-
PDF
- Split View
-
Views
-
Cite
Cite
Mohammad Saidur Rhaman, Toshiyuki Nakamura, Yoshimasa Nakamura, Shintaro Munemasa, Yoshiyuki Murata, The Myrosinases TGG1 and TGG2 Function Redundantly in Reactive Carbonyl Species Signaling in Arabidopsis Guard Cells, Plant and Cell Physiology, Volume 61, Issue 5, May 2020, Pages 967–977, https://doi.org/10.1093/pcp/pcaa024
Close - Share Icon Share
Abstract
Myrosinase (β-thioglucoside glucohydrolase, enzyme nomenclature, EC 3.2.1.147, TGG) is a highly abundant protein in Arabidopsis guard cells, of which TGG1 and TGG2 function redundantly in abscisic acid (ABA)- and methyl jasmonate-induced stomatal closure. Reactive carbonyl species (RCS) are α,β-unsaturated aldehydes and ketones, which function downstream of reactive oxygen species (ROS) production in the ABA signalling pathway in guard cells. Among the RCS, acrolein is the most highly reactive, which is significantly produced in ABA-treated guard cells. To clarify the ABA signal pathway downstream of ROS production, we investigated the responses of tgg mutants (tgg1-3, tgg2-1 and tgg1-3 tgg2-1) to acrolein. Acrolein induced stomatal closure and triggered cytosolic alkalization in wild type (WT), tgg1-3 single mutants and in tgg2-1 single mutants, but not in tgg1-3 tgg2-1 double mutants. Exogenous Ca2+ induced stomatal closure and cytosolic alkalization not only in WT but also in all of the mutants. Acrolein- and Ca2+-induced stomatal closures were inhibited by an intracellular acidifying agent, butyrate, a Ca2+ chelator, ethylene glycol tetraacetic acid (EGTA) and a Ca2+ channel blocker, LaCl3. Acrolein induced cytosolic free calcium concentration ([Ca2+]cyt) elevation in guard cells of WT plants but not in the tgg1-3 tgg2-1 double mutants. Exogenous Ca2+ elicited [Ca2+]cyt elevation in guard cells of WT and tgg1-3 tgg2-1. Our results suggest that TGG1 and TGG2 function redundantly, not between ROS production and RCS production, but downstream of RCS production in the ABA signal pathway in Arabidopsis guard cells.
Introduction
Stomatal pores, which are surrounded by pairs of specialized guard cells, serve as major gateways for regulating gas exchange and transpirational water loss. Guard cells respond to a variety of biotic and abiotic stimuli, such as plant hormones, light, drought and pathogen attack (Shimazaki et al. 2007, Murata et al. 2015, Ye et al. 2015). The phytohormone abscisic acid (ABA) induces stomatal closure, which reduces transpirational water loss (Schroeder et al. 2001, Munemasa et al. 2015).
ABA- and methyl jasmonate (MeJA)-induced stomatal closures are accompanied by cytosolic alkalization and cytosolic free calcium concentration ([Ca2+]cyt) elevation in Arabidopsis guard cells (Islam et al. 2010). In Arabidopsis thaliana, ABA-induced reactive oxygen species (ROS) production is catalyzed by the plasma membrane NADPH oxidases AtrbohD and AtrbohF (Murata et al. 2001, Kwak et al. 2003). ABA-induced ROS production plays a vital role in the activation of Ca2+-permeable cation (ICa) channels in the plasma membrane of guard cells (Pei et al. 2000, Murata et al. 2001, Kwak et al. 2003).
ROS are constitutively produced in plants under a variety of stresses, and the accumulating ROS can oxidize lipids, especially polyunsaturated fatty acids. Oxidized lipids decompose or degrade spontaneously to form various aldehydes and ketones (Mano 2012, Farmer and Mueller 2013). Alpha,beta-unsaturated carbonyls, such as acrolein and 4-hydroxy-E-2-nonenal, are termed as reactive carbonyl species (RCS) because of their high reactivity and high cytotoxicity (Mano 2012). In plants, there is a positive correlation between RCS accumulation and damage under stress conditions (Mano et al. 2010, Yin et al. 2010). RCS act as downstream components of ROS in the nuclear factor-κB-induced inflammatory response in animal cells (Yadav and Ramana 2013). RCS function downstream of ROS production in the ABA signaling pathway in guard cells using transgenic tobacco (Nicotiana tabacum) plants overexpressing the RCS-scavenging enzyme, 2-alkenal reductase (Islam et al. 2016). RCS act as signal mediators downstream of ROS production and regulate [Ca2+]cyt elevation in the ABA signaling pathway in Arabidopsis guard cells (Islam et al. 2019).
