-
PDF
- Split View
-
Views
-
Cite
Cite
Silvia Melina Velasquez, Elke Barbez, Jürgen Kleine-Vehn, José M. Estevez, Auxin and Cellular Elongation, Plant Physiology, Volume 170, Issue 3, March 2016, Pages 1206–1215, https://doi.org/10.1104/pp.15.01863
Close - Share Icon Share
Abstract
Auxin is a crucial growth regulator in plants. However, a comprehensive understanding of how auxin induces cell expansion is perplexing, because auxin acts in a concentration- and cell type-dependent manner. Consequently, it is desirable to focus on certain cell types to exemplify the underlying growth mechanisms. On the other hand, plant tissues display supracellular growth (beyond the level of single cells); hence, other cell types might compromise the growth of a certain tissue. Tip-growing cells do not display neighbor-induced growth constraints and, therefore, are a valuable source of information for growth-controlling mechanisms. Here, we focus on auxin-induced cellular elongation in root hairs, exposing a mechanistic view of plant growth regulation. We highlight a complex interplay between auxin metabolism and transport, steering root hair development in response to internal and external triggers. Auxin signaling modules and downstream cascades of transcription factors define a developmental program that appears rate limiting for cellular growth. With this knowledge in mind, the root hair cell is a very suitable model system in which to dissect cellular effectors required for cellular expansion.
MORPHOGENETIC AND ENVIRONMENTAL PROGRAMS CONTROL ROOT HAIR GROWTH
Root hairs as tip-growing protrusions that grow from root epidermal cells are crucial for nutrient and water uptake as well as for root anchoring and interaction with soil microorganisms. There are three major developmental steps during root hair development: (1) cell fate determination, (2) root hair initiation, and (3) cell elongation by polarized growth. In Arabidopsis (Arabidopsis thaliana), root hair cell (trichoblasts) and nonhair cell (atrichoblast) files differentiate from the epidermal cells in the meristematic and transition zones of the root (Dolan et al., 1993). In some monocots, including maize (Zea mays), a random and unpredictable process directs epidermal cells to develop into root hairs cells. However, in others, such as Brachypodium spp., trichoblasts can alternate with atrichoblasts along longitudinal epidermal cell files (Clowes, 2000). Arabidopsis has become a useful model for root system biology (Benfey et al., 2010; Libault et al., 2010) and appears especially suitable to unravel the molecular components that govern the root hair developmental program (Schiefelbein et al., 2009; Bruex et al., 2012). Cell fate determination is controlled by a multifaceted interaction between several transcription factor (TF) complexes. One complex, composed by WEREWOLF, GLABRA3-ENHANCER OF GLABRA3, and TRANSPARENT TESTA GLABRA, promotes the homeodomain protein GLABRA2 (GL2) expression, which is the main negative regulator of root hair differentiation. Recently, it was shown that GL2 in atrichoblast cells directly represses the expression of several root hair-specific TFs (e.g. ROOT HAIR DEFECTIVE6-LIKE1 [RSL1], RSL2, ROOT HAIR DEFECTIVE6 [RHD6], and Lotus japonicus ROOT HAIRLESS1-LIKE1 [LRL1]/LRL2; Lin et al., 2015a). On the other hand, the TFs CAPRICE (CPC), TRYPTYCHON, and ENHANCER OF TRYPTYCHON AND CAPRICE repress GL2 and promote hair cell fate. Several excellent reviews have covered this developmental process in detail (Pesch and Hülskamp, 2009; Schiefelbein et al., 2009; Wang et al., 2010). Here, we will focus on processes that determine root hair expansion.
Once root hair cell fate has been defined, root hairs protrude from the root hair cell surface. This process takes place in the root differentiation zone and is controlled by the interplay between several genes, including RSL1 and RHD6. RSL1 and RHD6 are class I RSL proteins and belong to the basic helix-loop-helix TF family (Menand et al., 2007; Pires et al., 2013). CPC activates the latter after the epidermis cell fate has been specified. Loss-of-function mutants for RHD6 and RSL1 do not develop root hairs (Menand et al., 2007). Class I RSL (RHD6/RSL1) TFs induce the expression of downstream TFs of the basic helix-loop-helix family, including RSL2, RSL4, and LRL3, ultimately triggering differentiation and the subsequent polarized tip growth of root hairs (Karas et al., 2009; Yi et al., 2010; Bruex et al., 2012). Recently, it was demonstrated that the intensity of RSL4 synthesis directly determines the final size of the root hair cells, highlighting the predominant role of this TF in directing the cell elongation of root hairs (Datta et al., 2015). In addition, other TFs, which possibly act independently of RDH6/RSL1, have been reported to affect root hair growth, such as LRL1 and LRL2 (Karas et al., 2009), a membrane-anchored R2R3-MYB (Slabaugh et al., 2011), the HD-ZIP IV HOMEODOMAIN GLABROUS11 (Xu et al., 2014), as well as the transcriptional coregulator MEDIATOR25/PHYTOCHROME AND FLOWERING TIME1 (Sundaravelpandian et al., 2013). Apart from the morphogenetic program described above, root hair outgrowth is modulated by a wide spectrum of environmental signals, such as nutrient availability, water status, carbon monoxide and carbon dioxide levels, phosphate depletion (Yi et al., 2010), as well as the availability of spore elements including manganese (Niu et al., 2014), vanadium (Lin et al., 2015b), and boron (Martín-Rejano et al., 2011). Root hair development is a highly dynamic process in which endogenous as well as environmental aspects are integrated, and the phytohormone auxin is central to this complex regulation (Lee and Cho, 2013). This review will discuss the particular molecular mechanisms by which auxin triggers root hair tip growth (Fig. 1). Examples of mutants that have an impact on auxin biosynthesis, conjugation, transport, and signaling with a consequent root hair defect in cell expansion have been selected to highlight the multiple and complex levels of auxin action on cell elongation (Table I).
