-
PDF
- Split View
-
Views
-
Cite
Cite
Oliver Xiaoou Dong, Pamela C. Ronald, Genetic Engineering for Disease Resistance in Plants: Recent Progress and Future Perspectives, Plant Physiology, Volume 180, Issue 1, May 2019, Pages 26–38, https://doi.org/10.1104/pp.18.01224
Close - Share Icon Share
BACKGROUND
Modern agriculture must provide sufficient nutrients to feed the world’s growing population, which is projected to increase from 7.3 billion in 2015 to at least 9.8 billion by 2050. This goal is made even more challenging because of crop loss to diseases. Bacterial and fungal pathogens reduce crop yields by about 15% and viruses reduce yields by 3% (Oerke and Dehne, 2004). For some crops, such as potatoes, the loss caused by microbial infection is estimated to be as high as 30% (Oerke and Dehne, 2004). As an alternative to the application of chemical agents, researchers are altering the genetic composition of plants to enhance resistance to microbial infections.
Conventional breeding plays an essential role in crop improvement but usually entails growing and examining large populations of crops over multiple generations, a lengthy and labor-intensive process. Genetic engineering, which refers to the direct alteration of an organism’s genetic material using biotechnology (Christou, 2013), possesses several advantages compared with conventional breeding. First, it enables the introduction, removal, modification, or fine-tuning of specific genes of interest with minimal undesired changes to the rest of the crop genome. As a result, crops exhibiting desired agronomic traits can be obtained in fewer generations compared with conventional breeding. Second, genetic engineering allows for interchange of genetic material across species. Thus, the raw genetic materials that can be exploited for this process is not restricted to the genes available within the species. Third, plant transformation during genetic engineering allows the introduction of new genes into vegetatively propagated crops such as banana (Musa sp.), cassava (Manihot esculenta), and potato (Solanum tuberosum). These features make genetic engineering a powerful tool for enhancing resistance against plant pathogens.
Most cases of plant genetic engineering rely on conventional transgenic approaches or the more recent genome-editing technologies. In conventional transgenic methods, genes that encode desired agronomic traits are inserted into the genome at random locations through plant transformation (Lorence and Verpoorte, 2004). These methods typically result in varieties containing foreign DNA. In contrast, genome editing allows changes to the endogenous plant DNA, such as deletions, insertions, and replacements of DNA of various lengths at designated targets (Barrangou and Doudna, 2016). Depending on the type of edits introduced, the product may or may not contain foreign DNA. In some areas of the world, including the United States (USDA, 2018), Argentina (Whelan and Lema, 2015), and Brazil (CTNBio, Brazil, 2018), genome-edited plants that do not contain foreign DNA are not subject to the additional regulatory measures applied to transgenic plants (Orozco, 2018). Regardless of differences in regulatory policies, both conventional transgenic techniques and genome editing continue to be powerful tools for crop improvement.
Plants have evolved multilayered defense mechanisms against microbial pathogens (Chisholm et al., 2006; Jones and Dangl, 2006). For example, preformed physical and physiological barriers and their reinforcements prevent potential pathogens from gaining access inside the cell (Collinge, 2009; Uma et al., 2011). Plasma membrane-bound and intracellular immune receptors initiate defense responses upon the perception of pathogens either directly through physically interacting with pathogen-derived immunogens or indirectly by monitoring modifications of host targets incurred by pathogens (Jones and Dangl, 2006; Bentham et al., 2017; Zhang et al., 2017; Kourelis and van der Hoorn, 2018). Plant-derived antimicrobial peptides and other compounds can suppress pathogenicity by direct detoxification or through inhibition of the activity of virulence factors (Kitajima and Sato, 1999; Thomma et al., 2002; Ahuja et al., 2012). Plants also employ RNA interference (RNAi) to detect invading viral pathogens and target the viral RNA for cleavage (Rosa et al., 2018).
On the other side, successful pathogens have evolved strategies to overcome the defense responses of their plant hosts. For instance, many bacterial and fungal pathogens produce and release cell-wall-degrading enzymes (Kubicek et al., 2014). Pathogens can also deliver effectors into the host cytoplasm (Xin and He, 2013; Franceschetti et al., 2017); some of these effectors suppress host defenses and promote susceptibility. To counteract plant RNAi-based defenses, almost all plant viruses produce viral suppressors of RNAi (Zamore, 2004). Some viruses also hijack the host RNAi system to silence host genes to promote viral pathogenicity (Wang et al., 2012).
Some aspects of host-microbe interactions provide opportunities for genetic engineering for disease resistance (Dangl et al., 2013). For example, genes that encode proteins capable of breaking down mycotoxins (Karlovsky, 2011) or inhibiting the activity of cell-wall-degrading enzymes (Juge, 2006) can be introduced into plants. Plants can be engineered to synthesize and secrete antimicrobial peptides or compounds that directly inhibit colonization (Osusky et al., 2000). The RNAi machinery can be exploited to confer robust viral immunity by targeting viral RNA for degradation (Rosa et al., 2018). Natural or engineered immune receptors that recognize diverse strains of a pathogen can be introduced individually or in combination for robust, broad-spectrum disease resistance (Fuchs, 2017). Essential defense hub regulatory genes can be reprogrammed to fine-tune defense responses (Pieterse and Van Loon, 2004). Susceptible host targets can be modified to evade recognition and manipulation by pathogens (Li et al., 2012). Similarly, host decoy proteins, which serve to trap pathogens, can be modified through genetic engineering for altered specificity in pathogen recognition (Kim et al., 2016). For a more comprehensive overview of various aspects of engineered disease resistance in plants, the readers are referred to previously published review articles (Collinge et al., 2010; Cook et al., 2015).
