instrument that measures the planned and unplanned dimensions of lifestyle physical activity | ✓ | ||
| Geneactiv accelerometers18 are research-grade wrist-worn devices that continuously record physical and sedentary behaviors without feedback provided to the wearer.33 We are developing algorithms specific to HD to transform the accelerometer data into summary data specifying periods of physical activity, sedentary, and sleep behaviors.34 Participants will be given the monitors at baseline assessment and will be asked to return them after 7 d of wear. Wear time will be 24 h/d, except when showering. For validation of sleep time compared with sedentary time, participants will be asked to complete and return sleep diaries for the 7-d period of physical activity assessment | ✓ | ✓ (RCT only) | ✓ | |
| International Physical Activity Questionnaire (IPAQ, Short Form) measures the intensity and duration of physical activity undertaken in the last 7 d. It will be used to assess 7-d physical activity and to validate outputs of the physical activity monitors35 | ✓ | ✓ (RCT only) | ✓ | |
| RCT sites only | ||||
| Motor and dual-task function | The Clinch Token Transfer Test (C3T)17 is a novel dual-task assessment of bilateral, upper motor function that consists of 3-coin transfer tasks that increase in difficulty (baseline simple, baseline complex, and a dual task). The time taken to pick up and transfer the coins from dominant to nondominant hand and place into a purpose-developed box is recorded. The addition of cognitive load increases the task complexity. This has been shown to be highly sensitive across all stages of HD | ✓ | ✓ | |
| Motor function | Q-Motor was developed in TRACK-HDb and TRACK-ON-HD where motor tasks are related to functionally relevant everyday tasks. All Q-Motor assessments are conducted using precalibrated and temperature-controlled force transducers and 3D-tracking19 sensors with very high sensitivity and test-retest reliability across sessions and sites in multicenter clinical trials involving participants with HD36,37 | ✓ | ✓ | |
| Cognitive function | Q-Cog assessments deploy the technology used in the Q-Motor system to benefit from the high accuracy of the sensors in tasks with high cognitive load with the goal of providing a sensitive quantitative assessment of cognitive deficits in HD. Q-Cog assessments have high discriminative ability.38 Longitudinal performance has not yet been evaluated | ✓ | ✓ | |
| Self-efficacy | The Lorig Self-Efficacy39 scale is a 6-item questionnaire validated for the assessment of self-efficacy in people with chronic conditions. In this study, it will be used to measure self-efficacy related to exercise (exercise subscale only) | ✓ | ✓ | ✓ |
| Construct . | Measure . | Time Point (month) . | ||
|---|---|---|---|---|
| 0 . | 6 . | 12 . | ||
| All sites | ||||
| Fitness | Predicted V̇o2max will be measured during stepwise incremental exercise test.29 The test is performed on a cycle ergometer with participants seated in a standardized position. Participants will attempt to maintain a cadence of 50 revolutions per minute (rpm), starting at 50 W and increasing by 25 W every 2 min until test termination. The test will be terminated when the participant reaches volitional exhaustion or cadence drops by 10 rpm. At the end of each minute, work-rate (W), rating of perceived exertion (Borg RPE scale), and heart rate will be recorded for analysis and conversion to predicted V̇o2max score | ✓ | ✓ (RCT only) | ✓ |
| Walking endurance | The 6-minute walk test will be used as a measure of walking endurance. This test evaluates the distance walked over a 6-min period, and has been validated for use in HD30 | ✓ | ✓ (RCT only) | ✓ |
| Participant-reported clinical symptoms | HD Pro-Triad,31 a quality-of-life measure specific to HD, will be used to assess disease-specific symptoms including cognitive decline, emotional/behavioral disturbance, and motor dysfunction | ✓ | ✓ | |
| Physical activity monitoring | Brunel Lifestyle Physical Activity Questionnaire32 is a validated self-report instrument that measures the planned and unplanned dimensions of lifestyle physical activity | ✓ | ||
