-
PDF
- Split View
-
Views
-
Cite
Cite
Francisco J. Esteva, Gabriel N. Hortobagyi, Integration of Systemic Chemotherapy in the Management of Primary Breast Cancer, The Oncologist, Volume 3, Issue 5, October 1998, Pages 300–313, https://doi.org/10.1634/theoncologist.3-5-300
Close - Share Icon Share
Abstract
Breast cancer cells can metastasize early in the development of primary tumors. Adjuvant chemotherapy improves disease-free survival and overall survival (OS) in patients with early-stage breast cancer, both in premenopausal and postmenopausal women. Tamoxifen improves OS in patients whose tumors are estrogen-receptor-positive, regardless of age. Although the relative risk reduction with these interventions is the same for all patients, the absolute benefit is more prominent in patients at the highest risk of relapse. All patients with involved lymph nodes should receive adjuvant chemotherapy if no contraindications occur. In patients with negative lymph nodes, adjuvant chemotherapy is recommended if the invasive tumor is moderately or poorly differentiated, the tumor size is larger than 1 or 2 cm, or hormone receptors are negative. Other prognostic and predictive indicators of response to chemotherapy and hormonal therapy remain investigational. Models that combine several factors to determine a patient's risk of relapse and death are presented.
Neoadjuvant chemotherapy in conjunction with radiation therapy and surgery is the treatment of choice for patients with locally advanced breast cancer. Recently, this approach has been extended to patients with small, operable tumors. Neoadjuvant chemotherapy can downstage tumors effectively, leading to improved cosmetic results and possibly to a reduction in local recurrence rate. Another advantage of neoadjuvant therapy is the in vivo assessment of tumor sensitivity to chemotherapy, which allows optimization of available therapeutic agents. One disadvantage of neoadjuvant chemotherapy is that preoperative treatment causes an alteration of information regarding lymph node status prior to systemic treatment.
Although the optimal chemotherapy regimen has not been established, doxorubicin-containing regimens are considered superior to non-doxorubicin-containing regimens. The role of high-dose chemotherapy is not well defined in the adjuvant setting and remains investigational. The taxanes paclitaxel and docetaxel are moving rapidly from the metastatic setting to the adjuvant setting.
A better understanding of the biology of breast cancer is providing molecular tools to study novel treatment approaches. Oncogenes and tumor suppressor genes are emerging as important indicators of response to chemotherapy. These molecules, in turn, become targets for therapy. Novel agents under development are presented.
Introduction
Breast cancer is the most prevalent malignancy among women in the Western industrialized world. It has been estimated that more than 180,000 women in the United States will be diagnosed with breast cancer in 1998, and more than 40,000 of these women will die of the disease [1]. Although the incidence of breast cancer remained constant from 1990 to 1995, mortality rates decreased 1.9% per year during the same period [2]. This is most likely due to earlier diagnosis through mammographic screening and the increased use of adjuvant therapy. Chemotherapy is often used in the adjuvant setting following surgical removal of the primary tumor in patients with early-stage breast cancer [3]. Patients with locally advanced breast cancer (LABC), however, receive chemotherapy as their initial or neoadjuvant treatment modality [4]. Recently, the use of neoadjuvant chemotherapy has been extended to patients with resectable tumors. This article attempts to clarify the roles of adjuvant and neoadjuvant chemotherapy as treatments for primary breast cancer. We also discuss ongoing efforts to introduce into the adjuvant setting novel agents now targeted for patients with metastatic disease.
The Role of Systemic Chemotherapy in the Treatment of Early-Stage Breast Cancer: Historical Background
During the first half of this century, breast cancer was considered a locally spreading disease. Surgery and radiation therapy were the mainstays of treatment, and patients with locally advanced disease were likely to undergo radical “en block” resection, which was pioneered by Dr. William Halsted at Johns Hopkins University [5]. However, despite aggressive local therapy, most patients with involvement of the axillary nodes relapsed and died. In the 1950s, the Halstedian principles were challenged. It was hypothesized that cancer cells can spread to distant sites through the bloodstream at the time the primary tumor was removed. Based on this hypothesis, it was predicted that radical local treatment would not improve survival, but chemotherapy might eradicate distant metastases and improve cure rates [6]. In a step-wise fashion, investigators from the National Surgical Adjuvant Breast and Bowel Project (NSABP) and other groups showed that lumpectomy with radiation therapy was adequate treatment for most patients with primary breast cancer [7-10]. The second challenge to the Halstedian paradigm came from studies evaluating the effectiveness of chemotherapy as an adjuvant to surgery. Initial trials targeted women with involved lymph nodes at the time of mastectomy, because most of them experienced recurrences and died of their disease. In one of the first randomized clinical trials, Fisher and associates [11] showed that administration of L-phenylalanine mustard (L-PAM) after surgery improved survival in premenopausal women with node-positive (stage II) breast cancer. The effect of adjuvant chemotherapy was even more striking when agents with different mechanisms of action were combined. Bonadonna and coworkers [12] employed CMF, a combination of cyclophosphamide, methotrexate and 5-fluorouracil (5-FU), that resulted in a significant improvement in survival among patients with node-positive breast cancer. A recent analysis of the first trial using CMF as adjuvant treatment indicated that the improvement in survival is sustained for over 20 years [13]. It is now clear that breast cancer cells metastasize early in the development of primary tumors and that chemotherapy can eradicate micrometastatic disease present at the time of diagnosis.