Increased knowledge regarding the molecular mechanism underlying plant-pathogen interactions and advancements in biotechnology have provided new opportunities for engineering resistance to microbial pathogens in plants. In this review, we describe recent breakthroughs in genetic engineering in plants for enhanced disease resistance and highlight several strategies that have proven successful in field trials.
HIGHLIGHTS OF RECENT BREAKTHROUGHS IN GENETIC ENGINEERING FOR DISEASE RESISTANCE IN PLANTS
Pathogen-Derived Resistance and RNAi
Researchers have long observed that transgenic plants expressing genes derived from viral pathogens often display immunity to the pathogen and its related strains (Lomonossoff, 1995). These results led to the hypothesis that ectopic expression of genes encoding wild-type or mutant viral proteins could interfere with the viral life cycle (Sanford and Johnston, 1985). More recent studies demonstrated that this immunity is mediated by RNAi, which plays a major role in antiviral defense in plants.
The detailed molecular mechanism of RNAi in antiviral defense has been described in several excellent reviews (Wang et al., 2012; Katoch and Thakur, 2013; Galvez et al., 2014). Activation of RNAi has proven to be an effective approach for engineering resistance to viruses (Lindbo and Dougherty, 2005; Lindbo and Falk, 2017) as they rely on the host cellular machinery to complete their life cycle. Most plant viruses contain single-stranded RNA as their genetic information. Double-stranded RNA (dsRNA) replicative intermediates often form during the replication of the viral genome mediated by RNA-dependent RNA polymerase, triggering RNAi in the host.
Transgenic overexpression of viral RNA often leads to the formation of dsRNA, which also triggers RNAi (Galvez et al., 2014). This process is often known as cosuppression (Box 1). By far, most existing cases of genetically engineered crops with resistance to viral pathogens that have been approved for cultivation and consumption were generated using this strategy (Table 1). Notably, transgenic squash (Cucurbita sp.) and papaya (Carica papaya) varieties created using this approach have been grown commercially in the United States for more than 20 years. Field data indicate that disease resistance achieved through RNAi-mediated strategies is highly durable (Fuchs and Gonsalves, 2007). The RNAi strategy has also been effective in generating squash varieties that are resistant to multiple viral species (Table 1). These varieties were produced by gene stacking (i.e. transgenic expression of two distinct RNAi constructs targeted to different viruses; Tricoli et al., 1995).
Events of genetically engineered food crops with enhanced disease resistance to microbial pathogens that have been approved for commercial production
Data collected from The International Service for the Acquisition of Agri-biotech Applications (ISAAA) and the U.S. Department of Agriculture-Animal and Plant Health Inspection Service (USDA-APHIS). MOA, Ministry of Agriculture, China; ARS, Agriculture Research Service; EMBRAPA, the Brazilian Agricultural Research Corporation; CTNBio, the Brazilian National Technical Commission on Biosafety.
| Crop Species . | Developer . | Initial Approval . | Target Pathogen . | Gene(s) Expresseda . | References . |
|---|---|---|---|---|---|
| Squash | Seminis and Monsanto | 1994 USDA | Watermelon mosaic virus 2 and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Squash | Seminis and Monsanto | 1996 USDA | Cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Papaya | Cornell University and the University of Hawaii | 1996 USDA | Papaya ringspot virus | Coat protein | Gonsalves, 1998, 2006 |
| Potato | Monsanto | 1998 USDA | Potato leafroll virus | Replicase and helicase | Thomas et al., 1997; Kaniewski and Thomas, 2004 |
| Sweet Pepper | Beijing University | 1998 MOA | Cucumber mosaic virus | Coat protein | Zhu et al., 1996 |
| Potato | Monsanto | 1999 USDA | Potato virus Y | Coat protein | Newell et al., 1991; Kaniewski and Thomas, 2004 |
| Tomato | Beijing University | 1999 MOA | Cucumber mosaic virus | Coat protein | Yang et al., 1995 |
| Papaya | South China Agricultural University | 2006 MOA | Papaya ringspot virus | Replicase | Ye and Li, 2010 |
| Plum | USDA/ARS | 2007 USDA | Plum pox virus | Coat protein | Scorza et al., 1994; Ilardi and Tavazza, 2015 |
| Papaya | University of Florida | 2009 USDA | Papaya ringspot virus | Coat protein | Davis and Ying, 2004 |
| Bean | EMBRAPA | 2011 CTNBio | Bean golden mosaic virus | +, − RNA of viral replication protein | Bonfim et al., 2007; Tollefson, 2011 |
| Potato | J.R. Simplot | 2015 USDA | Phytophthora infestans (late blight) | Resistance protein | Foster et al., 2009 |
| Crop Species . | Developer . | Initial Approval . | Target Pathogen . | Gene(s) Expresseda . | References . |
|---|---|---|---|---|---|
| Squash | Seminis and Monsanto | 1994 USDA | Watermelon mosaic virus 2 and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Squash | Seminis and Monsanto | 1996 USDA | Cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Papaya | Cornell University and the University of Hawaii | 1996 USDA | Papaya ringspot virus | Coat protein | Gonsalves, 1998, 2006 |
| Potato | Monsanto | 1998 USDA | Potato leafroll virus | Replicase and helicase | Thomas et al., 1997; Kaniewski and Thomas, 2004 |
| Sweet Pepper | Beijing University | 1998 MOA | Cucumber mosaic virus | Coat protein | Zhu et al., 1996 |
| Potato | Monsanto | 1999 USDA | Potato virus Y | Coat protein | Newell et al., 1991; Kaniewski and Thomas, 2004 |
| Tomato | Beijing University | 1999 MOA | Cucumber mosaic virus | Coat protein | Yang et al., 1995 |
| Papaya | South China Agricultural University | 2006 MOA | Papaya ringspot virus | Replicase | Ye and Li, 2010 |
| Plum | USDA/ARS | 2007 USDA | Plum pox virus | Coat protein | Scorza et al., 1994; Ilardi and Tavazza, 2015 |
| Papaya | University of Florida | 2009 USDA | Papaya ringspot virus | Coat protein | Davis and Ying, 2004 |
| Bean | EMBRAPA | 2011 CTNBio | Bean golden mosaic virus | +, − RNA of viral replication protein | Bonfim et al., 2007; Tollefson, 2011 |
| Potato | J.R. Simplot | 2015 USDA | Phytophthora infestans (late blight) | Resistance protein | Foster et al., 2009 |
Only genes relevant to pathogen resistance are listed.