| Geneactiv accelerometers18 are research-grade wrist-worn devices that continuously record physical and sedentary behaviors without feedback provided to the wearer.33 We are developing algorithms specific to HD to transform the accelerometer data into summary data specifying periods of physical activity, sedentary, and sleep behaviors.34 Participants will be given the monitors at baseline assessment and will be asked to return them after 7 d of wear. Wear time will be 24 h/d, except when showering. For validation of sleep time compared with sedentary time, participants will be asked to complete and return sleep diaries for the 7-d period of physical activity assessment | ✓ | ✓ (RCT only) | ✓ | |
| International Physical Activity Questionnaire (IPAQ, Short Form) measures the intensity and duration of physical activity undertaken in the last 7 d. It will be used to assess 7-d physical activity and to validate outputs of the physical activity monitors35 | ✓ | ✓ (RCT only) | ✓ | |
| RCT sites only | ||||
| Motor and dual-task function | The Clinch Token Transfer Test (C3T)17 is a novel dual-task assessment of bilateral, upper motor function that consists of 3-coin transfer tasks that increase in difficulty (baseline simple, baseline complex, and a dual task). The time taken to pick up and transfer the coins from dominant to nondominant hand and place into a purpose-developed box is recorded. The addition of cognitive load increases the task complexity. This has been shown to be highly sensitive across all stages of HD | ✓ | ✓ | |
| Motor function | Q-Motor was developed in TRACK-HDb and TRACK-ON-HD where motor tasks are related to functionally relevant everyday tasks. All Q-Motor assessments are conducted using precalibrated and temperature-controlled force transducers and 3D-tracking19 sensors with very high sensitivity and test-retest reliability across sessions and sites in multicenter clinical trials involving participants with HD36,37 | ✓ | ✓ | |
| Cognitive function | Q-Cog assessments deploy the technology used in the Q-Motor system to benefit from the high accuracy of the sensors in tasks with high cognitive load with the goal of providing a sensitive quantitative assessment of cognitive deficits in HD. Q-Cog assessments have high discriminative ability.38 Longitudinal performance has not yet been evaluated | ✓ | ✓ | |
| Self-efficacy | The Lorig Self-Efficacy39 scale is a 6-item questionnaire validated for the assessment of self-efficacy in people with chronic conditions. In this study, it will be used to measure self-efficacy related to exercise (exercise subscale only) | ✓ | ✓ | ✓ |
HD = Huntington disease; RCT = randomized controlled trial; V̇o2max = maximum oxygen uptake; 3D = 3-dimensional.
bTRACK-HD was a 3-year prospective, observational study of people with HD and controls where imaging, cognitive, and motor assessment data were collected. TRACK-ON-HD was an extension of TRACK-HD to include premanifest HD participants.
PACE-HD (Physical ACtivity and Exercise Outcomes in Huntington Disease) Specific Assessmentsa
| Construct . | Measure . | Time Point (month) . | ||
|---|---|---|---|---|
| 0 . | 6 . | 12 . | ||
| All sites | ||||
| Fitness | Predicted V̇o2max will be measured during stepwise incremental exercise test.29 The test is performed on a cycle ergometer with participants seated in a standardized position. Participants will attempt to maintain a cadence of 50 revolutions per minute (rpm), starting at 50 W and increasing by 25 W every 2 min until test termination. The test will be terminated when the participant reaches volitional exhaustion or cadence drops by 10 rpm. At the end of each minute, work-rate (W), rating of perceived exertion (Borg RPE scale), and heart rate will be recorded for analysis and conversion to predicted V̇o2max score | ✓ | ✓ (RCT only) | ✓ |
| Walking endurance | The 6-minute walk test will be used as a measure of walking endurance. This test evaluates the distance walked over a 6-min period, and has been validated for use in HD30 | ✓ | ✓ (RCT only) | ✓ |
| Participant-reported clinical symptoms | HD Pro-Triad,31 a quality-of-life measure specific to HD, will be used to assess disease-specific symptoms including cognitive decline, emotional/behavioral disturbance, and motor dysfunction | ✓ | ✓ | |
| Physical activity monitoring | Brunel Lifestyle Physical Activity Questionnaire32 is a validated self-report instrument that measures the planned and unplanned dimensions of lifestyle physical activity | ✓ | ||