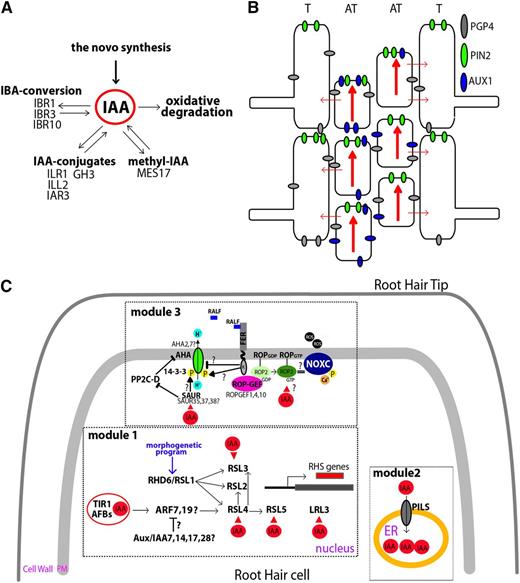
Auxin-mediated responses that trigger root hair growth. A, Auxin homeostasis pathways. Free indole-3-acetic acid (IAA) levels are maintained in plant cells by several mechanisms, including de novo biosynthesis and oxidative degradation. IAA-conjugate hydrolases hydrolyze IAA conjugates to free IAA. Indole-3-butyric acid (IBA) response genes (IBR1, IBR3, and IBR10) are necessary to metabolize IBA to free IAA. Methyl-IAA is converted to free IAA by MES17. B, Auxin transport in the root epidermis. PIN-FORMED2 (PIN2) and PGP4 are involved in auxin efflux in both trichoblast (T) and atrichoblast (AT) cells, whereas AUXIN RESISTANT1 (AUX1) is in charge of auxin influx located only in the atrichoblast cells. Auxin fluxes are indicated by red arrows. C, The main target of auxin in root hair cells. Auxin could trigger a transcriptional response in the nucleus via TIR/AFB-ARFs and downstream RSL/LRL3 activation of root hair-specific (RHS) genes (module 1). The morphogenetic program on root hair cells (trichoblasts) acts on RHD6/RSL1 to trigger root hair initiation growth. Auxin directly regulates the expression of several TFs, such as the central root hair regulator RSL4, as well as RSL3, RSL5, and LRL3. It is postulated, based on cell expression profile and mutant phenotypes, that ARF7 and ARF19 as well as AUX/IAA7, AUX/IAA14, AUX/IAA17, and AUX/IAA28 would be components of the auxin response in root hair cells. In addition, auxin can be regulated by its transport into and storage in the endoplasmic reticulum (ER; module 2) via PILS proteins (PILS2 and PILS5). Finally, auxin may trigger nontranscriptional responses (module 3) via plasma membrane H+-ATPases (AHA) activation affecting pH changes and FERONIA (FER)-ROP2-mediated NADPH oxidase (NOX) activation, thus increasing reactive oxygen species (ROS) production. FER could also interact with the RAPID ALKALINIZATION FACTOR1 (RALF1) peptide to trigger changes in apoplastic pH by inhibiting the H+-ATPase activity of AHA2 and suppressing cell expansion. In addition, at least for hypocotyl cells (it has not yet been validated for growing root hairs), it was demonstrated that auxin promotes the activation of AHA1 and AHA2 by the activity of SMALL AUXIN UP RNA19 (SAUR19), which interacts with and inhibits the activity of 2C protein phosphatase D1 (PP2C-D1). This phosphatase negatively regulates AHA1 activity, allowing the binding of 14-3-3 proteins to AHA1. In this model, SAUR and PP2C-D1 act antagonistically to control cell expansion (Spartz et al., 2014). It is hypothesized that SAUR35, SAUR37, and SAUR38 as well as AHA2 and AHA7, which are highly expressed in root hair cells, would be components that specifically act on this cell type. The red triangles indicate auxin activation at the transcriptional level of several TFs and SAUR proteins and, posttranscriptionally, by a still unknown mechanism to FER-ROP2.
Selected examples of genes that impact auxin homeostasis and are involved in root hair cell expansion
ox, Overexpressor line.