Data collected from The International Service for the Acquisition of Agri-biotech Applications (ISAAA) and the U.S. Department of Agriculture-Animal and Plant Health Inspection Service (USDA-APHIS). MOA, Ministry of Agriculture, China; ARS, Agriculture Research Service; EMBRAPA, the Brazilian Agricultural Research Corporation; CTNBio, the Brazilian National Technical Commission on Biosafety.
| Crop Species . | Developer . | Initial Approval . | Target Pathogen . | Gene(s) Expresseda . | References . |
|---|---|---|---|---|---|
| Squash | Seminis and Monsanto | 1994 USDA | Watermelon mosaic virus 2 and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Squash | Seminis and Monsanto | 1996 USDA | Cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Papaya | Cornell University and the University of Hawaii | 1996 USDA | Papaya ringspot virus | Coat protein | Gonsalves, 1998, 2006 |
| Potato | Monsanto | 1998 USDA | Potato leafroll virus | Replicase and helicase | Thomas et al., 1997; Kaniewski and Thomas, 2004 |
| Sweet Pepper | Beijing University | 1998 MOA | Cucumber mosaic virus | Coat protein | Zhu et al., 1996 |
| Potato | Monsanto | 1999 USDA | Potato virus Y | Coat protein | Newell et al., 1991; Kaniewski and Thomas, 2004 |
| Tomato | Beijing University | 1999 MOA | Cucumber mosaic virus | Coat protein | Yang et al., 1995 |
| Papaya | South China Agricultural University | 2006 MOA | Papaya ringspot virus | Replicase | Ye and Li, 2010 |
| Plum | USDA/ARS | 2007 USDA | Plum pox virus | Coat protein | Scorza et al., 1994; Ilardi and Tavazza, 2015 |
| Papaya | University of Florida | 2009 USDA | Papaya ringspot virus | Coat protein | Davis and Ying, 2004 |
| Bean | EMBRAPA | 2011 CTNBio | Bean golden mosaic virus | +, − RNA of viral replication protein | Bonfim et al., 2007; Tollefson, 2011 |
| Potato | J.R. Simplot | 2015 USDA | Phytophthora infestans (late blight) | Resistance protein | Foster et al., 2009 |
| Crop Species . | Developer . | Initial Approval . | Target Pathogen . | Gene(s) Expresseda . | References . |
|---|---|---|---|---|---|
| Squash | Seminis and Monsanto | 1994 USDA | Watermelon mosaic virus 2 and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Squash | Seminis and Monsanto | 1996 USDA | Cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus | Coat proteins | Tricoli et al., 1995 |
| Papaya | Cornell University and the University of Hawaii | 1996 USDA | Papaya ringspot virus | Coat protein | Gonsalves, 1998, 2006 |
| Potato | Monsanto | 1998 USDA | Potato leafroll virus | Replicase and helicase | Thomas et al., 1997; Kaniewski and Thomas, 2004 |
| Sweet Pepper | Beijing University | 1998 MOA | Cucumber mosaic virus | Coat protein | Zhu et al., 1996 |
| Potato | Monsanto | 1999 USDA | Potato virus Y | Coat protein | Newell et al., 1991; Kaniewski and Thomas, 2004 |
| Tomato | Beijing University | 1999 MOA | Cucumber mosaic virus | Coat protein | Yang et al., 1995 |
| Papaya | South China Agricultural University | 2006 MOA | Papaya ringspot virus | Replicase | Ye and Li, 2010 |
| Plum | USDA/ARS | 2007 USDA | Plum pox virus | Coat protein | Scorza et al., 1994; Ilardi and Tavazza, 2015 |
| Papaya | University of Florida | 2009 USDA | Papaya ringspot virus | Coat protein | Davis and Ying, 2004 |
| Bean | EMBRAPA | 2011 CTNBio | Bean golden mosaic virus | +, − RNA of viral replication protein | Bonfim et al., 2007; Tollefson, 2011 |
| Potato | J.R. Simplot | 2015 USDA | Phytophthora infestans (late blight) | Resistance protein | Foster et al., 2009 |
Only genes relevant to pathogen resistance are listed.
The knowledge that dsRNA effectively induces RNAi (Lindbo and Dougherty, 2005) inspired the design of transgenes encoding inverted repeat sequences, the transcripts of which form dsRNA (Box 1; Waterhouse et al., 1998). This strategy was used to develop a transgenic common bean variety exhibiting resistance to the DNA virus Bean golden mosaic virus (BGMV; Bonfim et al., 2007). BGMV contains single-stranded DNA as its genetic material, which is converted to dsDNA in the host, transcribed, and translated to produce the essential proteins required for its replication (Hanley-Bowdoin et al., 2013). To generate small interfering RNA targeting the viral transcripts, sense and antisense sequences of part of the BGMV replication gene AC1 were directionally cloned into an intron-spliced hairpin RNA expression cassette (Bonfim et al., 2007). The resulting genetically engineered bean exhibited strong and robust resistance in greenhouse conditions (Bonfim et al., 2007) as well as field conditions (Aragão and Faria, 2009). The BGMV-resistant bean was deregulated in 2011 in Brazil (Table 1), which allows farmers to grow the crop. It is to date the only example of a deregulated genetically engineered crop showing resistance to a DNA virus. Compared with the cosuppression strategy, transgenic expression of an artificially designed inverted repeat allows simultaneous generation of heterogenous small interfering RNAs targeting multiple transcripts in a relatively simple manner.
