-
PDF
- Split View
-
Views
-
Cite
Cite
Teresa J Kimberley, Cecília N Prudente, Navzer D Engineer, David Alexander Dickie, Teresa A Bisson, Ann Van de Winckel, Vagus Nerve Stimulation Paired With Mobility Training in Chronic Ischemic Stroke: A Case Report, Physical Therapy, Volume 103, Issue 12, December 2023, pzad097, https://doi.org/10.1093/ptj/pzad097
Close - Share Icon Share
Abstract
The purpose of this case report is to describe pairing vagus nerve stimulation (VNS) with mobility training in an individual after stroke.
A 53-year-old man with left hemiparesis 14.2 months after an ischemic stroke participated in a pilot study investigating the safety and feasibility of VNS paired with upper limb rehabilitation. In addition to upper limb impairment, the participant had impaired gait and wanted to improve his mobility. A single-subject design investigation of VNS paired with self-directed mobility training was conducted. Following the conclusion of the pilot study, the participant was instructed to complete daily sessions of self-activated VNS paired with walking or stationary biking. The 10-Meter Walk Test and timed distance (6-Minute Walk Test) were assessed at 4 baseline points and at 3 to 41 months after mobility training.
The participant had stable baseline values and was classified as a household ambulator with a quad cane. After VNS-paired mobility training, statistically significant improvements were observed in all measures, with the greatest improvements at 9 months exceeding the minimal detectable change: self-selected gait speed from 0.34 (standard deviation [SD] = 0.01) to 0.60 meters/second, fast gait speed from 0.37 (SD = 0.03) to 0.79 meters/second, and 6-Minute Walk Test distance from 106.91 (SD = 6.38) to 179.83 meters. The participant reported increased confidence and balance when walking. No falls or adverse events were reported.
The participant demonstrated improved gait speed and timed distance after VNS-paired mobility training. Randomized, blinded trials are needed to determine treatment efficacy.
This is the first documented case of VNS-paired mobility training in an individual with chronic poststroke gait impairments. VNS paired with mobility training may improve poststroke gait impairments.
Background and Purpose
An estimated 30 to 60% of people with stroke have long-term upper limb and lower limb impairments that substantially affect their functional activities and participation.1 Neurorehabilitation interventions can improve motor outcomes in people with chronic poststroke impairments; however, deficits persist despite intensive rehabilitation efforts.2 Vagus nerve stimulation (VNS) paired with rehabilitation is a novel intervention for motor impairment after stroke. In clinical studies, VNS paired with task-specific upper limb rehabilitation significantly improved impairment and function in chronic stroke survivors.3–6 The Vivistim Paired VNS System (MicroTransponder Inc, Austin, TX, USA) (Vivistim System) has been approved by the US Food and Drug Administration for treating moderate to severe upper limb impairment in people with chronic ischemic stroke. To date, however, no studies have reported on paired VNS combined with lower limb or mobility training.
Paired VNS therapy is based on the principle of targeted plasticity.7,8 When combined with repeated task-specific training, VNS-driven activation of neuromodulatory input facilitates long-term plasticity and motor learning.9 Evidence from upper limb trials, preclinical studies of motor training, and a case report with tactile training suggest that improvements after pairing VNS with training are specific to the trained modality.4–6,8–10 Since poststroke lower limb impairment should, in theory, be responsive to the same plasticity mechanisms as upper limb impairment, we hypothesized that VNS paired with mobility training would result in gait improvements. We report the unique case of an individual in whom VNS was paired with self-directed mobility training following the completion of the randomized phase of the VNS-paired upper limb pilot study.3,4
Case Description
This open-label, unblinded, single-case study occurred during the multicenter, blinded, controlled pilot trial investigating the effects of VNS paired with upper limb rehabilitation (ClinicalTrials.gov NCT02243020). Trial details and results have been published previously.3,4 The case study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Minnesota’s internal review board. The participant provided written informed consent, and Health Insurance Portability and Accountability Act (HIPAA) guidelines were followed.
Participant Characteristics
The participant was a 53-year-old right-handed man, 14.2 months after stroke, who enrolled in the pilot upper limb study.3 He had a right middle cerebral artery ischemic stroke that resulted in left-sided hemiparesis. He had severe upper limb impairment based on his total upper limb Fugl-Meyer Assessment score at baseline (19) in the VNS-paired upper limb rehabilitation trial.3 His initial gait speed classified him as a household ambulator,11 and he walked independently with a small-base quad cane.