| Gene Type . | Mutant/Transgenic Line . | Root Hair Phenotype . | Reference . |
|---|---|---|---|
| IAA transport | |||
| pin2 | Short root hairs | Cho et al. (2007a); Rigas et al. (2013) | |
| pEXP7a:PIN3ox | Long root hairs | Lee and Cho (2006) | |
| pEXP7a:PIN1/PIN2/PIN3/PIN7/PIN8 | Short root hairs | Ganguly et al. (2010) | |
| pEXP7a:PIN5 | Long root hairs | Ganguly et al.( 2010) | |
| pgp4 (abcb4) | Long root hairs | Santelia et al. (2005); Cho et al. (2007b, 2012) | |
| PGP4ox | Short root hairs | Cho et al. (2007b) | |
| aux1-7, aux1-T, aux1-22 | Short root hairs | Pitts et al. (1998); Ikeda et al. (2009); Jones et al. (2009) | |
| AUX1ox | Long root hairs | Ganguly et al. (2010) | |
| pEXP7a:PID | Short and fewer root hairs | Lee and Cho (2006) | |
| IAA ER storage | |||
| pils2 pils5 | Long root hairs | Barbez et al. (2012) | |
| PILS5-GFPox, EXP7a:PILS5-GFP | Short root hairs | Barbez et al. (2012) | |
| IAA metabolism | |||
| ibr1 ibr3 ibr10, ilr1 iar3 ill2 | Short root hairs | Davies et al. (1999); LeClere et al. (2002); Zolman et al. (2008); Strader et al. (2010b) | |
| echo2 ibr1ibr3 ibr10 | No root hairs | Strader et al. (2010b) | |
| ill2 ilr1 iar3 ibr1, ill2 iar3 ibr3 | Short root hairs | Spiess et al. (2014) | |
| mes17 ill2 iar3, mes17 ibr3 | Mild short root hair phenotype | Spiess et al. (2014) | |
| Iar4 | Short root hairs; rescued by YUCCA1ox | Quint et al. (2009) | |
| YUCCA1ox | Long root hair phenotype | Zhao et al. (2001) | |
| yucca1 yucca2 yucca4 yucca6 | Hairy root hair phenotype | Chen and Xiong (2009) | |
| IBA transport | |||
| cdcg36 (pen3, pdr8) | Long root hairs | Strader et al. (2011) | |
| IAA perception | |||
| tir1, tir1 afb2 afb3 | Short root hairs | Dharmasiri et al. (2005) | |
| pEXP7a:TIR1 | Longer root hairs | Ganguly et al. (2010) | |
| aux/iaa1 (axr5) | No root hairs | Park et al. (2002) | |
| aux/iaa7 (axr2) | No/short root hairs | Wilson et al. (1990); Timpte et al. (1994) | |
| aux/iaa14 (slr) | No/short root hairs | Fukaki et al. (2002) | |
| aux/iaa17 (axr3) | Short root hairs | Leyser et al. (1996) | |
| axr1 | Short root hairs | Pitts et al. (1998) | |
| iaa28 | Short root hairs | Rogg et al. (2001) | |
| IAA nontranscriptional response | |||
| fer | Short root hairs | Duan et al. (2010) | |
| ropGEF4/ropGEF10 | Short root hairs and reduced root hair density for ropGEF10 | Huang et al. (2013) | |
| IAA transcriptional response | |||
| rsl4/RSL4ox | Short root hairs/long root hairs | Yi et al. (2010) |
| Gene Type . | Mutant/Transgenic Line . | Root Hair Phenotype . | Reference . |
|---|---|---|---|
| IAA transport | |||
| pin2 | Short root hairs | Cho et al. (2007a); Rigas et al. (2013) | |
| pEXP7a:PIN3ox | Long root hairs | Lee and Cho (2006) | |
| pEXP7a:PIN1/PIN2/PIN3/PIN7/PIN8 | Short root hairs | Ganguly et al. (2010) | |
| pEXP7a:PIN5 | Long root hairs | Ganguly et al.( 2010) | |
| pgp4 (abcb4) | Long root hairs | Santelia et al. (2005); Cho et al. (2007b, 2012) | |
| PGP4ox | Short root hairs | Cho et al. (2007b) | |
| aux1-7, aux1-T, aux1-22 | Short root hairs | Pitts et al. (1998); Ikeda et al. (2009); Jones et al. (2009) | |
| AUX1ox | Long root hairs | Ganguly et al. (2010) | |
| pEXP7a:PID | Short and fewer root hairs | Lee and Cho (2006) | |
| IAA ER storage | |||
| pils2 pils5 | Long root hairs | Barbez et al. (2012) | |
| PILS5-GFPox, EXP7a:PILS5-GFP | Short root hairs | Barbez et al. (2012) | |
| IAA metabolism | |||
| ibr1 ibr3 ibr10, ilr1 iar3 ill2 | Short root hairs | Davies et al. (1999); LeClere et al. (2002); Zolman et al. (2008); Strader et al. (2010b) | |
| echo2 ibr1ibr3 ibr10 | No root hairs | Strader et al. (2010b) | |
| ill2 ilr1 iar3 ibr1, ill2 iar3 ibr3 | Short root hairs | Spiess et al. (2014) | |
| mes17 ill2 iar3, mes17 ibr3 | Mild short root hair phenotype | Spiess et al. (2014) | |
| Iar4 | Short root hairs; rescued by YUCCA1ox | Quint et al. (2009) | |
| YUCCA1ox | Long root hair phenotype | Zhao et al. (2001) | |
| yucca1 yucca2 yucca4 yucca6 | Hairy root hair phenotype | Chen and Xiong (2009) | |
| IBA transport | |||
| cdcg36 (pen3, pdr8) | Long root hairs | Strader et al. (2011) | |
| IAA perception | |||
| tir1, tir1 afb2 afb3 | Short root hairs | Dharmasiri et al. (2005) | |
| pEXP7a:TIR1 | Longer root hairs | Ganguly et al. (2010) | |
| aux/iaa1 (axr5) | No root hairs | Park et al. (2002) | |
| aux/iaa7 (axr2) | No/short root hairs | Wilson et al. (1990); Timpte et al. (1994) | |
| aux/iaa14 (slr) | No/short root hairs | Fukaki et al. (2002) | |
| aux/iaa17 (axr3) | Short root hairs | Leyser et al. (1996) | |
| axr1 | Short root hairs | Pitts et al. (1998) | |
| iaa28 | Short root hairs | Rogg et al. (2001) | |
| IAA nontranscriptional response | |||
| fer | Short root hairs | Duan et al. (2010) | |
| ropGEF4/ropGEF10 | Short root hairs and reduced root hair density for ropGEF10 | Huang et al. (2013) | |
| IAA transcriptional response | |||
| rsl4/RSL4ox | Short root hairs/long root hairs | Yi et al. (2010) |
ox, Overexpressor line.