The discovery of microRNAs (miRNAs), a class of endogenous noncoding regulatory RNAs (Ambros, 2001; Reinhart et al., 2002) led to further refinements of genetic engineering for viral resistance. The miRNA machinery (Xie et al., 2015) has been exploited in engineering resistance to RNA viruses by replacing specific nucleotides in the miRNA-encoding genes to alter targeting specificity (Box 1). Such artificial miRNAs have been used for engineering resistance to a wide range of plant viral pathogens including Turnip mosaic virus (Niu et al., 2006), Cucumber mosaic virus (Qu et al., 2007; Duan et al., 2008; Zhang et al., 2011), Potato virus X, and Potato virus Y (Ai et al., 2011). These results suggest that artificial-miRNA-based antiviral strategies are highly promising. Disease resistance engineered by this strategy awaits future field tests.
Harnessing CRISPR-Cas to Target Viral Pathogens Directly
In recent years, the bacterially derived clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated (Cas) technology has proven to be a promising approach for engineering resistance against plant viruses (Box 2; Wright et al., 2016). In many bacterial species, CRISPR-Cas acts as antiviral defense machinery. In this process, an RNA-guided nuclease (often a Cas protein) cleaves at specific target sites on the substrate viral DNA or RNA, leading to their degradation. The specificity of the cleavage is governed by base complementarity between the CRISPR RNA and the target DNA or RNA molecules. A number of Cas proteins with sequence-specific nuclease activity have been identified (Wu et al., 2018). For example, the RNA-guided endonuclease Cas9 from Streptococcus pyogenes (SpCas9) induces double-stranded breaks in DNA in vivo (Jinek et al., 2012) and the RNA-guided RNases Cas13a from Leptotrichia shahii (LshCas13a) or Leptotrichia wadei (LwaCas13a) target RNA in vivo (Abudayyeh et al., 2017; Cox et al., 2017). Cas9 from Francisella novicida (FnCas9) cleaves both DNA and RNA in vivo (Price et al., 2015).
The ability of CRISPR-Cas to act like molecular scissors, creating breaks at sequence-specific targets in the substrate DNA or RNA molecules makes it an excellent candidate tool for engineering for antiviral defense in plants. CRISPR-Cas platforms based on Cas9 or Cas13a have been successfully harnessed to engineer resistance to DNA viruses or RNA viruses in planta (Fig. 1).
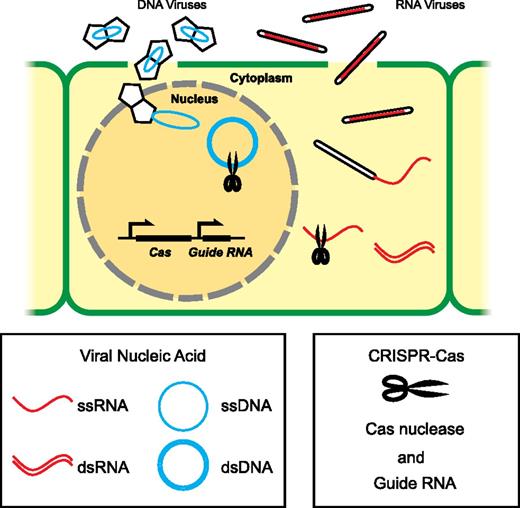
CRISPR-Cas platforms for engineering viral resistance in plants. Plant viruses complete their life cycles in the host cells. Viral particles with geminate capsids, and rod-shaped capsids are used as examples to illustrate DNA viruses and RNA viruses, respectively. One of the Cas nuclease genes (SpCas9, FnCas9, or LshCas13a) and the matching guide RNA(s) are expressed transgenically to generate sequence-specific ribonucleoproteins that target viral DNA or RNA substrates for degradation. SpCas9 targets nuclear dsDNA, while FnCas9 and LshCas13a have been used to target single-stranded RNA (ssRNA) substrates in the cytoplasm. For simplicity, only one generic Cas nuclease is drawn. ssDNA, single-stranded DNA.
The Tomato yellow leaf curl virus (TYLCV) belongs to Geminiviridae, a major family of plant viruses with single-stranded circular DNA genomes (Dry et al., 1993). The viral genome forms double-stranded intermediates during replication inside the host cell nuclei. Overexpression of SpCas9 and artificially designed guide RNAs targeting various regions of TYLCV conferred resistance to the virus in Nicotiana benthamiana and tomato (Solanum lycopersicum; Ali et al., 2015; Mahfouz et al., 2017). One potential caveat of this approach is that alterations to the viral DNA sequence may occur near the cleavage target due to DNA repair within the host cell. These mutations could shield the viral DNA from recognition by the guide RNA. Among all the guide RNAs used against TYLCV, the ones targeting the stem-loop sequence within the replication of origin were the most effective, possibly because of the reduced occurrence of viable escapee variants of the virus with mutations in this region (Ali et al., 2015, 2016). A separate lab study demonstrated that overexpression of SpCas9 and guide RNAs in N. benthamiana and Arabidopsis (Arabidopsis thaliana) conferred resistance to the geminivirus family member Beet severe curly top virus (Ji et al., 2015).
CRISPR-Cas has also been used to engineer resistance to RNA viruses, which comprise most known plant viral pathogens (Mahas and Mahfouz, 2018). For example, stable expression of the RNA-targeting nuclease Cas13a and the corresponding guide RNA in N. benthamiana conferred resistance to the RNA virus Turnip mosaic virus (Aman et al., 2018). Using Cas13a to target viral RNA substrates does not induce DNA breakage and thus would not introduce undesired off-target mutations to the host genome (Abudayyeh et al., 2017). Similarly, FnCas9 has been used to engineer resistance against RNA viruses Cucumber mosaic virus and Tobacco mosaic virus in N. benthamiana and Arabidopsis (Zhang et al., 2018).
