-
PDF
- Split View
-
Views
-
Cite
Cite
Zhipeng Fan, Hongfei Song, Rongli Yuan, Yangzhi Peng, Yong Jiang, Genetic predisposition to female infertility in relation to epithelial ovarian and endometrial cancers, Postgraduate Medical Journal, Volume 99, Issue 1168, February 2023, Pages 63–68, https://doi.org/10.1093/postmj/qgad009
Close - Share Icon Share
Abstract
The associations between female infertility and epithelial ovarian cancer (EOC) or endometrial cancer (EC) have been reported in observational studies, but its causal relationship remains unknown. We intended to assess the causal effect of female infertility on EOCs and ECs using a two-sample Mendelian Randomization (MR) approach.
Large pooled genome-wide association study (GWAS) datasets for female infertility (6481 cases and 68 969 controls), EOC (25 509 cases and 40 941 controls), and EC (12 906 cases and 108 979 controls) were derived from public GWAS databases and published studies. The Inverse Variance Weighted method, Weighted Median method, MR-Egger regression, and MR-Pleiotropy Residual Sum and Outlier test were adopted for MR analyses.
Our results suggested that genetically predicted infertility was positively associated with the risk of EOC (OR = 1.117, 95% CI = 1.003–1.245, P = .045), but did not find a causal relationship between infertility and EC (OR = 1.081, 95% CI = 0.954–1.224, P = .223). As to the reverse direction, our study did not obtain evidence from genetics that EOCs (OR = 0.974, 95% CI = 0.825–1.150, P = .755) and ECs (OR = 1.039, 95% CI = 0.917–1.177, P = .548) were associated with an increased risk of infertility.
This large MR analysis supported a causal association between female infertility and increased risk of EOCs, but did not find a causal relationship between infertility and ECs.
What is already known on this topic:
Many studies have revealed that some people are more susceptible to undergoing cancers than others. Understanding the risk drivers that confer cancer risk will provide vital information to help public health officials make decisions that will benefit these at-risk populations.
Observational studies have shown that female infertility is a high risk factor for ovarian cancer and endometrial cancer (EC), but the causal relationship has not been established.
What this study adds:
Our two-sample Mendelian Randomization study supported a causal association between female infertility and increased risk of epithelial ovarian cancers (EOCs), but did not find a causal relationship between infertility and ECs.
No clear evidence was found for a potential causal effect of EOCs and ECs on female infertility.
How this study might affect research, practice, or policy
Our findings suggested that patients with infertility were at higher risk of developing EOC compared to the general population. Increased knowledge and regular surveillance of EOC in infertile women may help to identify early EOC.
Introduction
Ovarian cancer (OC) is one of the deadliest gynecological malignancies in high-income countries [1]. Epithelial ovarian cancer (EOC) has a higher incidence and mortality rate than other OC [2]. According to the NCCN Clinical Practice Guidelines in Oncology (Ovarian Cancer, Version 2.2020), EOC was the fifth most common cause of cancer deaths in women in the USA [3], accounting for >95% of ovarian malignancies [4]. There are two main etiological mechanisms for the development of OC: (i) the theory of sustained ovulation, which refers to chronic cyclic damage and repair of the ovarian epithelium associated with the development of OC; and (ii) the theory of hypergonadotropic hormone, which refers to the increase of the estrogen level in the body due to hypergonadotropic hormone, which stimulates the proliferation and malignant transformation of ovarian epithelium. EOC is a heterogeneous disease that is further classified as benign, junctional, and malignant. A primary challenge in the treatment of EOC is that most sufferers have advanced disease at the time of initial diagnosis.
Endometrial cancer (EC) is the most widespread gynecological cancer in high-income countries and its incidence is on the rise globally [5]. Worldwide, 417 367 women were diagnosed with EC in 2020, accounting for 4.5% of all malignancies in women [6]. EC is predominant in postmenopausal women, with a trend toward younger women in recent years. Common risk factors for EC include increasing age, obesity, use of unagonized estrogens, polycystic ovary syndrome, and hereditary cancer syndromes (Lynch syndrome, Cowden syndrome). The main symptom of EC is abnormal uterine bleeding (AUB). However, it is not specific and only ~7.9% of postmenopausal AUB women and ~1.2% of premenopausal AUB women suffer from EC [7].