Cytosolic alkalization in guard cells is a common phenomenon in both ABA- and MeJA-induced stomatal closure in A. thaliana, Pisum sativum, Paphiopedilum supersuk (Royal Horticulture Society, 1973) and Paphiopedilum tonsum (Rchb.f) Stein (Irving et al. 1992, Gehring et al.1997, Suhita et al. 2004, Gonugunta et al. 2008, Islam et al. 2010). In Vicia faba guard cells, cytosolic alkalization activates outward K+ currents and inactivates inward K+ currents to promote the net efflux of K+ (Blatt and Armstrong 1993). These results suggest that cytosolic pH plays a role as an important regulator of stomatal movements.
Myrosinase (EC 3.2.1.147) is the β-thioglucoside glucohydrolase (TGG) enzyme abundantly present in crucifer plants and catalyzes the degradation of glucosinolates to produce isothiocyanates and so on. The glucosinolate–myrosinase system plays a major chemical defense mechanism in plants against bacteria, insects and herbivores (Bones and Rossiter 1996, Wittstock and Halkier 2002, Barth and Jander 2006). In the Arabidopsis genome, six myrosinase (TGG) genes have been identified and two functional myrosinase genes, TGG1 and TGG2, have been found (Xue et al. 1995, Husebye et al. 2002, Barth and Jander 2006). TGG1 is a strikingly abundant guard cell protein (Zhao et al. 2008). TGG1 is significantly expressed in guard cells and phloem cells, while TGG2 is strongly expressed in phloem associated cells (Husebye et al. 2002, Barth and Jander 2006). TGG3 is a pseudogene that is expressed in stamens and petals (Zhang et al. 2002). TGG4 and TGG5 are expressed in root tissues, and TGG6 is expressed in flowers, specifically in the stamens (Toufighi et al. 2005). Islam et al. (2009) showed that the myrosinases, TGG1 and TGG2, redundantly function downstream of ROS production and upstream of cytosolic Ca2+ elevation in ABA and MeJA signaling in Arabidopsis guard cells. However, to our knowledge, there is no report on whether the myrosinases, TGG1 and TGG2, function downstream or upstream of RCS production in ABA signaling in Arabidopsis guard cells. Therefore, we investigated stomatal movement, cytosolic alkalization and [Ca2+]cyt elevation in guard cells of the tgg1-3, tgg2-1 and tgg1-3 tgg2-1 mutants in response to RCS.
Results
TGG1 and TGG2 are involved in acrolein-induced stomatal closure
Acrolein inhibits light-induced stomatal opening (Islam et al. 2015) and induces stomatal closure in Arabidopsis (Islam et al. 2019). To clarify the involvement of TGG1 and TGG2 in the RCS-induced stomatal closure in Arabidopsis guard cells, we examined stomatal responses to acrolein of tgg1-3, tgg2-1 and tgg1-3 tgg2-1 mutants. Application of 10 µM acrolein significantly induced stomatal closure in WT (P < 0.03) in agreement with the previous result (Islam et al. 2019). Acrolein at 10 µM significantly induced stomatal closure in the tgg1-3 mutant (P < 0.02) and tgg2-1 mutant (P < 0.001) but not in the tgg1-3 tgg2-1 double mutant (P > 0.79) (Fig. 1A). These results suggest that TGG1 and TGG2 are redundantly involved in RCS-induced stomatal closure.
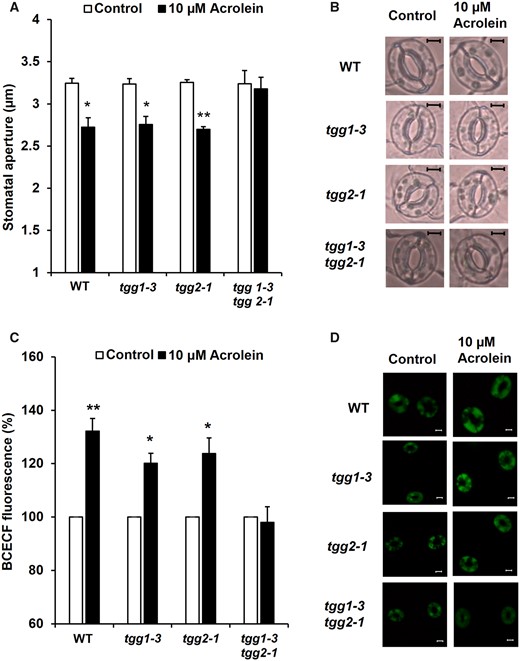
Effects of acrolein on stomatal aperture and cytosolic alkalization in Arabidopsis guard cells. (A) Acrolein-induced stomatal closure in WT and tgg mutants, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. (B) Representative images of the epidermal tissues containing guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Excised rosette leaves were treated with acrolein for 2 h. Averages from three (n = 3) independent experiments (60 total stomata per bar) are shown. Scale bar = 5 µm. (C) Effects of acrolein on BCECF fluorescence in guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. The vertical axis shows the percentage of BCECF fluorescence when fluorescent intensities of acrolein-treated cells are normalized to the control value taken as 100% for each experiment. Each data was obtained from at least 90 guard cells. (D) Representative BCECF fluorescence images of the epidermal tissues containing guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Scale bar = 20 µm. The error bar represents the standard error. Differences between treatments were analyzed by Student’s t-test: *P < 0.05 and **P < 0.01.