| Gene Type . | Mutant/Transgenic Line . | Root Hair Phenotype . | Reference . |
|---|---|---|---|
| IAA transport | |||
| pin2 | Short root hairs | Cho et al. (2007a); Rigas et al. (2013) | |
| pEXP7a:PIN3ox | Long root hairs | Lee and Cho (2006) | |
| pEXP7a:PIN1/PIN2/PIN3/PIN7/PIN8 | Short root hairs | Ganguly et al. (2010) | |
| pEXP7a:PIN5 | Long root hairs | Ganguly et al.( 2010) | |
| pgp4 (abcb4) | Long root hairs | Santelia et al. (2005); Cho et al. (2007b, 2012) | |
| PGP4ox | Short root hairs | Cho et al. (2007b) | |
| aux1-7, aux1-T, aux1-22 | Short root hairs | Pitts et al. (1998); Ikeda et al. (2009); Jones et al. (2009) | |
| AUX1ox | Long root hairs | Ganguly et al. (2010) | |
| pEXP7a:PID | Short and fewer root hairs | Lee and Cho (2006) | |
| IAA ER storage | |||
| pils2 pils5 | Long root hairs | Barbez et al. (2012) | |
| PILS5-GFPox, EXP7a:PILS5-GFP | Short root hairs | Barbez et al. (2012) | |
| IAA metabolism | |||
| ibr1 ibr3 ibr10, ilr1 iar3 ill2 | Short root hairs | Davies et al. (1999); LeClere et al. (2002); Zolman et al. (2008); Strader et al. (2010b) | |
| echo2 ibr1ibr3 ibr10 | No root hairs | Strader et al. (2010b) | |
| ill2 ilr1 iar3 ibr1, ill2 iar3 ibr3 | Short root hairs | Spiess et al. (2014) | |
| mes17 ill2 iar3, mes17 ibr3 | Mild short root hair phenotype | Spiess et al. (2014) | |
| Iar4 | Short root hairs; rescued by YUCCA1ox | Quint et al. (2009) | |
| YUCCA1ox | Long root hair phenotype | Zhao et al. (2001) | |
| yucca1 yucca2 yucca4 yucca6 | Hairy root hair phenotype | Chen and Xiong (2009) | |
| IBA transport | |||
| cdcg36 (pen3, pdr8) | Long root hairs | Strader et al. (2011) | |
| IAA perception | |||
| tir1, tir1 afb2 afb3 | Short root hairs | Dharmasiri et al. (2005) | |
| pEXP7a:TIR1 | Longer root hairs | Ganguly et al. (2010) | |
| aux/iaa1 (axr5) | No root hairs | Park et al. (2002) | |
| aux/iaa7 (axr2) | No/short root hairs | Wilson et al. (1990); Timpte et al. (1994) | |
| aux/iaa14 (slr) | No/short root hairs | Fukaki et al. (2002) | |
| aux/iaa17 (axr3) | Short root hairs | Leyser et al. (1996) | |
| axr1 | Short root hairs | Pitts et al. (1998) | |
| iaa28 | Short root hairs | Rogg et al. (2001) | |
| IAA nontranscriptional response | |||
| fer | Short root hairs | Duan et al. (2010) | |
| ropGEF4/ropGEF10 | Short root hairs and reduced root hair density for ropGEF10 | Huang et al. (2013) | |
| IAA transcriptional response | |||
| rsl4/RSL4ox | Short root hairs/long root hairs | Yi et al. (2010) |
| Gene Type . | Mutant/Transgenic Line . | Root Hair Phenotype . | Reference . |
|---|---|---|---|
| IAA transport | |||
| pin2 | Short root hairs | Cho et al. (2007a); Rigas et al. (2013) | |
| pEXP7a:PIN3ox | Long root hairs | Lee and Cho (2006) | |
| pEXP7a:PIN1/PIN2/PIN3/PIN7/PIN8 | Short root hairs | Ganguly et al. (2010) | |
| pEXP7a:PIN5 | Long root hairs | Ganguly et al.( 2010) | |
| pgp4 (abcb4) | Long root hairs | Santelia et al. (2005); Cho et al. (2007b, 2012) | |
| PGP4ox | Short root hairs | Cho et al. (2007b) | |
| aux1-7, aux1-T, aux1-22 | Short root hairs | Pitts et al. (1998); Ikeda et al. (2009); Jones et al. (2009) | |
| AUX1ox | Long root hairs | Ganguly et al. (2010) | |
| pEXP7a:PID | Short and fewer root hairs | Lee and Cho (2006) | |
| IAA ER storage | |||
| pils2 pils5 | Long root hairs | Barbez et al. (2012) | |
| PILS5-GFPox, EXP7a:PILS5-GFP | Short root hairs | Barbez et al. (2012) | |
| IAA metabolism | |||
| ibr1 ibr3 ibr10, ilr1 iar3 ill2 | Short root hairs | Davies et al. (1999); LeClere et al. (2002); Zolman et al. (2008); Strader et al. (2010b) | |
| echo2 ibr1ibr3 ibr10 | No root hairs | Strader et al. (2010b) | |
| ill2 ilr1 iar3 ibr1, ill2 iar3 ibr3 | Short root hairs | Spiess et al. (2014) | |
| mes17 ill2 iar3, mes17 ibr3 | Mild short root hair phenotype | Spiess et al. (2014) | |
| Iar4 | Short root hairs; rescued by YUCCA1ox | Quint et al. (2009) | |
| YUCCA1ox | Long root hair phenotype | Zhao et al. (2001) | |
| yucca1 yucca2 yucca4 yucca6 | Hairy root hair phenotype | Chen and Xiong (2009) | |
| IBA transport | |||
| cdcg36 (pen3, pdr8) | Long root hairs | Strader et al. (2011) | |
| IAA perception | |||
| tir1, tir1 afb2 afb3 | Short root hairs | Dharmasiri et al. (2005) | |
| pEXP7a:TIR1 | Longer root hairs | Ganguly et al. (2010) | |
| aux/iaa1 (axr5) | No root hairs | Park et al. (2002) | |
| aux/iaa7 (axr2) | No/short root hairs | Wilson et al. (1990); Timpte et al. (1994) | |
| aux/iaa14 (slr) | No/short root hairs | Fukaki et al. (2002) | |
| aux/iaa17 (axr3) | Short root hairs | Leyser et al. (1996) | |
| axr1 | Short root hairs | Pitts et al. (1998) | |
| iaa28 | Short root hairs | Rogg et al. (2001) | |
| IAA nontranscriptional response | |||
| fer | Short root hairs | Duan et al. (2010) | |
| ropGEF4/ropGEF10 | Short root hairs and reduced root hair density for ropGEF10 | Huang et al. (2013) | |
| IAA transcriptional response | |||
| rsl4/RSL4ox | Short root hairs/long root hairs | Yi et al. (2010) |
AUXIN BIOSYNTHESIS AND METABOLISM CONTROL ROOT HAIR ELONGATION
More than 40 years ago, the phytohormone auxin was posited as regulating root hair development in pea (Pisum sativum; Gaither, 1975). Later, in the 1990s, clear genetic and pharmacological evidence pinpointed the important role of the phytohormones auxin and ethylene in the formation and elongation of root hairs in Arabidopsis (Masucci and Schiefelbein, 1994, 1996; Tanimoto et al., 1995; Pitts et al., 1998). However, later work revealed that ethylene activates auxin biosynthesis, suggesting an indirect impact of ethylene on root hair development (Růžička et al., 2007; Swarup et al., 2007; Strader et al., 2010a). Additional evidence for its indirect involvement is the inability of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid to restore the lack of root hairs in seedlings where auxin biosynthesis is blocked (Soeno et al., 2010). These studies indicate auxin to be the main phytohormonal regulator in root hair elongation.