The link between gynecologic cancers and female infertility has been a controversial and challenging topic. Female infertility refers to infertility caused primarily by female factors encompassing: ovulatory disturbances; diminished ovarian reserve; anatomical, endocrine, genetic, functional, or immunological abnormalities of the reproductive system; chronic illness; and sexual conditions incompatible with coitus [8]. Infertility not only causes greater psychological and social stress to couples, but also has an impact on the stability of society [9]. Several studies have shown that infertility in women is a hazard factor for OC [10] and EC [11]. Rodriguez et al. [12] examined the relationship between self-reported infertility and death from OC among 676 526 female participants in the Cancer Prevention Study II (CPS-II) and found that infertility itself, without concomitant exposure to fertility drugs, may increase the risk of fatal OC among nulligravid women. Brinton et al. [13] observed a significantly higher incidence of OC in patients with infertility than in the general female population in 45 OC cases, with a higher risk in patients with primary infertility than in those with secondary infertility and a particularly high risk in those who never subsequently conceived. As for EC, Yang et al. [14] demonstrated that nulliparity and infertility may independently contribute to EC risk with data from 2 cohort studies and 12 case–control studies comprising 8153 cases and 11 713 controls. However, whether infertility is genetically causally linked to EOC and EC remains largely unclear.
Mendelian Randomization (MR) is an emerging approach to epidemiological research that uses genetic variation [in this case single nucleotide polymorphisms (SNPs)] as instrumental variables for causal inference [15]. Genetic variation is not affected by traditional confounding factors and could overcome the drawbacks of traditional observational epidemiological studies: unknown confounding factors and reverse causality. Here, we applied two-sample MR to assess the potential causal effect of female infertility on EOC and EC.
Materials and methods
Data source
Large genome-wide association study (GWAS) datasets were used in this study: female infertility, EOC, and EC. An overview of the study design is presented in Fig. 1. Patient consent for publication and ethics approval were not required.

Exposure sources
FinnGen Research [16] has become a global research project aimed at improving human health through genetic research and ultimately identifying new therapeutic targets and diagnostics for various diseases. It has brought together Finnish universities, hospitals and hospital districts, THL, blood services, biobanks, FINBB, and international pharmaceutical companies as well as hundreds of thousands of Finns. It has not only provided insights into new medicine and treatments, but also established a world-class resource for future research. We extracted a large summary-level GWAS dataset related to female infertility from FinnGen Biobank Analysis (Round 5), involving 6481 cases and 68 969 controls. In addition, the aforementioned GWAS datasets were all derived from the European populations (available from http://r5.finngen.fi/pheno/N14_FEMALEINFERT).
Outcome sources
EOC Phelan et al. [17] designed a custom Illumina array called “OncoArray” to identify new cancer susceptibility loci. OncoArray included ~533 000 variants (260 660 of which form the GWAS backbone) and had been used to genotype >500 000 samples, including the Ovarian Cancer Association Consortium (OCAC) EOC case–control study and the BRCA1 and BRCA2 mutation carriers of the Consortium of Investigators of BRCA1/2 Modifiers (CIMBA). We performed genetic association analysis using genotype data from OCAC for 25 509 population-based EOC cases and 40 941 controls. All participating studies were approved by the relevant research ethics committees, and all participants provided written informed consent.
EC O’Mara et al. [18] performed a meta-GWAS including 12 906 EC cases and 108 979 nationally matched controls of European ancestry from 17 studies identified through the Endometrial Cancer Association Consortium, the Endometrial Cancer Epidemiology Consortium, and the UK Biobank and reported 9 additional genome-wide significant EC genetic risk regions. This study doubled the number of known EC risk loci and revealed candidate causative genes for future studies.
Statistical analysis
The MR approach is based on the following assumptions: genetic variation is associated with exposure factors; genetic variation must be independent of any confounding factors associated with the outcome; and genetic variation must be through exposure factors and not otherwise. Therefore, we selected SNPs that were strongly associated with female infertility and without linkage disequilibrium (P < 5e−6, r2 < 0.01), and which were not linked to the outcome. We harmonized all SNPs to ensure that the effect estimates corresponded to the same alleles (see online supplementary material: Table S1). We used the F-statistic to gauge the strength of instrumental variables. Weak instrumental variables were defined as an F-statistic < 10 and all weak instrumental variables were excluded [19]. Finally, echo SNPs that would introduce ambiguity in exposing the identity of effect alleles in GWAS were also removed. After a series of rigorous screenings, the remaining SNPs were considered to be eligible for instrumental variables. We used Inverse Variance Weighting (IVW), MR-Egger, and Weighted Median to assess the causal relationship between female infertility and EOC or EC (see online supplementary material: Table S2).