| Geneactiv accelerometers18 are research-grade wrist-worn devices that continuously record physical and sedentary behaviors without feedback provided to the wearer.33 We are developing algorithms specific to HD to transform the accelerometer data into summary data specifying periods of physical activity, sedentary, and sleep behaviors.34 Participants will be given the monitors at baseline assessment and will be asked to return them after 7 d of wear. Wear time will be 24 h/d, except when showering. For validation of sleep time compared with sedentary time, participants will be asked to complete and return sleep diaries for the 7-d period of physical activity assessment | ✓ | ✓ (RCT only) | ✓ | |
| International Physical Activity Questionnaire (IPAQ, Short Form) measures the intensity and duration of physical activity undertaken in the last 7 d. It will be used to assess 7-d physical activity and to validate outputs of the physical activity monitors35 | ✓ | ✓ (RCT only) | ✓ | |
| RCT sites only | ||||
| Motor and dual-task function | The Clinch Token Transfer Test (C3T)17 is a novel dual-task assessment of bilateral, upper motor function that consists of 3-coin transfer tasks that increase in difficulty (baseline simple, baseline complex, and a dual task). The time taken to pick up and transfer the coins from dominant to nondominant hand and place into a purpose-developed box is recorded. The addition of cognitive load increases the task complexity. This has been shown to be highly sensitive across all stages of HD | ✓ | ✓ | |
| Motor function | Q-Motor was developed in TRACK-HDb and TRACK-ON-HD where motor tasks are related to functionally relevant everyday tasks. All Q-Motor assessments are conducted using precalibrated and temperature-controlled force transducers and 3D-tracking19 sensors with very high sensitivity and test-retest reliability across sessions and sites in multicenter clinical trials involving participants with HD36,37 | ✓ | ✓ | |
| Cognitive function | Q-Cog assessments deploy the technology used in the Q-Motor system to benefit from the high accuracy of the sensors in tasks with high cognitive load with the goal of providing a sensitive quantitative assessment of cognitive deficits in HD. Q-Cog assessments have high discriminative ability.38 Longitudinal performance has not yet been evaluated | ✓ | ✓ | |
| Self-efficacy | The Lorig Self-Efficacy39 scale is a 6-item questionnaire validated for the assessment of self-efficacy in people with chronic conditions. In this study, it will be used to measure self-efficacy related to exercise (exercise subscale only) | ✓ | ✓ | ✓ |
| Construct . | Measure . | Time Point (month) . | ||
|---|---|---|---|---|
| 0 . | 6 . | 12 . | ||
| All sites | ||||
| Fitness | Predicted V̇o2max will be measured during stepwise incremental exercise test.29 The test is performed on a cycle ergometer with participants seated in a standardized position. Participants will attempt to maintain a cadence of 50 revolutions per minute (rpm), starting at 50 W and increasing by 25 W every 2 min until test termination. The test will be terminated when the participant reaches volitional exhaustion or cadence drops by 10 rpm. At the end of each minute, work-rate (W), rating of perceived exertion (Borg RPE scale), and heart rate will be recorded for analysis and conversion to predicted V̇o2max score | ✓ | ✓ (RCT only) | ✓ |
| Walking endurance | The 6-minute walk test will be used as a measure of walking endurance. This test evaluates the distance walked over a 6-min period, and has been validated for use in HD30 | ✓ | ✓ (RCT only) | ✓ |
| Participant-reported clinical symptoms | HD Pro-Triad,31 a quality-of-life measure specific to HD, will be used to assess disease-specific symptoms including cognitive decline, emotional/behavioral disturbance, and motor dysfunction | ✓ | ✓ | |
| Physical activity monitoring | Brunel Lifestyle Physical Activity Questionnaire32 is a validated self-report instrument that measures the planned and unplanned dimensions of lifestyle physical activity | ✓ | ||
| Geneactiv accelerometers18 are research-grade wrist-worn devices that continuously record physical and sedentary behaviors without feedback provided to the wearer.33 We are developing algorithms specific to HD to transform the accelerometer data into summary data specifying periods of physical activity, sedentary, and sleep behaviors.34 Participants will be given the monitors at baseline assessment and will be asked to return them after 7 d of wear. Wear time will be 24 h/d, except when showering. For validation of sleep time compared with sedentary time, participants will be asked to complete and return sleep diaries for the 7-d period of physical activity assessment | ✓ | ✓ (RCT only) | ✓ | |