In agreement with these earlier findings, negative interference with nuclear auxin signaling imposes reduced root hair formation, whereas the root hair-specific elevation of auxin signaling enhances root hair elongation (Takeda et al., 2008; Farquharson, 2014). Accordingly, seedlings overexpressing the auxin biosynthesis gene YUCCA1 display enhanced root hair growth compared with wild-type seedlings (Zhao et al., 2001), phenocopying the exogenous application of auxin (Wilmoth et al., 2005; Lee and Cho, 2006; Jones et al., 2009). On the contrary, pharmacological inhibition of auxin biosynthesis using l-amino-oxyphenylpropionic acid blocks the formation of root hairs, which can be rescued by the exogenous application of IAA (Soeno et al., 2010).
Apart from biosynthesis, other auxin metabolic processes play developmentally important roles in the regulation of root hair elongation. Besides the free, active form of auxin, a large proportion of cellular auxin is conjugated to other molecules, including sugars, amino acids, peptides, and proteins (for review, see Ludwig-Müller, 2011). Auxin conjugates appear either as auxin storage molecules, which can release auxin upon hydrolysis, or as intermediate products of the auxin degradation pathway (Ljung et al., 2002). Seedlings defective in IAA-ALANINE RESISTANT4 (IAR4), a gene involved in the regulation of cellular auxin homeostasis, contain enhanced levels of auxin amino acid conjugates and display reduced root hair growth (Quint et al., 2009). In rice (Oryza sativa), overexpression of the drought-induced auxin conjugase OsGH3-2 results in similarly reduced root hair growth (Du et al., 2012). By analogy, auxin conjugate hydrolase mutants display reduced root hair elongation (Strader et al., 2010b). These data suggest the developmental importance of auxin conjugation machinery for the defined control of root hair growth. Another endogenous auxinic compound, IBA, is, like auxin conjugates, presumably inactive and is synthesized directly from IAA by the fatty acid-like elongation of its side chain (Epstein and Ludwig‐Müller, 1993). IBA can undergo peroxisomal β-oxidation, resulting in its conversion to IAA (Zolman et al., 2007, 2008). IBA has been described as promoting root hair elongation (Strader and Bartel, 2009), but only in the presence of functional IBA-to-IAA conversion machinery. Mutants defective in the three enzymes required for IBA-to-IAA conversion (ibr1, ibr3, and ibr10) display impaired root hair elongation (Strader et al., 2010b). The requirement of strictly controlled auxin conjugation rates, functional auxin conjugate hydrolysis, as well as correct IBA conversion (Fig. 1A) emphasizes the importance of auxin metabolic processes for the regulation/fine-tuning of root hair tip growth. It is currently unclear if IBA-to-IAA conversion takes place directly in root hair cells to promote root hair elongation. Notably, local conversion of IBA to IAA in lateral root cap cells positions lateral root founder cells (De Rybel et al., 2012; Xuan et al., 2015). A similar mechanism could also impact root hair elongation in a non-cell-autonomous manner.
INTERCELLULAR AUXIN TRANSPORT MEDIATES ROOT HAIR ELONGATION
The well-defined auxin levels in plant tissues depend not only on local auxin metabolism but also on intercellular auxin transport (for review, see Sauer et al., 2013). Various auxin transporters regulate cellular auxin levels. The most prominent auxin carriers include the PIN-FORMED (PIN) and ATP-BINDING CASSETTE B (ABCB) auxin efflux carriers as well as the auxin influx carriers from the AUXIN RESISTANT1 (AUX1)/LIKE AUX1 (LAX) family (Fig. 1B).
The PIN protein family consists of eight members in Arabidopsis, most of which have been demonstrated to transport auxin either in plants or in heterologous systems (Petrásek et al., 2006; Mravec et al., 2009; Yang and Murphy, 2009). The polar plasma membrane localization of PIN auxin efflux carriers determines the direction of intercellular (polar) auxin transport and has been suspected of imposing auxin gradients within tissues (for review, see Wabnik et al., 2011). PIN2 is the only family member expressed in root epidermal cells and is present in both root and nonroot hair cells (Luschnig et al., 1998). Notably, PIN2 shows higher trafficking and turnover rates in trichoblast cells, leading to higher PIN2 abundance in atrichoblasts (Löfke et al., 2015). PIN2 displays a polar localization to the apical (shoot-ward) side of the epidermal cells and, thereby, mediates the basipetal (shoot-ward) direction of the auxin flow (Wiśniewska et al., 2006). Seedlings lacking PIN2 display shorter root hairs compared with wild-type seedlings, indicating the importance of the basipetal auxin flow in the epidermis for root hair development (Cho et al., 2007a; Rigas et al., 2013).