Although a field test of CRISPR-Cas-based antiviral resistance on crop species has not been reported, the laboratory studies have demonstrated its potential as an antiviral tool. To ensure durable resistance, it is important to consider potential viral evasions from the surveillance by the specific guide RNA used. Choosing genomic targets essential for the replication or movement of the viral pathogen minimizes viral evasions (Ali et al., 2016). Multiplexing the guide RNAs can also improve the robustness. In addition, it has been hypothesized that the use of Cas12a (also known as Cpf1) may reduce the occurrence of escapee viral variants because mutations caused by CRISPR-Cas12a are less likely to abloish the recognition of the target by the orginal guide RNA (Ali et al., 2016). Future efforts on improving CRISPR-Cas for antivirus resistance may also be focused on establishing an in planta adaptive immunity by exploiting the spacer acquisition machinery in the CRISPR-Cas adaptive immune system in prokaryotes (McGinn and Marraffini, 2019).
Deploying Resistance Genes for Broad-Spectrum and Durable Resistance
Pioneering genetic studies in plant-pathogen interaction using flax and flax rust fungus by Flor in the early 1940s (Flor, 1956) established the classic “gene-for-gene” theory, which states that the outcome of any given plant-pathogen interaction is largely determined by a resistance (R) locus from the host and the matching avirulence factor (avr) from the pathogen (Flor, 1971). When both the R gene in the plant host and the cognate avr in the pathogen are present, the plant-pathogen interaction is incompatible and the host exhibits full resistance to the pathogen (Flor, 1971). The effectiveness of R gene-mediated resistance was first demonstrated by British scientist Rowland Biffen in wheat (Triticum sp.) breeding in the early twentieth century (Biffen, 1905).
Since that time, numerous R genes have been cloned and introduced into varieties of the same species (De Wit et al., 1985), across species boundaries (Song et al., 1995) and across genera (Tai et al., 1999). For example, the introduction of the maize (Zea mays) R gene Rxo1 into rice (Oryza sativa) conferred resistance to the bacterial streak pathogen Xanthomonas oryzae pv. oryzicola under lab conditions (Zhao et al., 2005). In tomato, multiyear fields trials under commercial type growth conditions have demonstrated that tomatoes expressing the pepper Bs2 R gene confer robust resistance to Xanthomonas sp. causing the bacterial spot disease (Horvath et al., 2015; Kunwar et al., 2018). Wheat transgenically expressing various alleles of the wheat resistance locus Pm3 exhibited race-specific resistance to powdery mildew in the field (Brunner et al., 2012). Potatoes transgenically expressing wild potato R gene RB or Rpi-vnt1.1 display strong field resistance to Phytophthora infestans, the causal agent of potato late blight (Halterman et al., 2008; Bradeen et al., 2009; Foster et al., 2009; Jones et al., 2014). Notably, transgenic potato expressing Rpi-vnt1.1 developed by J.R. Simplot is to date the only case of a genetically engineered crop with enhanced resistance to a nonviral pathogen that has been approved for commercial use (Table 1).
Successful pathogens often evade detection by host R genes (Jones and Dangl, 2006). Thus, disease resistance conferred by a single R gene often lacks durability in the field because pathogens can evolve to evade recognition by mutating the corresponding avr gene. For improved durability and to broaden the resistance spectrum, multiple R genes are often introduced simultaneously, which is commonly known as stacking (Li et al., 2001; Fuchs, 2017; Mundt, 2018). Resistance conferred by stacked R genes is predicted to be long lasting, as the evolution of a pathogen strain that could overcome resistance conferred by multiple R genes simultaneously is a low occurrence event. One approach to stack R genes is by cross breeding preexisting R loci (Fig. 2). Breeders can then use marker-assisted selection to identify the progeny with the desired R gene composition (Das and Rao, 2015). For example, bacterial blight in rice, caused by the bacterial pathogen Xanthomonas oryzae pv. oryzae (Xoo), is a serious disease in much of Asia and parts of Africa (Niño-Liu et al., 2006). Through cross breeding and marker-assisted selection, three R genes that confer resistance to bacterial blight in rice, Xa21, Xa5, and Xa13 were introduced into a deep-water rice cultivar called Jalmagna (Pradhan et al., 2015). The resulting line with the stacked R genes exhibited a higher level of field resistance to eight Xoo isolates tested (Pradhan et al., 2015). Although marker-assisted selection has largely improved the efficiency of the selection process, combining multiple loci through this approach can still be highly time consuming.
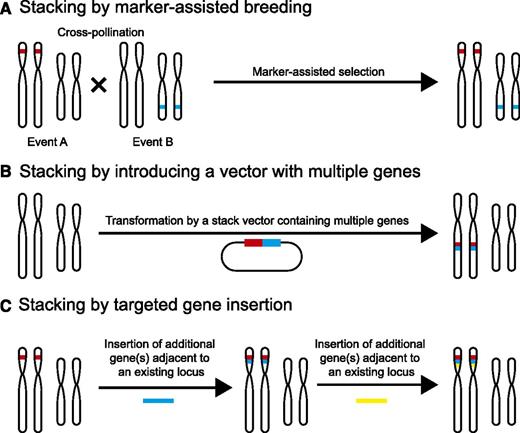
Methods of R gene stacking. A, Stacking by marker-assisted breeding is performed by cross pollinating individuals with existing trait loci followed by marker-assisted selection for progeny with combined trait loci. B, Stacking can be completed by combining multiple genes into a single stack vector and introducing them together as a single transgenic event. C, Stacking by targeted insertion aims at placing new gene(s) adjacent to an existing locus. This process can be performed iteratively to stack large numbers of genes. In B and C, the stacked genes are genetically linked and thus can be easily introduced into a different genetic background as a single locus through breeding.