Baseline Magnetic Resonance Imaging
The participant underwent 3-T structural and diffusion brain magnetic resonance imaging (MRI) at enrollment. Additional details on the MRI acquisition, analysis, and corticospinal tract segmentation were described previously.12,13
Structural MRI demonstrated a large, mainly cortical right middle cerebral artery infarct (Fig. 1). The lesion encompassed much of the premotor and primary motor cortices and extended into the temporal and parietal cortex. Ischemic tissue volume was approximately 38 mL, and total stroke volume was approximately 283 mL.
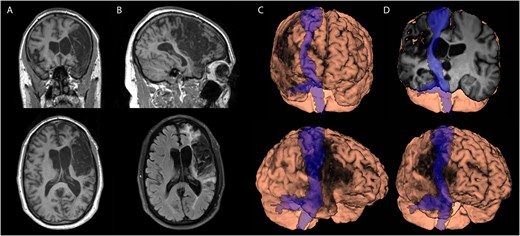
Participant’s magnetic resonance imaging scan showing a large right middle cerebral artery stroke with cortical and subcortical involvement. The stroke overlapped with the corticospinal tract and motor cortex. There was adjacent lateral ventricle expansion but little evidence of widespread tissue atrophy or small vessel disease. (A) Longitudinal relaxation time (T1-weighted image) scan, coronal (top), and axial (bottom) views. (B) T1-weighted scan, sagittal (top), and axial fluid-attenuated inversion recovery (bottom) views. (C) and (D) Three-dimensional brain renderings in various planes showing the overlaid corticospinal tract in blue.
Upper Limb Therapy
As part of the upper limb trial, the participant was implanted with a VNS device (Vivistim System).3 He was randomly assigned to the VNS group and received task-specific upper limb rehabilitation paired with 0.8-mA VNS over 19 in-clinic sessions. No lower limb exercises were included in the upper limb trial. The participant, therapist, and assessor were blinded to group assignment.
In accordance with the upper limb study protocol, after the in-clinic therapy, he began home-based upper limb therapy. During this therapy, he self-activated VNS with a magnet swipe over the implanted device, delivering a 0.5-second pulse every 10 seconds during a 30-minute task practice session including arm and hand functional activities. He did not do any mobility therapy paired with VNS during this randomized period of the upper limb VNS trial; however, baseline gait measures were collected during this time for this case study. At the end of the randomized phase, the participant’s group (VNS) was revealed. In accordance with the protocol, he was instructed to continue with home-based upper limb tasks paired with VNS and was monitored over a period of 4 years. His individual upper limb results are shown in the Supplementary Table.
Self-Directed Mobility Training Paired With VNS
The participant began the open-label, unblinded, single-case study of VNS paired with mobility training after the randomized phase of the upper limb trial (Suppl. Figure). He was selected for the case study because he was highly motivated to improve his gait and was willing to engage in unsupervised mobility training paired with VNS. He was instructed to activate his VNS device by swiping the magnet 1 or 2 times per day (30 minutes each) and perform home or community-based mobility training such as walking or using a stationary bike. In accordance with the upper limb protocol, he also was instructed to continue with the upper limb VNS-paired tasks at least once per day.
Gait Assessments
Gait assessments were performed to determine whether there was any carryover effect on gait measures during the upper limb clinical trial, as well as to establish a robust baseline prior to initiating the VNS-mobility training. Thus, gait speed and timed distance were measured after the upper limb tests at every assessment visit during the pilot trial. Gait speed was measured with the 10-meter walk test at both self-selected and fast speeds.14,15 Timed distance was measured with the 6-Minute Walk Test using a 12-meter walkway.11 Four baseline assessments took place at 18, 15, 12, and 0 weeks before the start of VNS-paired mobility training (Suppl. Figure 1). Posttest assessments occurred between 3 and 41 months thereafter. Assistive devices were allowed during these assessments, according to the participant’s choice, and were reported when used.