Acrolein-induced cytosolic alkalization in Arabidopsis guard cells
Both ABA and MeJA induce cytosolic alkalization, which leads to induced stomatal closure (Irving et al. 1992, Gehring et al. 1997, Suhita et al. 2004, Gonugunta et al. 2008, Islam et al. 2010). To examine the involvement of TGG1 and TGG2 in RCS signaling in Arabidopsis guard cells, we monitored cytosolic pH in guard cells of Arabidopsis tgg1-3, tgg2-1 and tgg1-3 tgg2-1 mutants using a pH-sensitive fluorescent dye, 2,7-bis-(2-carboxyethyl)-5,(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) (Islam et al. 2010).
Acrolein at 10 µM significantly increased BCECF fluorescence intensity in the WT guard cells (P < 0.002) (Fig. 1C), indicating that acrolein increases cytosolic pH in WT plants. Acrolein at 10 µM also increased guard cell BCECF fluorescence intensity in both tgg1-3 (P < 0.01) and tgg2-1 (P < 0.02) but not in tgg1-3 tgg2-1 (P > 0.74) (Fig. 1C), which is consistent with the results of stomatal response to acrolein (Fig. 1A). These results indicate that RCS-induced stomatal closure is accompanied by cytosolic alkalization in guard cells and suggests that the myrosinases, TGG1 and TGG2, function upstream of cytosolic alkalization in ABA signaling.
Ca2+-induced stomatal closure in Arabidopsis guard cells
We investigated the effects of exogenous Ca2+ on stomatal apertures of tgg1-3, tgg2-1 and tgg1-3 tgg2-1 mutants. Application of 1 mM CaCl2 significantly induced stomatal closure in all mutants (tgg1-3: P < 0.002; tgg2-1: P < 0.001; tgg1-3 tgg2-1: P < 0.01) as well as in WT (P < 0.0004) (Fig. 2A), which is consistent with previous results (Islam et al. 2009).
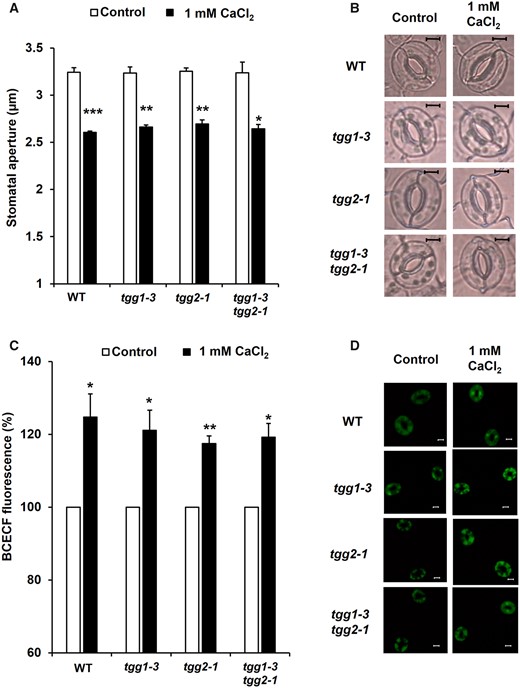
Exogenous Ca2+-induced stomatal closure and cytosolic alkalization in Arabidopsis guard cells. (A) Exogenous Ca2+ at 1 mM reduces stomatal aperture in WT and tgg mutants, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. (B) Representative images of the epidermal tissues containing guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Averages from three (n = 3) independent experiments (60 total stomata per bar) are shown. Scale bar = 5 µm. (C) Effects of exogenous Ca2+ at 1 mM on BCECF fluorescence in guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. The vertical axis shows the percentage of BCECF fluorescence when fluorescent intensities of Ca2+-treated cells are normalized to the control value taken as 100% for each experiment. Each data was obtained from at least 90 guard cells. (D) Representative BCECF fluorescence images of the epidermal tissues containing guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Scale bar = 20 µm. The error bar represents standard error. Differences between treatments were analyzed by Student’s t-test: *P < 0.05, **P < 0.01 and ***P < 0.001.
Ca2+-induced cytosolic alkalization in Arabidopsis guard cells
To analyze the role of TGG on Ca2+-induced cytosolic alkalization in guard cells, we examined cytosolic pH in WT and in the tgg mutants. Exogenous CaCl2 at 1 mM significantly increased BCECF fluorescence intensity in guard cells of tgg1-3 (P < 0.03), tgg2-1 (P < 0.002) and tgg1-3 tgg2-1 (P < 0.01) mutants as well as in WT (P < 0.03) (Fig. 2C). These results are consistent with the results that Ca2+ induced stomatal closure in WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1 mutants (Fig. 2A). These results indicate that exogenous Ca2+ triggers cytosolic alkalization in WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1 mutants and induces stomatal closure.