IVW was used as the main outcome. It merged the Wald ratio estimates for each SNP into one causal estimate for each risk factor, applying a random-effects model if heterogeneity existed [20]. In addition, we further adopted MR-Egger and Weighted Median methods to improve the robustness of the results. MR-Egger was used to detect potential pleiotropy and corrected for the resulting introduced bias [21]. The Weighted Median method required >50% of the weight in the meta-analysis to be derived from valid SNPs [22].
Sensitivity analysis was essential in MR studies to detect underlying pleiotropy, by which the heterogeneity of MR estimates could be seriously violated. We used heterogeneity markers from the IVW method (Cochran Q-derived P < 0.05) to indicate potential horizontal pleiotropy. The intercept derived from the MR-Egger regression was an indicator of directional pleiotropy (P < 0.05 was regarded as the existence of directional pleiotropy) [23]. The MR-Pleiotropy Residual Sum and Outlier approach (MR-PRESSO) was also performed to evaluate and correct for horizontal pleiotropy [24].
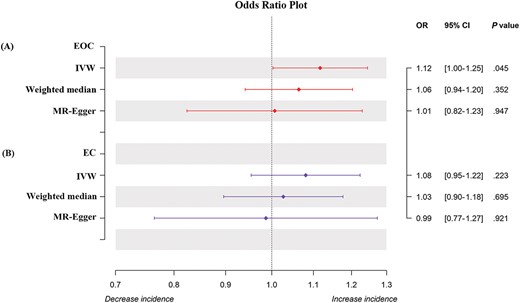
Forest plot of MR analysis between EOC and EC and female infertility.
The results were presented as ORs and their 95% confidence intervals (CIs). Statistical significance was considered to exist when P < 0.05. All analyses were performed by R statistical software version 4.2.1 with the R packages “TwoSampleMR” and “MRPRESSO.”
Results
Causal effect from female infertility to EOC and EC
After adopting MR-PRESSO (1 outlier removed), using 7 SNPs associated with infertility, we detected a positive association between female infertility and EOC (OR = 1.117, 95% CI = 1.003–1.245, P = .045), although the Weighted Median (OR = 1.064, 95% CI = 0.941–1.203, P = .352) and the MR-Egger methods (OR = 1.007, 95% CI = 0.824–1.230, P = .947) did not support this positive result (Fig. 2). We preferred to use the IVW estimates, because our results were not heterogeneous (MR-Egger, Q_P-val = .317; IVW, Q_P-val = .272) and there was no evidence of significant interception (intercept = 0.021; Standard Error (SE) = 0.018, P = .287) (see online supplementary material: Table S3). This suggested that our results were robust and credible. In conclusion, our results suggested that genetically predicted infertility was positively associated with the risk of EOC.
For EC, the IVW approach showed that genetically predicted increased infertility was not significantly associated with EC risk (OR = 1.081, 95% CI = 0.954–1.224, P = .223). This result was also supported by the Weighted Median (OR = 1.027, 95% CI = 0.896–1.177, P = .695) and the MR-Egger methods (OR = 0.987, 95% CI = 0.765–1.273, P = .921). No heterogeneity (MR-Egger, Q_P-val = .105; IVW, Q_P-val = .113) or significant interceptions (intercept = 0.016, SE = 0.020, P = .443) were observed. This indicated that the results were robust and convincing.
See the online supplementary material (Fig. S1) for an illustration of the MR regression slope for each of the SNPs across all SNPs. In addition, the funnel plots were symmetric, indicating no multidirectionality (see online supplementary material: Fig. S2).
Causal effect from EOC and EC on female infertility
After using 10 SNPs associated with EOC after MR-PRESSO, and applying the IVW method, we found no potential contribution of EOC to the increased risk of female infertility (OR = 0.974, 95% CI = 0.825–1.150, P = .755). Similarly, no clear evidence was found for a potential causal effect of EC on female infertility (OR = 1.039, 95% CI = 0.917–1.177, P = .548) (see online supplementary material: Table S4).
Discussion
Many studies have revealed that some people are more susceptible to undergoing cancer than others. Understanding the risk drivers that confer cancer risk will provide vital information to help public health officials make decisions that will benefit these at-risk populations. In this two-sample MR study, we found that genetically predicted female infertility was positively associated with EOC, but not with EC. Reverse MR analysis did not observe evidence of an association between EOC or EC and infertility in women.