The most extensively studied subgroup members of the ABCB auxin transport family are ABCB1/PGP1, ABCB4/PGP4, and ABCB19/PGP19. These nonpolar plasma membrane transporters export auxin in planta and upon heterologous expression in mammalian cells (Geisler et al., 2005; Petrásek et al., 2006; Cho et al., 2007b). pgp4 loss-of-function mutants display longer root hairs compared with the wild type, while root hair cell-specific PGP4 overexpression results in shorter root hairs compared with the wild type (Santelia et al., 2005; Cho et al., 2007b). These findings suggest that PGP4-dependent auxin export may primarily decrease the auxin levels directly in root hair cells, resulting in local control of root hair growth (Cho et al., 2007b).
Members from the AUX1/LAX protein family also localize to the plasma membrane and mediate auxin uptake into the cell (Yang et al., 2006; Swarup and Péret, 2012). The H+/IAA symporter AUX1 was demonstrated to facilitate auxin influx in planta and in heterologous systems (Yang et al., 2006; Yang and Murphy, 2009). AUX1 overexpression enhances root hair growth, presumably due to the increased auxin levels in the root hair cells (Ganguly et al., 2010). Intriguingly, in the root epidermis of wild-type plants, AUX1 is expressed only in nonhair cells (Jones et al., 2009). However, loss of the epidermal function of AUX1 leads to lower root hair elongation, suggesting that auxin transport in nonhair cells sustains root hair development (Lee and Cho, 2006; Jones et al., 2009).
Auxin not only controls root hair expansion but also depicts its polar outgrowth close to the basal (root-ward) base of the cell, defined as planar polarity (Ikeda et al., 2009). In an elegant study, it has been shown that ethylene-dependent control of auxin biosynthesis in the root meristem affects root hair positioning and root hair length (Ikeda et al., 2009). Intercellular auxin transport, utilizing at least AUX1/LAX auxin influx and PIN2 auxin efflux carriers, is required to establish the planar polarity of root hairs. Therefore, the auxin carriers involved mediate the redistribution of auxin from its local biosynthesis maximum in the root meristem into the differentiation zone (Ikeda et al., 2009). Hence, it appears that the very same mechanism controlling planar polarity also controls root hair elongation.
It seems that multiple auxin transport and metabolism processes jointly contribute to root hair development. Such a complex regulation of cellular auxin levels in root hair cells allows for the flexible adaptation of auxin-dependent root hair length in response to a plethora of developmental and environmental signals.
INTRACELLULAR AUXIN TRANSPORT PROCESSES AT THE ENDOPLASMIC RETICULUM CONTRIBUTE TO ROOT HAIR ELONGATION
Based on the current literature, it can be concluded that auxin biosynthesis in the meristem and intercellular auxin transport, particularly in nonhair cells, determine the polar positioning and elongation rates of root hairs cells in the differentiation zone. Besides intercellular auxin transport, intracellular auxin transport processes appear to impact root hair elongation. Using an in silico-based screen, the novel PIN-LIKE (PILS) family of putative auxin carriers was identified (Barbez et al., 2012). PILS proteins are evolutionarily conserved in the plant lineage and affect intracellular auxin accumulation in plants and heterologously in yeast (Barbez et al., 2012; Feraru et al., 2012). PILS proteins localize to the endoplasmic reticulum, where they presumably transport auxin from the cytosol into the endoplasmic reticulum lumen (Barbez et al., 2012; Barbez and Kleine-Vehn, 2013), thereby reducing the nuclear auxin availability and, hence, genomic auxin signaling (Fig. 1C, module 2; Barbez et al., 2012).
PILS2 and PILS5 are expressed in the root epidermis and redundantly control root organ and root hair growth (Barbez et al., 2012). pils2 pils5 double mutants show longer root hairs, whereas PILS5 overexpression reduces root hair expansion (Barbez et al., 2012). In response to exogenously applied auxin, PILS loss and gain of function lead to hypersensitive and resistant root hair elongation, respectively, suggesting that PILS activity determines cellular sensitivity to auxin (Barbez et al., 2012). Hence, not only auxin production/decay and its intercellular distribution, but also its intracellular compartmentalization, appear central to auxin-dependent cellular growth regulation.
Notably, auxin-sensitive root hair growth was also utilized in an elegant bioassay to indirectly illustrate auxin carrier activity (Lee and Cho, 2006; Ganguly et al., 2010). In this approach, root hair-specific expression of auxin carriers in the differentiation zone was used to modulate root hair elongation. The root hair-specific expression of putative auxin carriers, such as PILS1/3/5, and auxin efflux carriers, such as PIN1/2/3/7/8 or ABCB4, reduced root hair length (Dharmasiri et al., 2005; Barbez et al., 2012), which is consistent with their negative impact on nuclear auxin signaling and/or cellular auxin content. Moreover, these findings illustrate that the actual auxin levels in mature root hair cells indeed control root hair elongation. PILS proteins are also expressed in mature root hair cells, possibly enabling these cells to modulate auxin-dependent elongation, and in part are independent of the auxin signals derived from the root meristem.
AUXIN TRANSCRIPTIONAL RESPONSES IN ROOT HAIR CELLS
Auxin impacts plant cells via fast nongenomic and slower genomic mechanisms (for review, see Badescu and Napier, 2006). Auxin induces a rapid, nongenomic physiological response, leading to membrane hyperpolarization, and protoplast swelling (Sauer and Kleine-Vehn, 2011). These and other fast auxin responses have been associated with AUXIN-BINDING PROTEIN1 (ABP1), which has been described as a putative auxin receptor (for review, see Sauer and Kleine-Vehn, 2011). However, recent data are currently questioning the developmental importance of ABP1 in Arabidopsis (Gao et al., 2015; Tena, 2015).