Acrolein- and Ca2+-induced stomatal closure were repressed by EGTA and LaCl3
We examined the effects of EGTA (Ca2+ chelator) and LaCl3 (Ca2+ channel blocker) on acrolein- and Ca2+-induced stomatal closure. The acrolein-induced stomatal closure was significantly inhibited by EGTA and LaCl3 in WT (EGTA: P < 0.02; LaCl3: P < 0.01), tgg1-3 (EGTA: P < 0.02; LaCl3: P < 0.03) and tgg2-1 (EGTA: P < 0.002; LaCl3: P < 0.002) mutants (Fig. 3A, C, E). Acrolein did not induce stomatal closure in the tgg1-3 tgg2-1 double mutant in the presence or absence of EGTA and LaCl3 (EGTA: P < 0.92; LaCl3: P < 0.98) (Fig. 3G). By contrast, the Ca2+-induced stomatal closure was also significantly suppressed by EGTA and LaCl3 in WT (EGTA: P < 0.002; LaCl3: P < 0.009) and all mutants (EGTA: P < 0.003; LaCl3: P < 0.0007 in tgg1-3, EGTA: P < 0.003; LaCl3: P < 0.0006 in tgg2-1, EGTA: P < 0.002; LaCl3: P < 0.007 in tgg1-3 tgg2-1) (Fig. 3A, C, E, G). These results suggest that [Ca2+]cyt functions downstream of myrosinase in the ABA signal pathway in guard cells and that the myrosinases, TGG1 and TGG2, function redundantly between the RCS production and [Ca2+]cyt elevation stages.
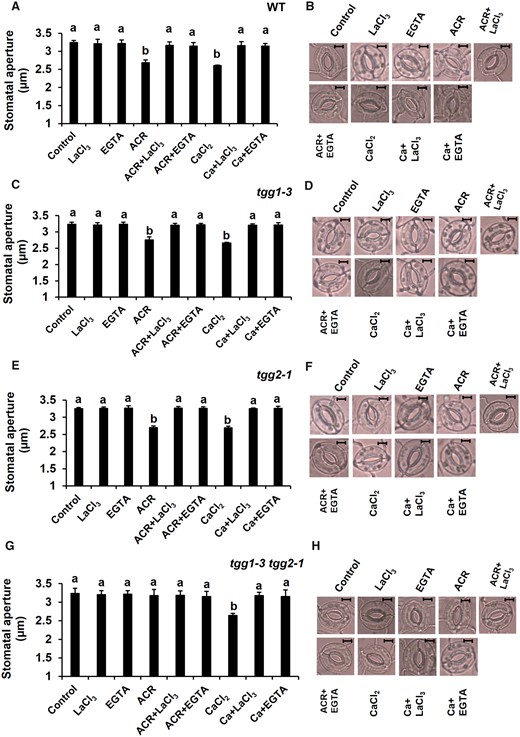
Effects of EGTA (Ca2+ chelator) and LaCl3 (Ca2+ channel blocker) on acrolein (ACR)- and Ca2+ (CaCl2)-induced stomatal closure. (A, C, E, G) Effects of EGTA and LaCl3 on ACR- and Ca2+-induced stomatal closure in WT and tgg mutants, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Excised rosette leaves were incubated for 2 h in light. Light-treated leaves were further treated with ACR and Ca2+. EGTA and LaCl3 were applied 30 min before the application of ACR and Ca2+. Averages from three (n = 3) independent experiments (60 total stomata per bar) are shown. (B, D, F, H) Representative images of the epidermal tissues containing guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Scale bar = 5 µm. The error bar represents standard error. Differences among treatments were analyzed by Tukey’s test: P < 0.05.
Effects of butyrate on acrolein- and Ca2+-induced stomatal closure
We examined the effects of butyrate on acrolein- and Ca2+-induced stomatal closure to clarify whether they required cytosolic alkalization. Acrolein-induced stomatal closure was significantly inhibited by butyrate in WT (P < 0.002), tgg1-3 (P < 0.02) and tgg2-1 (P < 0.003) mutants (Fig. 4A, C, E). Acrolein did not induce stomatal closure in the tgg1-3 tgg2-1 (P < 0.89) double mutant in the presence or absence of butyrate (Fig. 4G). The Ca2+-induced stomatal closure was also significantly inhibited by butyrate in WT (P < 0.003) and in all mutants (tgg1-3: P < 0.003; tgg2-1: P < 0.01; tgg1-3 tgg2-1: P < 0.0003) (Fig. 4A, C, E, G). Application of butyrate did not affect stomatal apertures of either WT or mutants untreated with acrolein or Ca2+. These results indicate that the acrolein- and Ca2+-induced stomatal closure are mediated by cytosolic alkalization. Taken together with other results in this study, it appears that cytosolic alkalization is the downstream component of [Ca2+]cyt elevation in ABA-induced stomatal closure and that the myrosinases, TGG1 and TGG2, function redundantly between the RCS production, [Ca2+]cyt elevation and cytosolic alkalization stages in the ABA signaling pathway.