| International Physical Activity Questionnaire (IPAQ, Short Form) measures the intensity and duration of physical activity undertaken in the last 7 d. It will be used to assess 7-d physical activity and to validate outputs of the physical activity monitors35 | ✓ | ✓ (RCT only) | ✓ | |
| RCT sites only | ||||
| Motor and dual-task function | The Clinch Token Transfer Test (C3T)17 is a novel dual-task assessment of bilateral, upper motor function that consists of 3-coin transfer tasks that increase in difficulty (baseline simple, baseline complex, and a dual task). The time taken to pick up and transfer the coins from dominant to nondominant hand and place into a purpose-developed box is recorded. The addition of cognitive load increases the task complexity. This has been shown to be highly sensitive across all stages of HD | ✓ | ✓ | |
| Motor function | Q-Motor was developed in TRACK-HDb and TRACK-ON-HD where motor tasks are related to functionally relevant everyday tasks. All Q-Motor assessments are conducted using precalibrated and temperature-controlled force transducers and 3D-tracking19 sensors with very high sensitivity and test-retest reliability across sessions and sites in multicenter clinical trials involving participants with HD36,37 | ✓ | ✓ | |
| Cognitive function | Q-Cog assessments deploy the technology used in the Q-Motor system to benefit from the high accuracy of the sensors in tasks with high cognitive load with the goal of providing a sensitive quantitative assessment of cognitive deficits in HD. Q-Cog assessments have high discriminative ability.38 Longitudinal performance has not yet been evaluated | ✓ | ✓ | |
| Self-efficacy | The Lorig Self-Efficacy39 scale is a 6-item questionnaire validated for the assessment of self-efficacy in people with chronic conditions. In this study, it will be used to measure self-efficacy related to exercise (exercise subscale only) | ✓ | ✓ | ✓ |
HD = Huntington disease; RCT = randomized controlled trial; V̇o2max = maximum oxygen uptake; 3D = 3-dimensional.
bTRACK-HD was a 3-year prospective, observational study of people with HD and controls where imaging, cognitive, and motor assessment data were collected. TRACK-ON-HD was an extension of TRACK-HD to include premanifest HD participants.
Randomization and Blinding
At RCT sites, on completion of baseline assessments, participants will be randomly assigned 1:1 to receive the 12-month activity intervention or activity as usual. Random assignment will be performed via the web-based study database using a minimization technique to maintain allocation concealment. The randomization allows for balancing by age (above or below 50 years of age), sex, and motor impairment (above or below a Unified Huntington Disease Rating score [total motor score] of 40) at baseline and stratified by country. For pragmatic reasons (including the nature of the intervention) neither participants nor assessors will be blinded to allocation.
Physical Activity Intervention
The PACE-HD intervention is a physical activity behavioral-change intervention based on knowledge developed in 2 previous studies: Exert-HD9 and ENGAGE-HD.20 The intervention will be delivered by trained, licensed physical therapists focusing on promoting strategies to engage in aerobic exercise and physical activity (Fig. 2). The program uses a disease-specific workbook and participant-coach interaction that emphasizes relatedness and promotes participant autonomy and competence.21 The intervention framework is underpinned by self-determination theory,22 a fundamental component of which is collaborative regulation that specifically considers social-contextual disease-specific conditions. This emphasizes the importance of working in a collaborative way with participants, as well as understanding the progressive nature of HD and the changing needs of the participant so that aspects such as access to health care and fitness facilities, social situation, and adaptation to a participant's ongoing cognitive and behavioral status are all taken into account during intervention delivery.
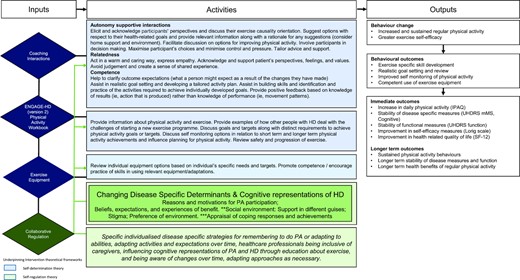
Logic model for the PACE-HD (Physical ACtivity and Exercise Outcomes in Huntington Disease) intervention. HD = Huntington disease; IPAQ = International Physical Activity Questionnaire; PA = physical activity; SF-12 = 12-item quality of life short form survey; UHDRS = Unified Huntington's Disease Rating Scale; UHDRS mMS = Unified Huntington's Disease Rating Scale (modified Motor Score).