The slow genomic auxin response is mediated by the receptors of the TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX (TIR1/AFB) family (Dharmasiri et al., 2005; Kepinsky and Leyser, 2005). Auxin binding on TIR1/AFB and its coreceptor AUXIN RESISTANT/INDOLE-3-ACETIC ACID INDUCIBLE (AUX/IAA) induces the proteasome-dependent degradation of the latter. AUX/IAA depletion results in the subsequent release of ARF TFs, which modify the auxin-dependent expression profile. Seedlings displaying root hair-specific TIR1 overexpression (Ganguly et al., 2010) and tir1 afb1 afb3 mutants (Dharmasiri et al., 2005) showed opposite phenotypes (longer and shorter root hairs, respectively), suggesting that TIR1-dependent signaling steers specific root hair genes. In agreement, other mutants in SCFTIR1 complex components, such as axr1, display a short root hair phenotype (Lincoln et al., 1990; Pitts et al., 1998). AXR1 has been described to encode for the N terminus of the ubiquitin-activating enzyme E1 (Leyser et al., 1993) and to interact with ECR1, which is required for the SCFTIR1 complex (del Pozo et al., 2002). Suppressors of the axr1 mutation (SAR) restore the impaired root hair phenotype (Cernac et al., 1997). SAR1 and SAR3 have been described as nucleoporins and may have specific roles in protein import and/or mRNA export, as evidenced by the lack of nuclear localization of AXR3 (Parry et al., 2006).
In Arabidopsis, gain-of-function mutants of AUX/IAAs, such as IAA7 (AXR2; Lee and Cho, 2006), IAA17 (AXR3; Knox et al., 2003), and IAA28 (Rogg et al., 2001), showed reduced growth in root hair cells, highlighting that ARF activation is required to allow root hair tip growth (Fig. 1C, module 1). These studies suggest that auxin needs to be sensed in situ in the root hair cells to trigger cell expansion. However, it cannot be excluded that a non-cell-autonomous contribution of auxin signaling may affect root hair development as well.
Auxin-dependent root hair expansion also appears to integrate external signals. Auxin sensitivity is enhanced in inorganic phosphate-deprived plants by an increased expression of TIR1, which consequently enhances the degradation of AUX/IAA proteins. Inorganic phosphate deficiency similarly impacts lateral root development, which depends on TIR1 through the action of ARF7/ARF19 (Wilmoth et al., 2005; Okushima et al., 2007). A gain-of-function mutant of IAA14 that makes it insensitive to SCFTIR1-mediated degradation, srl1, showed impaired lateral root and root hair formation (Fukaki et al., 2002). A later study suggested that IAA14 could be the direct repressor of ARF7 and ARF19 (Fukaki et al., 2005). In agreement, ARF7 and ARF19 are the most abundant ARFs expressed in root hair cells (Bargmann et al., 2013); therefore, a similar AUX/IAA-ARF pathway could be hypothesized to control and trigger cell expansion in root hairs. Downstream of ARFs, auxin treatments promote root hair growth by positively regulating the expression of several TFs, such as RSL4 (Yi et al., 2010), RSL3 and RSL5 (Pires et al., 2013), and LRL3 (Karas et al., 2009). It is still unclear if, upon auxin-reliant derepression, ARF7/ARF19 would directly enhance the expression of these TFs or if there are intermediate TFs linking both elements in the auxin-mediated response. Intriguingly, these root hair growth-determining TFs are hierarchically linked. RSL4 promotes the expression of RSL2, RSL3, and RSL5, while RHD6-RSL1 appears to be the master regulator of all of them (Pires et al., 2013). In addition, there are also other TFs, such as LRL3, involved in root hair growth (Karas et al., 2009) that are responsive to auxin (Tam et al., 2015), possibly independently of RHD6-RSL1, highlighting a complex scenario of the auxin-mediated response at the transcriptional level (Fig. 1C, module 1).
AUXIN SIGNALING TARGETS THAT TRIGGER CELL EXPANSION
Based on the acid growth theory (Rayle and Cleland, 1970), auxin stimulates cells to pump protons into the apoplast (by the activity of AHA), where the acidic pH activates cell wall-modifying enzymes and hyperpolarizes the plasma membrane, ultimately loosening the cell wall and promoting the turgor pressure needed to drive cell expansion. Among the genes most rapidly induced by auxin are several member of the SAUR gene family (Ren and Gray, 2015). Several SAUR proteins, such as the SAUR19 to SAUR24 group, promote cell expansion (Farquharson, 2014; Spartz et al., 2014). Recently, a robust mechanism was proposed where auxin promotes the activation of AHA1/AHA2 by the activity of SAUR19. More precisely, SAUR19 interacts with and inhibits the activity of PP2C-D1. This phosphatase negatively regulates AHA1 activity by dephosphorylating its C-terminal domain (autoinhibitory domain), allowing the binding of 14-3-3 proteins to AHA1. In this model, SAUR and PP2C-D1 act antagonistically to control cell expansion. So far, this mechanism has been proposed for dark-grown hypocotyls (Spartz et al., 2014) but has not yet been validated for growing root hairs. Several SAUR genes are highly expressed in root hairs (e.g. SAUR35, SAUR37, and SAUR38; Bargmann et al., 2013) and, hence, could possibly activate plasma membrane AHAs (Fig. 1C, module 3). In agreement, overexpressors of GsGF14o (a Glycine soja 14-3-3 protein) induce shorter root hairs in Arabidopsis (Sun et al., 2014), but it was not elucidated whether this affects AHA activity. Other proteins also modify AHA protein activity by phosphorylation (e.g. the SALT OVERLY SENSITIVE2-like protein PKS5 [CIPK11]; Fuglsang et al., 2007).