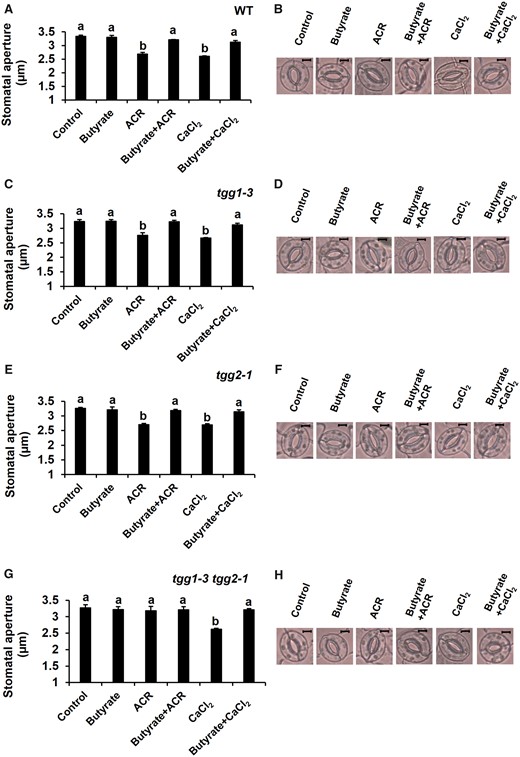
Effects of butyrate on the inhibition of acrolein (ACR)- and Ca2+-induced stomatal closure. (A, C, E, G) Effects of butyrate on ACR- and Ca2+-induced stomatal closure in WT and tgg mutants, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Excised rosette leaves were incubated for 2 h in light. Light-treated leaves were further treated with ACR and Ca2+. Butyrate was applied 30 min before the application of ACR and Ca2+. Averages from three (n = 3) independent experiments (60 total stomata per bar) are shown. (B, D, F, H) Representative images of the epidermal tissues containing guard cells of WT, tgg1-3, tgg2-1 and tgg1-3 tgg2-1. Scale bar = 5 µm. The error bar represents standard error. Differences among treatments were analyzed by Tukey’s test: P < 0.05.
Acrolein- and Ca2+-induced [Ca2+]cyt elevation in Arabidopsis guard cells
Application of ABA, MeJA and Ca2+ leads to stomatal closure through [Ca2+]cyt elevation in Arabidopsis guard cells (McAinsh et al. 1990, Allen et al. 2000, Pei et al. 2000, Allen et al. 2001, Allen et al. 2002, Suhita et al. 2004, Munemasa et al. 2011). We examined the effects of acrolein and Ca2+ on [Ca2+]cyt elevation in guard cells using the Yellow Cameleon (YC) technique (Nagai et al. 2004, Mori et al. 2006, Islam et al. 2010, Hossain et al. 2011, Munemasa et al. 2011). When WT guard cells were treated with 10 µM acrolein, 82.8% (n = 30) of the guard cells showed [Ca2+]cyt elevations (Fig. 5A, C). When tgg1-3 tgg2-1 guard cells were treated with 10 µM acrolein, 22.7% (n = 22) of the guard cells showed [Ca2+]cyt elevations (Fig. 5B, C). There was a significant difference in [Ca2+]cyt elevations between WT and tgg1-3 tgg2-1 (P = 0.0001). When WT and tgg1-3 tgg2-1 double mutant guard cells were treated with 1 mM Ca2+, 88.3% (n = 18) and 87.5% (n = 16) of the guard cells showed [Ca2+]cyt elevations (Fig. 5D–F). There was no significant difference in Ca2+-induced [Ca2+]cyt elevations between WT and tgg1-3 tgg2-1 (P = 0.65). These results indicate that acrolein-induced [Ca2+]cyt elevation was impaired by the tgg1-3 tgg2-1 double mutation and that the myrosinases, TGG1 and TGG2, function downstream of RCS production and upstream of [Ca2+]cyt elevation in the ABA signal pathway in Arabidopsis guard cells.