As an alternative to gene stacking through marker-assisted selection, scientists can assemble multiple R gene cassettes onto one plasmid and then introduce this R gene cluster en bloc at a single genetic locus through plant transformation (Fig. 2; Dafny-Yelin and Tzfira, 2007; Que et al., 2010). This approach, called molecular stacking, simplifies the selection process, as all the R genes introduced this way are inherited as a single genetic locus. As an example of molecular stacking, Zhu et al. (2012) introduced molecular stacking of three broad-spectrum potato late blight R genes Rpi-sto1, Rpi-vnt1.1, and Rpi-blb3 through Agrobacterium-based transformation of a susceptible potato cultivar. The resulting triple gene transformants displayed broad-spectrum resistance equivalent to the sum of the strain-specific resistance conferred by all three individual Rpi genes under greenhouse conditions (Zhu et al., 2012). In a related study, a single DNA fragment harboring Rpi-vnt1.1 and Rpi-sto1 was introduced into three different potato varieties through Agrobacterium-mediated transformation (Jo et al., 2014). The R gene-stacked lines showed broad-spectrum late blight resistance because of the introduction of both R genes (Jo et al., 2014). Notably, no foreign DNA such as a selectable marker gene was included in the inserted DNA fragment in the double gene study (Jo et al., 2014), which may reduce the number of regulatory approvals needed for the resulting products.
Resistance to the late blight pathogen in both the double gene-stacked and the triple gene-stacked potatoes mentioned above was confirmed under field conditions (Haverkort et al., 2016). It is estimated that proper spatial and temporal deployment of late blight R gene stacks in potatoes can reduce the amount of fungicide used on this crop by over 80% (Haverkort et al., 2016). Similarly, in a field study of two African highland potato varieties in Uganda, Ghislain et al. (2018) reported that significant field resistance to the late blight pathogen could be achieved by the molecular stacking of three R genes (RB, Rpi-blb2, and Rpi-vnt1.1). The yields of these R gene stacked potato varieties were three times higher than the national average. These results demonstrate that resistance achieved by this strategy does not negatively affect yield (Ghislain et al., 2018). The above studies show the simplicity and effectiveness of molecular stacking for engineering broad-spectrum disease resistance, especially in vegetatively propagated crop species, where breeding stacks are not practical.
Despite the advantages of molecular stacking, the number of genes that can be introduced through molecular stacking is often restrained by the limit in the length of the DNA insert that can be put into a vector (Que et al., 2010). This limitation can be overcome if DNA fragments can be sequentially inserted at the same genomic target (Fig. 2). Recent breakthroughs in genome-editing technologies in plants enable such targeted insertion of DNA fragments in diverse crop species (Puchta and Fauser, 2013; Rinaldo and Ayliffe, 2015; Kumar et al., 2016; Voytas, 2017). This technology allows multiple R gene cassettes to be inserted at a single locus in multiple rounds (Ainley et al., 2013). As genome-editing platforms in plants continue to be improved, the efficiency of targeted insertion, the size limit of the DNA inserts, and the number of applicable plant species are increasing. Future advancements in targeted gene insertion would offer new opportunities for stacking of large numbers of R genes and engineered viral resistance at a single locus for broad-spectrum, durable disease resistance and convenience in breeding.
Enriching the Known Repertoire of Immune Receptors
Plants possess immune receptors that perceive pathogens and trigger cellular defense responses. Discovery of novel immune receptors recognizing major virulence factors will enrich the repertoire of known immune receptor genes that may be deployed in the field. The nucleotide-binding leucine-rich repeats (NLR) family proteins comprise a major category of intracellular immune receptors conserved across the plant and animal kingdoms (Maekawa et al., 2011; Li et al., 2015; Bentham et al., 2017; Zhang et al., 2017). A distinct hallmark for NLR-mediated defense is the onset of localized programmed cell death known as the hypersensitive response, which plays a crucial role in restricting the movement of pathogens (del Pozo and Lam, 1998; Pontier et al., 1998). The hypersensitive response is often used as a marker to screen diverse plant germplasm for novel functional NLRs recognizing known effectors (Vleeshouwers and Oliver, 2014). During the screening, core virulence factors are delivered into plant leaves either as transiently expressed genes through Agro-infiltration (Du et al., 2014) or as genes expressed in laboratory bacterial strains with a functional secretion system (Zhang and Coaker, 2017).
Once a collection of germplasm exhibiting various degrees of resistance to a particular pathogen strain is identified, comparative genomics tools such as resistance gene enrichment sequencing (RenSeq; Jupe et al., 2013, 2014) can be applied to identify genomic variants in NLR genes that are linked to disease phenotypes (Box 3). This leads to the cloning of new NLR genes and their potential deployment in crop protection through genetic engineering. For example, RenSeq was successfully applied in the accelerated identification and cloning of an anti-P. infestans NLR gene Rpi-amr3i from Solanum americanum, a wild potato relative (Witek et al., 2016). Transgenic expression of Rpi-amr3i in potato conferred full resistance to P. infestans in greenhouse conditions (Witek et al., 2016). In a more recent study, Chen et al. described the rapid mapping of a newly identified anti-potato-late-blight NLR gene Rpi-ver1 by RenSeq in the wild potato species Solanum verrucosum (Chen et al., 2018).
In addition to its application in potato research, RenSeq has also been employed in the cloning of wheat NLR resistance genes against the stem rust fungus Puccinia graminis f. sp. tritici. As an example, Arora et al. (2019) used RenSeq to survey accessions of a wild progenitor species of bread wheat, Aegilops tauschii ssp. strangulata for trait-linked. This led to the rapid cloning of four stem rust (Sr) resistance genes (Arora et al., 2019). In a related study, Steuernagel et al. (2016) applied RenSeq to a mutagenized hexaploid bread wheat population to identify mutations disrupting resistance to the stem rust fungus. The study revealed the identity of two stem rust NLR genes, Sr22 and Sr45, which confer resistance to commercially important races of the stem rust pathogen (Steuernagel et al., 2016). Although future field experiments are required to evaluate the potential of the deployment of these newly identified NLR genes, the above lab studies demonstrate that RenSeq is a powerful tool to rapidly identify novel NLR genes.
