-
PDF
- Split View
-
Views
-
Cite
Cite
Ritu Singh, CRISPRing EDR1: Harmonizing grapevine defense and growth dynamics, Plant Physiology, Volume 195, Issue 3, July 2024, Pages 1762–1764, https://doi.org/10.1093/plphys/kiae181
Close - Share Icon Share
Powdery mildew, caused by the fungus Erysiphe necator, remains a significant concern for grape yields worldwide, persisting for over a century and posing ongoing challenges for growers. Effective management strategies demand integrated approaches, incorporating cultural practices, strategic pesticide application, and the development of resistant varieties (van Esse et al. 2020). Traditionally, efforts to breed resistant varieties have mainly focused on identifying and integrating specific resistance genes (R genes) (Dangl and Jones 2001). However, reliance solely on single R genes has proven ineffective over time because pathogens can evolve to overcome them (Dangl et al. 2013) and incorporating multiple R genes is time-consuming (Xiao et al. 2019).
Alternatively, researchers have explored exploiting recessive mutations in host susceptibility genes (S genes) to confer resistance (van Schie and Takken 2014). Unlike R genes, resistance mediated by S genes tends to be more durable. Moreover, disrupting a single S gene is simpler and faster than integrating multiple R genes. Enhanced disease resistance 1 (EDR1) is one such S gene identified in an Arabidopsis mutant, which showed increased disease resistance to Golovinomyces cichoracearum, a causal agent of powdery mildew (Frye and Innes 1998). EDR1 is highly conserved across plant species and negatively regulates plant defense responses (Shen et al. 2011; Ma et al. 2021).
In this issue of Plant Physiology, Yu et al. (2024) identified an ortholog of the Arabidopsis EDR1 gene in grapevine (VviEDR1) through multiple sequence alignment and phylogenetic analysis. Expression analysis in susceptible Thompson Seedless grapevine leaves suggests continual downregulation of VviEDR1 after E. necator inoculation, indicating the role of VviEDR1 in grapevine powdery mildew infection. To delve deeper, the authors generated VviEDR1 mutants through CRISPR-Cas9. Most of the obtained VviEDR1 mutant plants exhibited growth defects and did not survive. The only edited regenerated plants that showed wild-type growth levels were VviEDR1-chimeric edited lines, with more than 2 different mutations in a single plant.
To understand the role of VviEDR1 in grapevine powdery mildew response, the authors infected the leaves of VviEDR1-chi and wild-type (WT) plants with E. necator. The VviEDR1-chi mutant leaves showed more resistance against powdery mildew compared to WT. Furthermore, when VviEDR1-chi and WT plants were grown in the greenhouse infected with E. necator for over 2 years, VviEDR1-chi lines showed enhanced resistance to powdery mildew compared to WT, without compromising growth. This suggests that VviEDR1-chi mutants provide resistance against E. necator without growth penalty.
To further investigate the mechanism by which VviEDR1-chi confers resistance to powdery mildew without affecting plant growth and development, the authors analyzed the types and proportions of VviEDR1 mutations in various tissues of VviEDR1-chi lines using next-generation sequencing and high-throughput tracking of mutations platforms. Interestingly, the authors found that in the newly unfolded leaves, the proportion of mutated VviEDR1 was < 20%, and as leaf growth progressed, the proportion of mutated VviEDR1 increased, with mature leaves containing > 60% mutated VviEDR1. Similar trends were observed in stem tissues, with higher mutation proportions in lower-positioned and older tissues compared to newly developing tissues. Additionally, very few mutations were detected in the terminal and axillary buds (<1.0%), indicating that VviEDR1 mutations were absent or rare in the shoot apical meristem. Furthermore, approximately 10% of the mutated VviEDR1 occurred in lateral roots, whereas no mutations were detected in aerial and adventitious roots of VviEDR1-chi lines. Overall, the results suggest that a higher ratio of mutated cells in the lower-positioned and older senescent tissues than in the newly developing tissue from the apical meristem. Further, the authors also find consistent mutation regulation and a relatively stable mutation ratio among the different clones, indicating that the chimeric trait of VviEDR1-chi could be inherited by clonal propagation.
Because the number of mutations varies at different developmental stages of leaves, the authors evaluated powdery mildew resistance of both young and mature leaves by natural infection in the greenhouse. Whitish mildew colonies were observed on young leaves of both WT and VviEDR1-chi lines, whereas mature leaves of VviEDR1-chi exhibited strong resistance to powdery mildew with no visible colonies. Transcriptomic analysis of mature leaves of WT and VviEDR1-chi lines suggested that VviEDR1 edited lines regulate resistance to powdery mildew by activating multiple defense pathways, including callose deposition, increased salicylic acid and ethylene production, H2O2 production, and host cell death.
Overall, the study suggests that VviEDR1 mutation increases powdery mildew resistance at the expense of severe growth defects. The VviEDR1 homozygous and bi-allelic edited lines died due to the induction of a severe spontaneous hypersensitivity reaction. In VviEDR1-chi plants, almost no mutated VviEDR1 existed in the apical meristem, so the cells of the shoot apical meristem could divide and differentiate normally to maintain plant growth (Fig. 1). While the VviEDR1 mutation ratio increased with increasing leaf age, abundant mutations accumulated in mature leaves, conferring leaf resistance to powdery mildew by hypersensitive response-like cell death and H2O2 accumulation in the impacted epidermal cells and neighboring cells. Thus, the VviEDR1-chi maintains the balance between growth and defense.
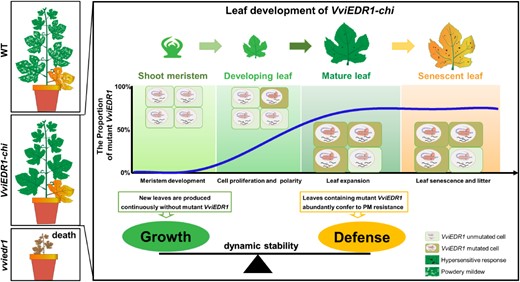
Schematic model depicting how VviEDR1-chi lines enhance resistance to powdery mildew without compromising growth (Figure 8 of Yu et al. 2024). The VviEDR1-chi lines express both the wild-type VviEDR1 and mutant Vviedr1 alleles as chimera (VviEDR1-chi), ensuring the dynamic stability of the VviEDR1 mutation. Almost no VviEDR1 mutation is detected in the apical meristem, allowing cells of the shoot apical meristem to divide and differentiate normally for plant growth. As leaves mature, the VviEDR1 mutation ratio increases, resulting in abundant accumulation of mutation in mature leaves and conferring resistance against powdery mildew.
Data availability
No data is generated in this study.
References
Author notes
Conflict of interest statement. None declared.