Data Analysis
The mean (SD) baseline values were calculated by averaging data from the 4 baseline assessments. The ±2SD-band method was used; with this method, at least 2 consecutive data points in the intervention phase above the 2SD band indicate statistical improvement.16,17 Results were also compared with minimal detectable change values for chronic stroke to ensure changes were not due to measurement error. The minimal detectable change values were 0.18 meters/second (self-selected speed),18 0.13 meters/second (fast speed),18 and 34.4 meters (6-Minute Walk Test).19 Even though the minimal clinically important difference (MCID) values for gait speed have not been established for chronic stroke, we chose to also compare changes with the MCID because many clinicians still use the subacute MCID value. The MCID values used were 0.16 meters/second (self-selected speed, subacute stroke)20 and 34.4 meters (6-Minute Walk Test, chronic stroke).21
Outcomes
Self-Directed Mobility Training Paired With VNS
The participant reported activating the VNS device and practicing mobility activities primarily while walking with his small-base quad cane (outside or at the mall with friends) or stationary biking for 30 minutes 1 to 3 times per day during the first year (from approximately the fourth baseline assessment at 0 weeks to the 9-month assessment). After the first year, his intensity and frequency of training decreased because of personal reasons unrelated to health concerns or the study. The VNS device was activated only during activity.
Gait Speed
The participant’s baseline gait speeds are shown in the Table. Significant improvements in both gait speed measures were observed from 6 to 33 months (Fig. 2). The greatest improvements were observed at 9 months, when self-selected and fast speeds increased by 0.26 and 0.42 meters/second, respectively, which exceeded the minimal detectable change (Table).18 At 41 months, both speeds decreased (self-selected: 0.33 meter/second; fast: 0.35 meter/second) and were not significantly different from baseline speeds. Self-selected speed at 9 months also exceeded the MCID (Table).20
Gait Speed and Timed Distance in a Single Participant After Mobility Training Paired With Vagus Nerve Stimulationa
| Visit . | Self-Selected Speed (m/s) . | Fast Speed (m/s) . | Timed Distance (m) . | |||
|---|---|---|---|---|---|---|
| Absolute . | Change . | Absolute . | Change . | Absolute . | Change . | |
| B1 | 0.34 | 0.34 | 99.06 | |||
| B2 | 0.35 | 0.42 | 105.46 | |||
| B3 | 0.33 | 0.35 | 108.81 | |||
| B4 | 0.33 | 0.36 | 114.30 | |||
| Mean (SD) for B1–B4 | 0.34 (0.01) | 0.37 (0.03) | 106.91 (6.38) | |||
| 3 mo | 0.45 | 0.11 | 0.33 | −0.03 | 118.11 | 11.20 |
| 6 mo | 0.43 | 0.09 | 0.64 | 0.27b | 143.26 | 36.35b,c |
| 9 mo | 0.60 | 0.26b,c | 0.79 | 0.42b | 179.83 | 72.92b,c |
| 21 mo | 0.44 | 0.10 | 0.54 | 0.17b | 132.59 | 25.68 |
| 33 mo | 0.49 | 0.15 | 0.52 | 0.15b | 155.00 | 48.09b,c |
| 41 mo | 0.33 | −0.01 | 0.35 | −0.02 | 130.00 | 23.09 |
| Visit . | Self-Selected Speed (m/s) . | Fast Speed (m/s) . | Timed Distance (m) . | |||
|---|---|---|---|---|---|---|
| Absolute . | Change . | Absolute . | Change . | Absolute . | Change . | |
| B1 | 0.34 | 0.34 | 99.06 | |||
| B2 | 0.35 | 0.42 | 105.46 | |||
| B3 | 0.33 | 0.35 | 108.81 | |||
| B4 | 0.33 | 0.36 | 114.30 | |||
| Mean (SD) for B1–B4 | 0.34 (0.01) | 0.37 (0.03) | 106.91 (6.38) | |||
| 3 mo | 0.45 | 0.11 | 0.33 | −0.03 | 118.11 | 11.20 |
| 6 mo | 0.43 | 0.09 | 0.64 | 0.27b | 143.26 | 36.35b,c |
| 9 mo | 0.60 | 0.26b,c | 0.79 | 0.42b | 179.83 | 72.92b,c |
| 21 mo | 0.44 | 0.10 | 0.54 | 0.17b | 132.59 | 25.68 |
| 33 mo | 0.49 | 0.15 | 0.52 | 0.15b | 155.00 | 48.09b,c |
| 41 mo | 0.33 | −0.01 | 0.35 | −0.02 | 130.00 | 23.09 |
B1–B4 = baseline assessments at 18, 15, 12, and 0 wk, respectively, before the start of vagus nerve stimulation-paired mobility training.