In addition to the identification of useful immune receptors from diverse plant germplasm, researchers have attempted to engineer known immune receptors for new ligand specificity. For example, the fusion of the ectodomain of the Arabidopsis pattern recognition receptor EF-Tu receptor (EFR) to the intracellular domain of the phylogenetically related rice receptor Xa21 yielded a functional chimeric receptor in both rice and Arabidopsis (Holton et al., 2015; Schwessinger et al., 2015). This receptor triggers defense markers when transgenic tissues are treated with elf18, the ligand of EFR, although whole-plant resistance to the microbe was weak or undetectable (Holton et al., 2015; Schwessinger et al., 2015). Conversely, expression of a fusion receptor consisting of the ectodomain of Xa21 and the cytoplasmic domain of EFR in rice conferred robust resistance to Xoo expressing the cognate ligand of Xa21 (Thomas et al., 2018). Two related studies reported that chimeric receptors generated by fusing the rice chitin-binding protein Chitin Elicitor-Binding Protein and the intracellular protein kinase domain of the rice receptor-like kinase Xa21 or Pi-d2 confers disease resistance to the rice blast fungus (Kishimoto et al., 2010; Kouzai et al., 2013). Although these studies have not been advanced to field trials, they demonstrate that domain swapping among immune receptors may be an attractive approach in engineering broadened recognition specificity.
NLRs often perceive pathogens indirectly by monitoring the modification of host target proteins by pathogen-derived virulence factors (Jones and Dangl, 2006). Some of these host target proteins have evolved to serve as decoys that are targeted by virulence factors (van der Hoorn and Kamoun, 2008). For example, the NLR protein RESISTANCE TO PSEUDOMONAS SYRINGAE 5 (RPS5), activates defense responses in Arabidopsis (Simonich and Innes, 1995). Defense is triggered when the plant decoy protein PBS1, a kinase, is cleaved by the protease AvrPphB secreted by the bacterial pathogen Pseudomonas syringae into the plant cell (Ade et al., 2007). Kim et al. (2016) altered the protease cleavage site in PBS1 to expand the resistance spectrum of RPS5 so that it could recognize other pathogens. For example, they found that they could remove the cleavage site of PBS1 that is recognized by the AvrPphB protease and replace it with cleavage site variants recognized by other pathogen proteases such as the AvrRpt2 protease from Pseudomonas syringae, or the Nla protease from Turnip mosaic virus (Kim et al., 2016). The engineered forms of PBS1 are cleaved in vivo by these proteases, which activates RPS5 defense in response to the corresponding pathogen strains (Kim et al., 2016). These successful examples make decoy modification an attractive approach to engineer resistance to new pathogens (Kourelis et al., 2016).
Altered specificity and activity of immune receptors can also be achieved by directed molecular evolution (Lassner and Bedbrook, 2001). During this process, immune receptor genes are subjected to mutagenesis to generate multiple variants, which are screened for their immune functions. The process can be performed iteratively for continuous improvement in the activity of the gene product. Two pioneering studies demonstrated the potential value of directed molecular evolution in expanding the pool of available immune receptor genes. In a search for gain-of-function mutants of the potato late blight NLR gene R3a, Segretin et al. (2014) screened a library of R3a variants generated through PCR-based mutagenesis and identified R3a mutants recognizing additional Avr3a isoforms from P. infestans and the related blight pathogen Phytophthora capsici. In a follow-up study, the newly identified mutation in R3a was transferred into its tomato ortholog I2 and expanded the recognition spectrum of the NLR encoded when transiently expressed in N. benthaminana (Giannakopoulou et al., 2015).
Recently, a CRISPR-based mutagenesis platform known as EvolvR was developed for the directed evolution of a precisely defined window of genomic DNA in vivo (Halperin et al., 2018; Sadanand, 2018). EvolvR employs the mutagenesis activity of a fusion of the Cas9 nickase and an error-prone DNA polymerase to introduce nucleotide diversification at specific genomic regions defined by guide RNAs (Halperin et al., 2018). For example, EvolvR may potentially be used to develop novel alleles of the rice immune receptor Xa21 with the ability to recognize a broader array of Xoo strains, which secrete ligand variants (Pruitt et al., 2015; Luu et al., 2019). Variants of Xa21 receptors carrying amino acid substitutions predicted to alter ligand specificity can be screened for and introduced into susceptible rice cultivars. Future development of high-throughput functional screening methods to quickly identify gene variants conferring resistance phenotypes would broaden the application of directed molecular evolution for enhanced disease resistance in plants.
Modifying Susceptibility Genes to Attenuate Pathogenicity
Many plant pathogens hijack host genes to facilitate the infection process. These targeted host genes are often referred to as susceptibility genes (van Schie and Takken, 2014). The modification or removal of host susceptibility genes may, therefore, be an effective strategy to achieve resistance by preventing their manipulation by the pathogens. RNAi and genome-editing platforms (Box 2) enable efficient modification of endogenous susceptibility genes in a relatively simple manner (Rinaldo and Ayliffe, 2015).
The highly conserved eukaryotic translation initiation factor 4E (eIF4E) in many plant species is manipulated by a number of plant viruses to facilitate the viral infection process (Robaglia and Caranta, 2006). Naturally occurring recessive alleles of eIF4E confer viral resistance due to abolished protein-protein interaction with a specialized viral virulence protein (Cavatorta et al., 2008). Based on this knowledge, Cavatorta et al. (2011) demonstrated that transgenic overexpression of a site-directed-mutagenized viral-resistant allele of the eIF4E conferred resistance to Potato virus Y in potato. In a more recent study, Chandrasekaran et al. (2016) knocked out the eIF4E gene in cucumber using CRISPR-Cas and obtained homozygous mutant plants that are resistant to a variety of viral pathogens under greenhouse conditions.