![Effects of acrolein and exogenous Ca2+ on [Ca2+]cyt elevations in WT and tgg1-3 tgg2-1 double mutant guard cells. The fluorescence ratio (535/480 nm) was measured in guard cells expressing YC3.6. (A) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 10 µM acrolein-treated WT guard cells (n = 30). (B) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 10 µM acrolein-treated tgg1-3 tgg2-1 double mutant guard cells (n = 22). (C) Percentage bar chart showing the number of acrolein-induced [Ca2+]cyt elevations in the WT and the tgg1-3 tgg2-1 double mutant guard cells. (D) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 1 mM Ca2+-treated WT guard cells (n = 18). (E) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 1 mM Ca2+-treated tgg1-3 tgg2-1 double mutant guard cells (n = 16). (F) Percentage bar chart showing the number of Ca2+-induced [Ca2+]cyt elevations in the WT and the tgg1-3 tgg2-1 double mutant guard cells. [Ca2+]cyt transient elevations were counted when changes in [Ca2+]cyt ratios were ≥0.1 units.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/61/5/10.1093_pcp_pcaa024/2/m_pcaa024f5.jpeg?Expires=1747905690&Signature=nhK1H2Y-TMWYcN-rMwpJPquBkc5uCSQcg94S2RV7TQhe~wFmmQsBo~~~HT6Dy~OlpxDdTNasHezUEAYKWcRsKzDga2INk6XAql4WjzbGJWrOAApJK0v2CiSoOWHFn13vfsIgznMW7I466JsokWq90G8JDxqRt3NLM4bFAo-gobGaxKTgoUqGOzoswdDdRkb36-45Q5L0PzxZ1ICNCnk2MbqG2JfMR14vzCSdb63MvLVzGgvu5BQeICZo7GmQsFZQ~MeqcGF-1LtwhopNuE2aZ-zy1uPygq5bGCBPSKPnUrk3rr-D69nq48E7W~uxUka7r~daUaKTIXJMjShHf9lu6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Effects of acrolein and exogenous Ca2+ on [Ca2+]cyt elevations in WT and tgg1-3 tgg2-1 double mutant guard cells. The fluorescence ratio (535/480 nm) was measured in guard cells expressing YC3.6. (A) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 10 µM acrolein-treated WT guard cells (n = 30). (B) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 10 µM acrolein-treated tgg1-3 tgg2-1 double mutant guard cells (n = 22). (C) Percentage bar chart showing the number of acrolein-induced [Ca2+]cyt elevations in the WT and the tgg1-3 tgg2-1 double mutant guard cells. (D) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 1 mM Ca2+-treated WT guard cells (n = 18). (E) Representative fluorescence emission ratio (535/480 nm) showing [Ca2+]cyt elevations in 1 mM Ca2+-treated tgg1-3 tgg2-1 double mutant guard cells (n = 16). (F) Percentage bar chart showing the number of Ca2+-induced [Ca2+]cyt elevations in the WT and the tgg1-3 tgg2-1 double mutant guard cells. [Ca2+]cyt transient elevations were counted when changes in [Ca2+]cyt ratios were ≥0.1 units.
Discussion
Zhao et al. (2008) reported that TGG1 is a strikingly abundant protein in Arabidopsis guard cells and that inhibition of light-induced stomatal opening by ABA was disrupted in tgg1 mutants. However, ABA-induced stomatal closure functions in the tgg1 single mutant, while not only ABA-induced stomatal closure but also MeJA-induced stomatal closure were impaired in the tgg1-3 tgg2-1 double mutant (Islam et al. 2009). In our study, we found that acrolein induced stomatal closure in WT, tgg1-3 and tgg2-1, but not in the tgg1-3 tgg2-1 double mutant (Fig. 1A). These results suggest a redundant function of TGG1 and TGG2 in the response to RCS in guard cells.
Both ABA and MeJA induce stomatal closure, which is accompanied by cytosolic alkalization in Arabidopsis guard cells (Irving et al. 1992, Gehring et al. 1997, Suhita et al. 2004, Gonugunta et al. 2008, Islam et al. 2010). Acrolein induced cytosolic alkalization and stomatal closure in WT, tgg1-3 and tgg2-1 (Fig. 1A, C) while acrolein did not induce cytosolic alkalization or stomatal closure in the tgg1-3 tgg2-1 double mutant (Fig. 1A, C). Butyrate inhibited the acrolein-induced stomatal closure in WT, tgg1-3 and tgg2-1 (Fig. 4A, C, E), which indicates that acrolein-induced stomatal closure is accompanied by cytosolic alkalization. However, acrolein did not induce stomatal closure in tgg1-3 tgg2-1 double mutants in the presence or absence of butyrate (Fig. 4G). These results indicate that cytosolic alkalization is required for RCS-induced stomatal closure and suggests that the myrosinases, TGG1 and TGG2, are involved in the regulation of cytosolic pH downstream of RCS production in the ABA signal pathway in guard cells. These results also strengthen the notion that TGG1 and TGG2 function redundantly downstream of RCS production and upstream of cytosolic alkalization in guard cell ABA signaling.