Several receptor-like kinases are also able to activate or repress AHA2 in a ligand-dependent manner by modifying the C-terminal phosphorylation status of AHAs (Fuglsang et al., 2014; Haruta et al., 2014). In the case of the plant PEPTIDE-CONTAINING SULFATED TYROSINE RECEPTOR1 (PSYR1), the binding to the peptide PSY1 (Amano et al., 2007) enhances AHA2 activity via its phosphorylation of Thr-881, consequently triggering cell elongation (Fuglsang et al., 2014). On the other hand, in the case of the receptor-like kinase FER, the binding of RALF1 inhibits root growth via the phosphorylation of Ser-899 in AHA2 by an unknown kinase (Haruta et al., 2014). Such opposite effects on the same molecular target suggest that AHAs are central integrators of external and internal signals, enabling plant cells to respond immediately to fluctuating conditions. In addition, it was shown that FER forms a complex with the guanine nucleotide exchange factor ROP-GEF1 (and possibly others, such as ROP-GEF4 or ROP-GEF10) to recruit and activate the plant RHO GTPase ROP2. Spatially active ROP2 triggers ROS responses and consequently enhances tip growth (Duan et al., 2010). The active form of ROP is able to bind directly to the N terminus of an NADPH oxidase known as RBOH (for respiratory burst oxidase homolog protein) and enhance its enzymatic activity (Oda et al., 2010; Takahashi et al., 2012). In root hairs, RBOHC is the main ROS-producing enzyme and, possibly, the ROP2 target to trigger ROS-mediated root hair growth (Foreman et al., 2003; Takeda et al., 2008). In fer mutant seedlings, the levels of active ROPs (and ROS) are reduced. Interestingly, fer mutants with very short root hairs do not respond to auxin. High levels of ROP2 are able to rescue the fer phenotype, but they cannot restore auxin responses (Duan et al., 2010; Huang et al., 2013). These data suggest that FER is required for auxin-induced root hair growth and that it functions upstream of ROP signaling to trigger root hair cell expansion (Fig. 1C, module 3). In addition, rop-gef4,10 double mutants are still responsive to auxin and other environmental conditions, suggesting that alternative pathways may exist downstream of FER (Huang et al., 2013) to trigger cell expansion.
Auxin also impacts the shape of the biggest plant organelle, the vacuole (Löfke et al., 2015). High doses of auxin induce smaller luminal vacuoles, which are required for the negative impact of auxin on cellular elongation (Löfke et al., 2015). This growth mechanism appears to rely on the vacuolar occupancy of the cell (Scheuring et al., 2016), suggesting that the vacuole has a space-filling function during cellular growth (Dünser and Kleine-Vehn, 2015; Löfke et al., 2015). Vacuolar morphogenesis is also highly dynamic in tip-growing cells (Hicks et al., 2004). Intriguingly, processes that are required for auxin-dependent vacuolar morphogenesis, including vacuolar SNARES, phosphatidylinositol 4-kinases (Löfke et al., 2015), as well as actin and myosins (Scheuring et al., 2016), are also implicated in root hair expansion (Ringli et al., 2002; Preuss et al., 2006; Park and Nebenführ, 2013; Larson et al., 2014). Hence, it is tempting to speculate that vacuoles may also be important cellular effectors contributing to root hair expansion.
CONCLUSION
Root hair cells are highly important for the uptake of nutrients and water from the surroundings. However, under laboratory conditions, root hair development is not essential for plant development. Therefore, root hair development is highly pertinent for genetic studies with potential agronomic implications. Moreover, the root epidermis is easily accessible for highly sophisticated imaging approaches, such as superresolution microscopy and time-lapse imaging (Kleine-Vehn et al., 2010), and allows the application of various systems biology methodologies (Libault et al., 2010). Besides its outstanding contribution to tissue patterning (Schiefelbein et al., 2009; Wang et al., 2010; Bruex et al., 2012), the root epidermis has been singled out as a suitable cellular model system for essential cellular processes, such as cellular polarization, polar tip growth, cellular expansion, cell wall remodeling, and spatially defined vesicle trafficking (Velasquez et al., 2011; Ischebeck et al., 2013; Löfke et al., 2013, 2015; Ichikawa et al., 2014; Kiefer et al., 2015). Naturally, all these processes jointly contribute to root hair performance, enabling the surface definition of the root-soil interface. The phytohormone auxin has been shown to contribute to all of these processes; accordingly, root hair cells are a decidedly proper model system in which to further advance our knowledge of auxin (signaling, metabolism, and transport) and its contribution to the cellular processes that guide growth. It is currently germane to stitch certain insights together and learn more about the downstream targets of ARFs that control the regulation of growth. Similarly, the link between auxin and cell wall modifications is apparent, but most auxin-dependent cell wall effectors remain to be unraveled. Another unsolved question is why root hair cells show auxin-induced tip growth in the differentiation zone but auxin-repressed epidermal tissue expansion in the elongation zone. Accordingly, root hair cells undergo a sensitivity switch, whose underlying mechanism remains, for the time being, completely concealed. Our review urges a stronger focus on root hairs as an appropriate cellular system in which to generate such fundamental insights into the auxin-dependent regulation of cellular expansion.
Glossary
LITERATURE CITED
Author notes
This work was supported by ANPCyT (grant nos. PICT2011–054, PICT2013–003, and PICT2014–0504 to J.M.E), the Vienna Science and Technology Fund (Vienna Research Group grant to J.K.-V.), the Austrian Science Fund (grant nos. P26568–B16 and P26591–B16 to J.K.-V.), and the European Research Council (grant no. 639478–AuxinER to J.K.-V.)
These authors contributed equally to the article.
Present address: Gregor Mendel Institute of Molecular Plant Biology, Dr.-Bohr-Gasse 3, 1030 Vienna, Austria.
Address correspondence to [email protected] and [email protected].
J.K.-V. conceived the project and wrote the article with contributions of all the authors; J.M.E conceived the project and wrote the article with contributions of all the authors; S.M.V. provided technical assistance and wrote the article; E.B. provided technical assistance and wrote the article.