Gait Speed and Timed Distance in a Single Participant After Mobility Training Paired With Vagus Nerve Stimulationa
| Visit . | Self-Selected Speed (m/s) . | Fast Speed (m/s) . | Timed Distance (m) . | |||
|---|---|---|---|---|---|---|
| Absolute . | Change . | Absolute . | Change . | Absolute . | Change . | |
| B1 | 0.34 | 0.34 | 99.06 | |||
| B2 | 0.35 | 0.42 | 105.46 | |||
| B3 | 0.33 | 0.35 | 108.81 | |||
| B4 | 0.33 | 0.36 | 114.30 | |||
| Mean (SD) for B1–B4 | 0.34 (0.01) | 0.37 (0.03) | 106.91 (6.38) | |||
| 3 mo | 0.45 | 0.11 | 0.33 | −0.03 | 118.11 | 11.20 |
| 6 mo | 0.43 | 0.09 | 0.64 | 0.27b | 143.26 | 36.35b,c |
| 9 mo | 0.60 | 0.26b,c | 0.79 | 0.42b | 179.83 | 72.92b,c |
| 21 mo | 0.44 | 0.10 | 0.54 | 0.17b | 132.59 | 25.68 |
| 33 mo | 0.49 | 0.15 | 0.52 | 0.15b | 155.00 | 48.09b,c |
| 41 mo | 0.33 | −0.01 | 0.35 | −0.02 | 130.00 | 23.09 |
| Visit . | Self-Selected Speed (m/s) . | Fast Speed (m/s) . | Timed Distance (m) . | |||
|---|---|---|---|---|---|---|
| Absolute . | Change . | Absolute . | Change . | Absolute . | Change . | |
| B1 | 0.34 | 0.34 | 99.06 | |||
| B2 | 0.35 | 0.42 | 105.46 | |||
| B3 | 0.33 | 0.35 | 108.81 | |||
| B4 | 0.33 | 0.36 | 114.30 | |||
| Mean (SD) for B1–B4 | 0.34 (0.01) | 0.37 (0.03) | 106.91 (6.38) | |||
| 3 mo | 0.45 | 0.11 | 0.33 | −0.03 | 118.11 | 11.20 |
| 6 mo | 0.43 | 0.09 | 0.64 | 0.27b | 143.26 | 36.35b,c |
| 9 mo | 0.60 | 0.26b,c | 0.79 | 0.42b | 179.83 | 72.92b,c |
| 21 mo | 0.44 | 0.10 | 0.54 | 0.17b | 132.59 | 25.68 |
| 33 mo | 0.49 | 0.15 | 0.52 | 0.15b | 155.00 | 48.09b,c |
| 41 mo | 0.33 | −0.01 | 0.35 | −0.02 | 130.00 | 23.09 |
B1–B4 = baseline assessments at 18, 15, 12, and 0 wk, respectively, before the start of vagus nerve stimulation-paired mobility training.
![Participant’s gait speed (A) and (B) and timed distance (C) over time. The x-axis represents assessment time points. Baseline assessments were completed at B1–B4 (18, 15, 12, and 0 wk, respectively, before the start of vagus nerve stimulation [VNS]-paired mobility training). Mobility training paired with VNS was initiated the day after B4 (black square) and continued for 41 mo (gray shaded area). Posttest assessments occurred between 3 and 41 mo. The horizontal dashed lines represent the mean ± 2 SDs of baseline values, with values above the top line indicating a statistical improvement. The participant self-selected the use of his assistive device (see text for details). *Greater than minimal detectable change. ^Assessments with no assistive device. 6MWT = 6-Minute Walk Test.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ptj/103/12/10.1093_ptj_pzad097/1/m_pzad097f2.jpeg?Expires=1750201890&Signature=ITLsk0rSORxVCOY1KRsvhRptKZD1HcRf1MwLceFTA-dpq5jE4901Tg5Uj2tH8av7Owp6Qbs1EFvOCI5DWE0v8hb5v~uMAD67JDtG8DHK3OemfRg7NTjKkjDH5JqC1D7Idp1EzaSqZglHqYw2SZDwUhtEqHhXNo8ju-i10eW2tLkKgBtbWSl04th9wVGrHmDFD1MejRUWC5sMP1GzWV06PY3C2pHgmRFmPqDkVMjROkxxtz3PNm5BPfhJWjl8rjbuK5T6zoY0L2f5wFlVGDcySd5t8RiLpBug4DuWbzHrcan4XBXQoo6CObvQRStBCNb-kEj-P2IhYNPtrX90AF9jMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Participant’s gait speed (A) and (B) and timed distance (C) over time. The x-axis represents assessment time points. Baseline assessments were completed at B1–B4 (18, 15, 12, and 0 wk, respectively, before the start of vagus nerve stimulation [VNS]-paired mobility training). Mobility training paired with VNS was initiated the day after B4 (black square) and continued for 41 mo (gray shaded area). Posttest assessments occurred between 3 and 41 mo. The horizontal dashed lines represent the mean ± 2 SDs of baseline values, with values above the top line indicating a statistical improvement. The participant self-selected the use of his assistive device (see text for details). *Greater than minimal detectable change. ^Assessments with no assistive device. 6MWT = 6-Minute Walk Test.