Both ABA and MeJA induce [Ca2+]cyt elevation in guard cells, leading to stomatal closure (Allen et al. 2000, Pei et al. 2000, Allen et al. 2001, Allen et al. 2002, Suhita et al. 2004, Munemasa et al. 2011). In this study, acrolein elicited [Ca2+]cyt elevations in the WT guard cells but not in the tgg1-3 tgg2-1 double mutant guard cells (Fig. 5A–C). Exogenous Ca2+ elicited [Ca2+]cyt elevations in the guard cells of WT plants and tgg1-3 tgg2-1 (Fig. 5D–F), suggesting that TGG1 and TGG2 function downstream of RCS production and upstream of [Ca2+]cyt elevations in the ABA signal pathway in guard cells.
Zhao et al. (2008) proposed that isothiocyanate might modify plasma membrane inward-rectifying K+ channels in the guard cells, leading to the inhibition of stomatal opening through inactivation of the inward-rectifying K+ channels. This study shows that acrolein induces stomatal closure in WT, tgg1-3 and tgg2-1, but not in the tgg1-3 tgg2-1 double mutant, and that exogenous Ca2+ induces stomatal closure (Fig. 2A) and elicits [Ca2+]cyt elevation (Fig. 5D–F) in all lines. Both EGTA and LaCl3 significantly inhibited the acrolein- and Ca2+-induced stomatal closure (Fig. 3). This difference in stomatal responses in the tgg1-3 tgg2-1 double mutant between acrolein and exogenous Ca2+ may be accounted for by the difference in the concentrations of extracellular Ca2+. In other words, an extracellular substrate of the plasma membrane Ca2+-permeable channels, Ca2+, at higher concentrations may overcome the reduction in the activities of Ca2+-permeable channels because TGG1 and TGG2 are unlikely to totally inhibit the channels. Butyrate inhibited acrolein- and Ca2+-induced stomatal closure (Fig. 4). This result indicates that acrolein- and Ca2+-induced stomatal closures require cytosolic alkalization and that the cytosolic alkalization is the downstream signaling event of [Ca2+]cyt in Arabidopsis guard cells (Fig. 2A, C), which is consistent with previous results (Islam et al. 2010). Islam et al. (2009) reported that TGG1 and TGG2 can function downstream of ROS production in ABA signaling in Arabidopsis guard cells. ABA induced stomatal closure in WT, tgg1-3 and tgg2-1, but not in the tgg1-3 tgg2-1 double mutant (Islam et al. 2009), while ABA induced ROS production in all lines (Supplementary Fig. S1; Islam et al. 2009). This study clearly indicates that TGG1 and TGG2 function downstream of RCS and upstream of cytosolic Ca2+ elevation and cytosolic alkalization in the ABA signaling in Arabidopsis guard cells. Taken together with the previous results, these findings allow us to propose a simple model for the position of TGG1, TGG2 and RCS production in the ABA signaling pathway in Arabidopsis guard cells (Fig. 6), where TGG1 and TGG2 function downstream of RCS and upstream of both cytosolic Ca2+ elevation and cytosolic alkalization.
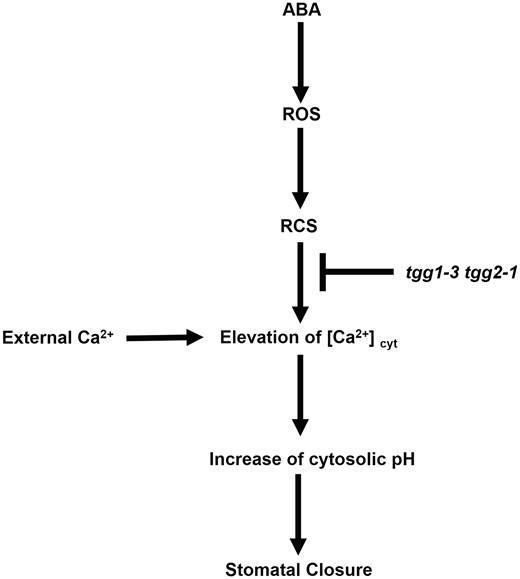
Simplified model for the ABA signaling pathway in Arabidopsis guard cells. The myrosinases TGG1 and TGG2 function downstream of RCS and upstream of cytosolic Ca2+ elevation and cytosolic alkalization in ABA signaling in Arabidopsis guard cells.
Myrosinases are spatially separated from their substrates, glucosinolates, in plants. Upon herbivore attack, plants use myrosinases to convert glucosinolates to isothiocyanates and so on. However, it is difficult for ABA to induce the breakdown of glucosinolate mediated by TGG1 and TGG2 because ABA is unlikely to release the separation of TGG1 and TGG2 from glucosinolates. Hence, the myrosinase activities of TGG1 and TGG2 may not be essential, but TGG1 and TGG2 may directly interact with ABA signal component(s), such as Ca2+ permeable channels, and may regulate the component(s) by an unknown mechanism.
Conclusion
It is concluded that two myrosinases, TGG1 and TGG2, function redundantly downstream of RCS and upstream of cytosolic Ca2+ elevation along with cytosolic alkalization in ABA signaling in Arabidopsis guard cells.