The program will consist of 18 face-to-face, one-to-one physical therapy sessions over 12 months. The timing and location (at the participant's home or rehabilitation facilities at site) of these sessions will be determined by participants in consultation with their therapist. The suggested timing of intervention sessions are 3 sessions in months 1 and 2, two sessions in months 3 and 6, and monthly sessions in months 4, 5, and 7 to 12. A therapist manual will be used to provide a structured approach to sessions, focusing on exercise uptake (specifically aerobic and strengthening exercises) and engagement in regular physical activity. In partnership with the therapist, participants will develop activity goals that will be monitored and adjusted throughout. The therapist will work with the participants, using the agreed activity goals as a focus to tailor their exercise programs to build up to regular 30-minute periods of exercise 3 to 5 times per week and strengthening exercises 2 to 3 times per week. Participants will be asked to keep monthly physical activity diaries (in paper format or directly entered into the study database) to record the amount and type of physical activity undertaken. Participants will be given the option to use wearable activity monitors (Fitbit Charge 2; Fitbit Inc, San Francisco, CA, USA) to be used throughout the 12-month period as a means to facilitate/monitor activity and sedentary behaviors. We anticipate that some participants will choose not to use a Fitbit and so the activity diaries will form the principal method of monitoring exercise. We are not using the Fitbit devices as an outcome assessment, but measures elicited from Fitbit use will be explored as part of the overall feasibility assessment. Therapists will send regular reminders for attending upcoming appointments, completing exercise diaries, and uploading of Fitbit data via app-syncing. Preferred communication methods will be discussed at the first session, and can be by phone, email, or text messaging.
People with HD need flexibility in terms of access to exercise facilities,23 and this is critical to successful adherence to exercise in this population. Therefore, each participant will be provided with a choice of exercise equipment options to be used for independent sessions in the home, or participants can choose to exercise in a gym setting.
Participants allocated to activity as usual will not receive the physical activity intervention and will be asked to continue with their usual level of physical activity for the 12-month follow-up period. When participants are randomized to activity as usual they will be referred to the Huntington's Disease Society of America webpages for advice on healthy eating (https://hdsa.org/find-help/living-well-with-hd/nutrition/). They will not receive visits but will be asked to record their activity in monthly diaries. These participants will also receive monthly prompts via their preferred method of communication to remind them to complete and return the diaries to their local site. The intervention is fully described in the Template for Intervention Description and Replication (TiDiER)24 format (eAppendix B).
Data Collection, Management, and Linkage
All participants will be assigned a unique participant identification number, which is used to identify all data relating to that participant and ensure confidentiality. Data for all PACE-HD assessments will be entered in real time into a custom-built database accessed via a secure web interface, after which they are automatically stored in a structured-query-language database. The database is engineered with built-in validations and warning messages that are rigorously tested for use to ensure data quality.
The Clinch Token Transfer Test assessment will be completed via a native Android application on a tablet. On test completion, data are transferred to the Clinch Token Transfer Test structured-query-language live server and PACE-HD structured-query-language live server simultaneously. Data from Geneactiv monitors will be transferred to the coordinating center where a preprogrammed algorithm will be applied to derive variables for analysis. Variables will be uploaded into the study database.
Participants randomized to the intervention who choose to use Fitbit monitors will be assigned a unique username and password for their devices, and will be asked to sync their devices regularly (approximately every 5 days). We will use a backend service platform, FitaBase, to aggregate participants’ Fitbit data, and on completion of the study will export summary activity into the study database. Additional clinical data will be obtained from Enroll-HD via a specific data request detailing all the required variables from the extended battery dataset. Enroll-HD data exports will be identified using the PACE-HD participant identification number, and the Enroll-HD participant identifier will not be shared. All sites will maintain participant identification number/Enroll-HD participant identifier–linking information. Data exports will be obtained at 3 time points: after recruitment of 5 participants per site, at the close of recruitment when all baseline data have been completed, and at the completion of all follow up visits. No follow-up data will be collected from participants who have decided to withdraw from the study.
Monitoring
Data will be monitored remotely for completeness and validity throughout recruitment and follow-up via the study database. A log of all data queries and their resolution will be maintained. Safety events will be monitored by site staff at each interaction with participants. Trial progress in regard to screening, recruitment, retention, noncompliances, and safety events will be monitored on a monthly basis at Trial Management Group meetings and by the independent Trial Steering Committee meeting every 6 months. This is a low-risk study therefore no formal data-monitoring committee will be convened. Onsite monitoring will be performed by Enroll-HD data monitors for the dual purposes of verifying validity of consent and accuracy of the participant identification number/Enroll-HD participant identifier–linking documentation. Participant allocation will be monitored for balance when approximately half the RCT participants have been randomized.