The causes of infertility are not only medical but also psychosocial. Infertility causes sufferers to feel anxious or depressed, have low self-esteem, or experience other negative emotions that can seriously affect their quality of life. There are increased risks of all-cause and cancer-related deaths in infertile women [25]. Scientific management of infertile patients is vital to avoid adverse events. Conflicting results from observational studies of infertility and OC risk have been reported so far. Lerner-Geva et al. [26] found no association between infertility and increased risk of OC over 30 years of follow-up. However, 9 prospective cohort studies involving 10 383 OC cases and 62 883 subjects found that female infertility was associated with an increased risk of OC (summary RR = 1.51, 95% CI = 1.35–1.69) with low heterogeneity [27]. This is consistent with our results. For patients with EC, it was found that infertility placed women at risk of developing EC at a younger age [28]. Our MR analysis showed no evidence to support a clear causal relationship between infertility and EC, implying that the observed association may be due to confounding factors, such as obesity [29–31].
As to the reverse direction, EOC and EC are typically diseases of postmenopausal age. Nevertheless, ~10% of EOC [32] and 5%–29% of EC cases are detected in patients aged 45 years or younger. Maintaining the fertility of these young women has been a challenging issue for doctors. Observational research studies on infertility due to EOC are sparse, with most studies focusing on infertility caused by the anticancer drugs, chemotherapy, and radiotherapy involved. Our work supported the lack of a potential genetic causal link between EOC and infertility. As for EC, Leone Roberti Maggiore et al. [33] found that among 41 EC patients who achieved total surgical remission, only 14 (34.14%) achieved pregnancy (6 spontaneous pregnancies and 8 pregnancies by in vitro fertilization). Reasons may be related to the use of anticancer drugs, chemotherapy, and radiotherapy. Studies have found that anticancer treatments can cause permanent damage to the uterus and impair its ability to allow and maintain a healthy pregnancy [34]. In particular, exposure to radiation therapy at a young age had greater radiation consequences for the uterus and led to a lower chance of pregnancies in the future [35]. Our study supported the lack of a potential causal genetic link between EC and infertility.
Our study had several advantages. First of all, MR analysis can mimic a randomized controlled trial in an observational context. Randomized controlled trials are broadly recognized in the study of causality, but are quite costly and often impractical. However, MR studies can successfully prevent the confounding bias of randomly assigned SNPs at the time of conception. Also, MR can avoid reverse causality effects. Secondly, our findings may influence health care policy in women with infertility and EOC or EC. Given the serious consequences of infertility, EOC, and EC, uncovering the causal relationship between infertility and EOC or EC could influence public health policies regarding early prevention and timely intervention. Our findings imply that enhanced screening for EOC in genetically predicted infertility is strongly warranted, whereas it may not be useful for EC.
However, there were some limitations. Firstly, the majority of all GWAS datasets in this study are from European populations. It remains to be validated whether the findings we describe are applicable to other populations. Secondly, we should be aware of the diversity of cancer sufferers and a wider study including EOC and EC subgroups could be considered in the future.
Conclusion
This is the first MR study to explore the causality of female infertility on EOC and EC. Our two-sample MR study supported a causal association between female infertility and increased risk of EOC, but did not find a causal relationship between infertility and EC.
Acknowledgements
We acknowledge the participants and investigators of the FinnGen study. We also thank Phelan et al. [17] and O’Mara et al. [18] for conducting the GWASs and making the summary statistics publicly available.
Conflict of interest statement: None declared.
Funding
This research was funded by the National Natural Science Foundation of China (no. 82104655), “Xinglin Scholar” Discipline Talent Research Promotion Program of Chengdu University of Traditional Chinese Medicine (BSH2020015), Inheritance Innovation Development Research Funds of Basic Medical College of Chengdu University of Traditional Chinese Medicine (CCCXYB202206), and Natural Science Foundation of Sichuan Province (2022NSFSC0638).
Data availability statement
All data used in the current study are from publicly available GWAS summary datasets. The data underlying this article are available in the article and in its online supplementary material.
Author contributions
Y.J., Y.P., and Z.F. contributed to the conception, study design, and data interpretation and wrote the manuscript. Z.F. and H.S. contributed to data collection and analysis. R.Y., Z.F., and H.S. contributed to drafting the text and preparing figures.
References
Author notes
Zhipeng Fan and Hongfei Song contributed equally and fully in this study.