Materials and Methods
Plant materials and growth conditions
Arabidopsis ecotype Columbia-0 was used as the WT plant. In controlled growth conditions, WT, tgg1-3 (SAIL_786_B08), tgg2-1 (SALK_038730) and tgg1-3 tgg2-1 mutant plants (Barth and Jander 2006) were grown in plastic pots filled with soil:vermiculite (v/v) 1:1 mixture under a 16-h light period with 80 μmol m−2 s−1 photon flux density and a 8-h dark period. The temperature and relative humidity in the growth chamber were 21 ± 2°C and 70%, respectively. Water containing 0.1% Hyponex (Hyponex Japan, Osaka, Japan) nutrient solution was applied to the plant growth tray one time per 2 weeks. The mutant lines were confirmed by genomic PCR (Supplementary Fig. S2). The left genomic primer, right genomic primer and T-DNA border primer were as follows: tgg1-3: CTCTCACATGTACCACTCTATT, CCACTCACTTGATGACTTTACT and TAGCATCTGAATTTCATAACCAATCTCGATACAC; tgg2-1: GGAGATTCTTCCACCGAACCC, CGTTTAACATCCCTCTTGGGTGG and ATTTTGCCGATTTCGGAAC.
Acrolein preparation
Acrolein was prepared from acrolein diethyl acetal (Tokyo Chemical Industry Co. Ltd.). Acrolein diethyl acetal was dissolved in 0.1 M HCl, and the solution was incubated at 40°C for 40 min. Then, NaHCO3 was added to the solution until bubbles are no longer produced. The neutralized solution was used for experiments in this study.
Measurement of stomatal aperture
Stomatal apertures were measured as reported by Munemasa et al. (2007) with modifications. Excised leaves of 4- to 6-week-old plants were floated on an assay solution containing 5 mM KCl, 50 μM CaCl2 and 10 mM MES-Tris (pH 6.15). The leaves were incubated for 2 h under light, acrolein was added to the pre-incubated leaves and then again leaves were incubated in the light for 2 h. For the measurement of stomatal apertures, the leaves were shredded in a commercial blender for 25 s and epidermal tissues were collected using a nylon mesh (pore size, 60 µm). Twenty stomatal apertures were measured for each individual experiment.
Measurement of cytosolic pH
Changes in pH in guard cells were analyzed by using a pH-sensitive fluorescent dye, BCECF-AM (Islam et al. 2010), with modifications. Five or six leaves from 4- to 6-week-old plants were shredded in a commercial blender for 40 s, and epidermal tissues were collected using a nylon mesh (pore size, 60 µm). The collected epidermal tissues were incubated for 2 h in the medium containing 5 mM KCl, 50 μM CaCl2 and 10 mM MES-Tris (pH 6.15) and then 20 μM BCECF-AM was added to this medium. Then, epidermal tissues were again incubated for 30 min in the dark at room temperature to load the dye. After 30 min from the dye loading, the excess dye was washed out with distilled deionized water and then 10 μM acrolein was added. Twenty minutes later, fluorescence was observed and imaged under a fluorescence microscope and analyzed using ImageJ software.
Measurement of [Ca2+]cyt elevations
Four- to six-week-old leaves of Arabidopsis YC3.6-expressing plants were employed as a Ca2+ indicator to monitor [Ca2+]cyt in Arabidopsis guard cells as described previously (Munemasa et al. 2011). The excised leaves were gently mounted on a glass slide by a medical adhesive to remove the adaxial epidermis and the mesophyll tissue. The mounted abaxial epidermal peels were placed in a 6-wall plate containing 5 mM KCl, 50 μM CaCl2 and 10 mM MES-Tris (pH 6.15) for 2 h under light incubation. The turgid guard cells were treated with 10 μM acrolein using a pipette at 5 min from the start of measurement. We selected 6–8 guard cells from one sample leaf for each experiment and repeated the experiment using different sample leaves on different days. Monitoring of YC3.6 was done by dual-emission ratio imaging. For the imaging of dual-emission ratio, we used a 440AF21 excitation filter, a 445DRLP dichroic mirror and two emission filters 480DF30 for cyan fluorescent protein (CFP) and 535DF25 for yellow fluorescent protein (YFP). The CFP and YFP fluorescent intensities of guard cells were imaged and analyzed using AQUA COSMOS software (Hamamatsu Photonics).
Statistical analysis
The significance of differences between mean values was assessed using Student’s t-test and Tukey’s test. Differences in the frequency of [Ca2+]cyt elevations in the WT and tgg1-3 tgg2-1 mutants induced by acrolein and Ca2+ were determined by χ2 test. We regarded differences at the level of P < 0.05 as statistically significant.
Funding
Japan Society for the Promotion of Science (JSPS) KAKENHI [18K05557 to S.M.].
Disclosures
The authors have no conflicts of interest to declare.