Timed Distance
The participant’s mean baseline timed distance was 106.91 (SD = 6.38) meters (Table). After he paired VNS with mobility training, his timed distance significantly improved from 6 to 41 months (Fig. 2). The peak improvement in 6-Minute Walk Test occurred at 9 months, when timed distance increased by 72.92 meters, which exceeded the minimal detectable change and MCID (Table).19,21
Participant Subjective Report
The participant reported his gait becoming progressively better, such that he no longer used a small-base quad cane at home. He chose to use the small-base quad cane at all assessments except between 3 and 33 months, when he felt he no longer needed it. The absence of an assistive device on those visits did not decrease his gait performance. The participant also reported improved confidence and balance with walking. He reported no falls or adverse events related to VNS-paired mobility training.
Discussion
VNS paired with mobility training significantly improved gait speed and timed distance in an individual with chronic poststroke mobility impairments. According to his self-selected gait speed, he progressed from a “household ambulator” at baseline to a “limited community ambulator” at 9 and 33 months,11 representing a notable difference in participation. However, mobility measures declined for all outcomes after 9 months, and by 41 months his gait speed had returned to baseline. Though causation cannot be determined from this case report, it is notable that a decrease in speed corresponded to the participant’s report that he had decreased his mobility practice after 1 year. Nonetheless, significant improvements in walking endurance lasted until the final (41 month) assessment.
The results of the multiple baseline tests conducted over the approximately 4-month period during which the individual was participating in upper limb VNS-paired therapy indicated that his gait performance was stable, and that no generalization to gait outcomes occurred during the upper limb therapy period. Gait improvements were observed after the participant added mobility training paired with VNS to his home exercise routine, reinforcing the hypothesis that improvements in outcomes are specific to rehabilitation tasks paired with VNS.9
Motivation and patient engagement during goal-oriented training are important factors that may contribute to improving motor skills. Importantly, gains observed in this report resulted from self-directed mobility training, suggesting a high level of motivation by the participant. This participant showed good adherence to therapy and continued to show incremental upper limb and lower limb long-term gains. More specifically, his upper limb score changes were at the high end of the range for participants in the pilot trial3,4 (ie, a 27-point change in the upper limb Fugl-Meyer Assessment), and in this study, he showed significant gains in mobility. Although motivation certainly encouraged his high adherence, it is unclear whether VNS could have modulated mood states to enhance motivation. It is unknown how robust the effects would be for someone with less adherence to the intervention or whether VNS contributed to enhanced motivational states. Future studies should explore the interaction between motivated states, mood, and VNS.