The SWEET family genes encode cross-membrane sugar transporters, many of which are exploited by plant pathogens for virulence (Chen et al., 2010; Streubel et al., 2013). Often, when SWEET genes are activated, more sugar is transported outside of the cell and made available to the bacteria (Chen et al., 2010, 2012). For example, the promoters of rice SWEET11 and SWEET14 are targets of various transcriptional activator-like (TAL) effectors from Xoo (Yang et al., 2006; Antony et al., 2010). Li et al. (2012) used a TAL effector-like nuclease to mutate predicted TAL effector-binding sites within the SWEET14 promoter (Li et al., 2012). The resulting rice exhibits enhanced resistance to at least two strains of Xoo due to the lack of induction of SWEET14 by the pathogens. In a follow-up study, Zhou et al. (2014) successfully generated rice lines carrying mutations in the promoter of SWEET11 using CRISPR-Cas. Mutations within the SWEET14 promoter in rice protoplasts by CRISPR-Cas have also been demonstrated (Jiang et al., 2013). In addition to the genome-editing approach, RNAi has been employed to knock down SWEET11 in rice for enhanced resistance to an Xoo strain carrying the cognate TAL effector (Yang et al., 2006).
The disease susceptibility gene for citrus bacterial canker disease, Citrus sinensis Lateral Organ Boundary1 (CsLOB1) encodes a transcription factor that regulates plant growth and development (Hu et al., 2014). Deletion of the effector binding element within the promoter of the susceptibility gene CsLOB1 by CRISPR-Cas conferred resistance to the bacterial canker pathogen Xanthomonas citri spp. citri (Xcc) in orange (Citrus sinensis; Peng et al., 2017). Similarly, disrupting the coding region of CsLOB1 by CRISPR-Cas in grapefruit (Citrus paradisi) resulted in enhanced resistance to Xcc (Jia et al., 2017). In both studies, the plants were raised in controlled environmental conditions. The effectiveness of resistance gained through mutating CsLOB1 in citrus plants awaits further evaluation by field experiments.
Susceptibility genes are often conserved among plant species (Huibers et al., 2013). Therefore, knowledge of susceptibility genes in one plant species can facilitate the discovery of new susceptibility genes in another plant species. The Arabidopsis susceptibility gene DOWNY MILDEW RESISTANT6 (DMR6) encodes an oxygenase, and its expression is up-regulated during pathogen infection (Van Damme et al., 2005; van Damme et al., 2008; Zeilmaker et al., 2015). Through phylogeny and gene expression analysis, Thomazella et al. (2016) identified a single DMR6 ortholog in tomato (SlDMR6-1). Knocking out SlDMR6-1 in tomato using CRISPR-Cas led to increased resistance to the bacterial pathogen P. syringae under greenhouse conditions (Thomazella et al., 2016). In another study, Sun et al. (2016) selected 11 Arabidopsis genes that had been previously shown to correlate with susceptibility to one or more of six common plant pathogens. In a search for gene products with similar amino acid sequences within the potato genome, they identified 11 candidate susceptibility genes in potato (Sun et al., 2016). Silencing of six of these candidate genes individually in potato using RNAi conferred enhanced or complete resistance to the late blight pathogen in controlled environmental conditions (Sun et al., 2016). These studies show that advancements in Arabidopsis biology and identification of an increasing number of susceptibility genes have enabled the discovery of additional susceptibility genes in a large variety of crop species.
Modifying susceptibility genes to thwart virulence strategies of pathogens holds great potential in crop protection. Because a given allele that confers susceptibility to one pathogen may confer resistance to another or have other essential biological functions (Lorang et al., 2012; McGrann et al., 2014), it is important to evaluate the potential side-effects of modifying susceptibility genes. It remains to be determined whether the observed resistance in the instances discussed in the laboratory studies is robust or durable under field conditions or if the plants perform well in the field.
CONCLUSION
Since the first report of a genetically engineered crop conferring resistance to disease in 1986, a virus-resistant tobacco expressing a viral coat protein gene (Abel et al., 1986), there have been tremendous breakthroughs both in labs and in the field. In terms of successful field applications, antiviral resistance has had a clear head start with most of the commercialized genetically engineered crops for disease resistance thus far falling into this category. However, with new technologies that enable rapid identification of novel immune receptor genes, such as RenSeq and directed molecular evolution, the pool of deployable genes for enhanced resistance to other microbes has expanded substantially. In parallel, advancements in molecular stacking and targeted gene insertion through genome editing are expected to play a major role in generating broad-spectrum resistance against both viral and nonviral pathogens in the near future. Furthermore, the increasingly versatile, accurate, and efficient genome-editing tools such as CRISPR-Cas enable accurate modification of endogenous genes for disease resistance, including, but not limited to, susceptibility and the decoy genes.
In the Outstanding Questions Box, we present some examples of challenges faced in genetic engineering for resistance. Answers to these questions will fill important knowledge gaps and can be used to develop new strategies for engineering plant disease resistance.
ACKNOWLEDGMENTS
We thank Dr. Mawsheng Chern and Dr. Michael Steinwand for valuable suggestions on the manuscript.
LITERATURE CITED
Author notes
This work was supported by research grants from the Innovative Genomics Institute (49411), the National Institutes of Health (R01GM122968) and the United States Department of Agriculture (NIFA 2017-67013-26590) to P.C.R.
Author for contact: [email protected].
Senior author.
O.X.D. and P.C.R. wrote the article.
Articles can be viewed without a subscription.