Intervention Fidelity Monitoring
Fidelity of the intervention will be measured using a combination of physical therapist self-report checklists (indicating whether the content of each of the sessions was consistent with that specified in the protocol and training manual) and therapist self-assessment completed using a purpose-developed rating scale.25 Intervention adherence will be assessed as part of the fidelity assessment in the process evaluation.
Analysis
Descriptive statistics will summarize key demographic characteristics of all participants in the study (n = 120). Summary data for recruitment, retention, and data completeness will be tabulated for the cohort and nested RCT. Adherence rates, safety, and fidelity will be summarized for the RCT. The primary feasibility outcome is the proportion of randomized participants that were followed up at 12 months, and this will be presented with 95% CIs along with all other feasibility outcomes. Secondary efficacy outcomes from the RCT will be explored and presented with effect sizes and 95% CIs.
Comparability of the observation-only (n = 60) and RCT (n = 60) participants at baseline will be explored using Enroll-HD data. Drop-out bias for the RCT will be assessed by comparing baseline data for those who were followed up and those who were not at 12 months. No statistical tests will be performed on these data and only appropriate summary data tabulated. For the analysis of secondary outcomes, we will examine the differences in mean scores between arms at 12 months postrandomization with 95% CIs. Linear models will be used for continuous outcomes where the distributional assumptions of the methods have been met and logistic regression for dichotomous outcomes with results presented as odds ratios and 95% CIs. Covariates in the models will include individual participant characteristics used to balance the randomization (age, sex, Unified Huntington Disease Rating Scale [UHDRS] total motor scale score) and the baseline score for each respective outcome. Analysis will be intention to treat and complete case.
A mediation analysis will be performed on V̇o2max recorded at 6 months to assess the role of physical activity and fitness on cognitive, motor, and functional abilities. A sensitivity analysis could be conducted using a mixed model to explore the extent of clustering in potential primary outcome variables by country if numbers permit.
Progression criteria to proceed to further evaluation will be based on the following: over 60% dropout from the RCT at 12 months will mean no progression; between 40% and 60% dropout will mean that changes to the intervention and/or follow-up procedures will be required; and less than 30% dropout at 12 months will indicate that the intervention is suitable for further evaluation without modification.
Summary data of weekly number of steps, average intensity and total intensity of exercise, hours of sleep, average heart rate, and peak heart rate as gathered from Fitbit activity monitors (intervention group only) will be explored for association with fitness (V̇o2max) at 6 and 12 months. We will also explore data on usual activity from self-reported physical activity assessments in relation to blinded Geneactiv activity data.
Principal component analysis will be used to explore the validity of a composite outcome for HD. Recently it has been proposed that a composite outcome has greater validity in detecting disease modification in HD than through the selection of a single primary outcome measure.26 Where it is hypothesized that exercise interventions will impact the triad of HD symptoms (motor, cognitive, and behavioral), a composite outcome would be more sensitive in detecting any meaningful disease modification, thus making this exploratory analysis of importance for interventions such as those being studied here. The composite will be based on motor and cognitive outcomes as suggested by Schobel et al26 and will be compared between arms using CIs.
We will also use propensity score weights to create a pseudorandomized trial of the intervention that compares the individuals in the intervention arm of the RCT with individuals in our Enroll-HD reference cohort. The propensity score weights will aim to make the 2 groups comparable on a range of important characteristics that are associated with HD progression. The treatment effect estimates from the propensity score-matched pseudorandomized groups will be compared with the treatment effect estimate from the randomized groups as a way to assess the utility of propensity score methodology relative to RCTs for obtaining treatment effect estimates for exercise interventions.
Role of the Funding Source
This study has been funded by the Jacques and Gloria Gossweiler Foundation. The funder plays no role in the design or conduct of this study.
Ethics and Governance
PACE-HD is sponsored by Cardiff University ([email protected]), and Cardiff University retains overall responsibility for the trial, which is currently recruiting (opened February 2018). Approval for the study has been obtained from the local institutional review board at each of the 6 sites (see supplementary materials for details of each site and their institutional review boards).