VNS paired with rehabilitation for chronic stroke differs from other neuromodulation interventions, both in how the stimulation is delivered and in its mechanism of action. Direct stimulation of the left vagus nerve leads to bilateral activation of the nucleus of the solitary tract and its projections to other nuclei involved in the ascending neuromodulatory system, resulting in the release of acetylcholine, norepinephrine, and serotonin throughout the cortex.9 Activation of these neuromodulatory systems, after repeatedly pairing VNS with task-specific motor training, drives long-lasting targeted plasticity (ie, cortical reorganization and synaptic plasticity in brain areas related to the trained task),8,9 thereby improving the brain’s ability to relearn impaired motor tasks. Therefore, VNS is based on enhancing plasticity across neurons engaged in a task and this neuronal network may be distributed across wide brain areas, including the opposite hemisphere. This bottom-up approach can modulate wide regions of the cortical network and, thus, VNS does not require localized stimulation to a specific cortical area in the brain, unlike the focal activation of specific cortical areas required for other neuromodulation methods such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation. This is particularly relevant for mobility training given that the cortical representation of the lower limb is a difficult region to directly stimulate.22
The stimulation settings for mobility training were the same as those used in the pilot study for upper limb home therapy (ie, cyclical stimulation of a 0.5-second pulse every 10 seconds for 30 minutes).3,4 Even though the cyclical VNS paradigm has less precise timing than the VNS pairing utilized in the in-clinic phase of the upper limb trial (on-demand stimulation triggered by the therapist), improvements in gait measures occurred over several months of VNS-paired mobility training. Because walking or biking continually engages several muscle groups across temporal time frames of minutes, it is possible that cyclical VNS may be sufficient for improvement if VNS overlaps with movement. Further work needs to determine the optimal temporal relationships of VNS to target movement to enhance motor recovery.
We cannot unequivocally conclude that the gait benefits were due to paired VNS versus the walking practice alone. The participant reported that he was walking and biking regularly before the VNS implant and that he increased his practice when he began pairing it with VNS. His improvements were comparable to reported values for average increase in gait speed and timed distance achieved with other high-intensity, supervised interventions for chronic poststroke mobility impairments.23,24
Structural MRI revealed a large ischemic stroke that resulted in both cortical and subcortical lesions with substantial corticospinal tract involvement. The participant had severe upper limb and mobility impairments but made significant gains after paired VNS (Suppl. Table and Fig. 1). It is possible that the participant’s severe stroke precluded gait improvement with training alone (there were no gains before VNS implantation) or during upper limb training paired with VNS (when gait training was not paired with VNS). Future studies should formally assess whether paired VNS can induce large-scale synaptic reorganization and subsequent functional improvements in severe stroke that may be unattainable with training alone.
Limitations
This case report has limitations. The participant did not practice supervised mobility training, nor was there a defined mobility training protocol beyond instructing the participant to walk and bike with VNS pairing. Nonetheless, the gait improvements observed support the participant’s report of regular self-directed mobility practice paired with VNS. These results represent outcomes of a single case and thus cannot be generalized to all individuals with poststroke mobility impairments. We also cannot rule out an enhanced benefit due to the participant’s ongoing home-based VNS-paired upper limb practice occurring in parallel with the regular VNS-paired walking practice. Additional studies will be needed to verify whether similar benefits are observed in other persons.
Conclusions
This is the first report demonstrating results after VNS combined with mobility training in an individual with chronic poststroke impairments. Paired VNS therapy may have implications for physical therapist practice. Our results suggest that VNS paired with mobility training may improve gait speed and endurance after stroke and provide preliminary data to support a randomized and blinded trial.
Author Contributions
Teresa J. Kimberley (Conceptualization [lead], Formal analysis [equal], Investigation [lead], Project administration [equal], Supervision [lead], Writing—original draft [lead], Writing—review & editing [equal]), Cecilia N. Prudente (Conceptualization [equal], Formal analysis [equal], Investigation [equal], Project administration [equal], Writing—original draft [equal], Writing—review & editing [equal]), Navzer D. Engineer (Conceptualization [equal], Formal analysis [equal], Methodology [equal], Writing—review & editing [equal]), David A. Dickie (Formal analysis [equal], Writing—review & editing [equal]), Teresa A. Bisson (Conceptualization [equal], Investigation [equal], Writing—review & editing [equal]), and Ann Van de Winckel (Investigation [equal], Project administration [equal], Writing—review & editing [equal])
Acknowledgments
The authors would like to thank the study participant, site personnel (Danielle K. Kline, PT, DPT, NCS; Kate Frost, PhD), the MicroTransponder team (David Pierce, MSEE; Brent Tarver, BSEE; Reema Adham Hinds, PhD; Natasha Green, ELS), and Jennifer Holmes, ELS (medical writing support) for their contributions.
Funding
No funding was received for this work.
Ethics Approval
The case study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Minnesota’s internal review board.
Data Availability
Data will be available upon reasonable request to the corresponding author.
Disclosures
C.N.P. and N.D.E. are employees of MicroTransponder Inc, in which they hold stock or stock options. D.A.D. was paid by MicroTransponder Inc to provide brain analysis and consultation.




Comments