The protocol was written according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement,27 and this article is based upon version 2.0 (November 23, 2017) of the study protocol. All protocol amendments will be communicated to participating sites and regulatory authorities by the coordinating center. Study results will be published on clinicaltrials.gov and in peer-reviewed literature.28
Discussion
This trial incorporates several novel aspects that could transform how we approach the evaluation of long-term exercise interventions in participants with neurodegenerative diseases. HD is an excellent paradigm for other research in neurodegenerative diseases and movement disorders. HD is a well-characterized, single-gene disorder, as opposed to a cluster of symptomatically aligned disorders with differing etiologies. This allows the development and evaluation of research methodologies and therapeutic interventions that could have high applicability to other related diseases.
This study uses a TWiC design with the first longitudinal evaluation of a well-defined exercise intervention in people with HD linking to a global cohort study supplemented with specific exercise-related outcome measures. PACE-HD employs a number of innovative methods for assessing lifestyle factors across a large, heterogeneous cohort. Incorporating routine data (from Enroll-HD) allows the use of a large, clinically relevant dataset without the need for additional tests thereby reducing participant burden. This is particularly important in a rare disease population, members of which are likely to be asked to be involved in multiple research studies. Wearable technologies are being employed to provide objective measures of physical activity. In combination with prospective activity and clinical data collection, this aspect allows further validation of reliable and sensitive tools for assessing physical activity in real-world settings. Propensity score matching allows assessment of the suitability of pseudorandomized study designs, which could be valuable in rare conditions where implementing randomized studies is difficult or unfeasible. The innovative systematic approach proposed for this study will have wide-reaching impact that will advance the development of new therapeutic options. It will deliver a realistic, clinically applicable therapeutic option for people with HD that can be implemented in a variety of health care settings and can be used as a methodological model for evaluating similar lifestyle interventions in other disease populations.
Author Contributions and Acknowledgments
Concept/idea/research design: C.J.G. Drew, L. Quinn, K. Hamana, R. Playle, B.A. Griffin, M. Kelson, R. Reilmann, A. Rosser, M. Busse
Writing: C.J.G. Drew, L. Quinn, K. Hamana, R. Williams-Thomas, B.A. Griffin, L. Muratori, A. Rosser, M. Busse
Data collection: R. Williams-Thomas, L. Marsh, R. Schubert, L. Muratori
Data analysis: L. Quinn, P. Dimitropoulou, R. Playle, B.A. Griffin, M. Kelson, M.Busse
Project management: C.J.G. Drew, L. Quinn, R. Williams-Thomas, L. Muratori, R. Reilmann, A. Rosser, M. Busse
Fund procurement: C.J.G. Drew, L. Quinn, R. Reilmann, A. Rosser, M. Busse
Providing participants: R. Reilmann
Providing institutional liaisons: L. Quinn, R. Reilmann, A. Rosser, M. Busse
Consultation (including review of manuscript before submitting): K. Hamana, P. Dimitropoulou, B.A. Griffin, R. Schubert, R. Reilmann, A. Rosser, M. Busse
We acknowledge the research staff at PACE-HD recruiting sites and Enroll-HD staff for their support and collaboration. Enroll-HD would not be possible without the vital contribution of the research participants and their families.
Ethics Approval
PACE-HD is sponsored by Cardiff University ([email protected]), and Cardiff University retains overall responsibility for the trial, which is currently recruiting (opened February 2018). Approval for the study has been obtained from the local institutional review board at each of the 6 sites (see supplementary materials for details of each site and their institutional review boards). Written informed consent will be obtained by appropriately trained and delegated site staff.
Funding
PACE-HD is funded by the Jacques and Gloria Gossweiler Foundation. Enroll-HD is sponsored by Cure Huntington's Disease Initiative Foundation, a nonprofit biomedical research organization exclusively dedicated to developing therapeutics for HD.
Clinical Trial Registration
This trial has been prospectively registered at clinicaltrials.gov (NCT03344601).
Disclosure and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
PACE-HD was presented at the European Huntington's Disease Network biannual plenary meeting in September 2018 in Vienna, Austria, and at a meeting of Enroll-HD centers in Quebec, Canada, May 2018.
References




Comments