Neoadjuvant chemotherapy, also known as primary or induction chemotherapy, was developed in parallel with the postoperative approach. Early trials included patients with LABC, for whom local treatments had limited success. From the beginning, it became apparent that induction chemotherapy could downstage tumors effectively [4]. Furthermore, survival was improved over that of patients treated with local modalities only (surgery, radiotherapy, or both) [14]. The scientific rationale for using preoperative chemotherapy came from theoretical models and animal studies performed in the United States and Canada. The mathematical model of Goldie and Coldman [15] predicts that the probability of developing drug-resistant clones is proportional to the tumor size, and when tumors are clinically detectable (>106 cells), it is likely that at least one cell line is resistant to chemotherapy. These authors hypothesized that multiple non-cross-resistant chemotherapeutic agents should be given as soon as possible to achieve cure. Experimental data indicate that the best outcome is obtained when chemotherapy is administered early and that a delay in surgery following tumor transplantation in mice is associated with a lower cure rate [16]. In the nude mouse model, removal of a primary tumor is associated with an increased proliferation rate of residual tumors. Fisher and colleagues [17] showed that the kinetic changes induced by removal of the primary tumor could be prevented by administration of chemotherapy, tamoxifen, or radiation prior to surgery. These experiments provided the scientific basis for the first trials of preoperative chemotherapy in early breast cancer patients.
Indications for Chemotherapy in the Adjuvant Setting
The decision to use adjuvant therapy must be made on a patient-by-patient basis to avoid excessive toxicity to patients who are likely to be cured by local treatment alone. The decision regarding adjuvant therapy should take into account: A) estimation of the patient's risk for relapse or death without further therapy based on known prognostic indicators; B) estimation of the benefit associated with a particular therapy in terms of absolute risk reduction of recurrence and death, and C) estimation of the risk associated with chemotherapy or hormonal therapy based on the patient's comorbid conditions. Randomized clinical trials have shown that all age groups benefit from adjuvant chemotherapy, and although patients who develop breast cancer are considered at high risk of relapse, age alone is not considered a factor to influence the choice of the type of adjuvant treatment (chemotherapy or endocrine therapy). The optimal therapy is determined by the patient's menopausal status and the hormone receptor status of the tumor (Table 1).
| Node-positive breast cancer . | Negative ER/PR . | Positive ER/PR . |
|---|---|---|
| Premenopausal or postmenopausal woman (older or younger than 50 years) | Chemotherapy | Chemotherapy → Tamoxifen × 5 yrs |
| Node-positive breast cancer . | Negative ER/PR . | Positive ER/PR . |
|---|---|---|
| Premenopausal or postmenopausal woman (older or younger than 50 years) | Chemotherapy | Chemotherapy → Tamoxifen × 5 yrs |
| Node-negative breast cancer . | Low risk . | High risk . |
|---|---|---|
| Premenopausal | ||
| ER + | No therapy | Chemotherapy ± Tamoxifen |
| ER - | ? | Chemotherapy |
| Postmenopausal | ||
| ER + | No therapy | Chemotherapy → Tamoxifen |
| ER - | ? | Chemotherapy |
| Node-negative breast cancer . | Low risk . | High risk . |
|---|---|---|
| Premenopausal | ||
| ER + | No therapy | Chemotherapy ± Tamoxifen |
| ER - | ? | Chemotherapy |
| Postmenopausal | ||
| ER + | No therapy | Chemotherapy → Tamoxifen |
| ER - | ? | Chemotherapy |
Low risk: Tumor size <1cm, low S-phase, well-differentiated tumor.
ER = estrogen receptor; PR = progesterone receptor.
| Node-positive breast cancer . | Negative ER/PR . | Positive ER/PR . |
|---|---|---|
| Premenopausal or postmenopausal woman (older or younger than 50 years) | Chemotherapy | Chemotherapy → Tamoxifen × 5 yrs |
| Node-positive breast cancer . | Negative ER/PR . | Positive ER/PR . |
|---|---|---|
| Premenopausal or postmenopausal woman (older or younger than 50 years) | Chemotherapy | Chemotherapy → Tamoxifen × 5 yrs |
| Node-negative breast cancer . | Low risk . | High risk . |
|---|---|---|
| Premenopausal | ||
| ER + | No therapy | Chemotherapy ± Tamoxifen |
| ER - | ? | Chemotherapy |
| Postmenopausal | ||
| ER + | No therapy | Chemotherapy → Tamoxifen |
| ER - | ? | Chemotherapy |
| Node-negative breast cancer . | Low risk . | High risk . |
|---|---|---|
| Premenopausal | ||
| ER + | No therapy | Chemotherapy ± Tamoxifen |
| ER - | ? | Chemotherapy |
| Postmenopausal | ||
| ER + | No therapy | Chemotherapy → Tamoxifen |
| ER - | ? | Chemotherapy |
Low risk: Tumor size <1cm, low S-phase, well-differentiated tumor.
ER = estrogen receptor; PR = progesterone receptor.
The Oxford Meta-Analysis
In 1985 [18], and again in 1991 [19], investigators at Oxford University obtained detailed information from 75,000 women with breast cancer treated with a variety of adjuvant therapies in prospective randomized clinical trials. A systematic overview (meta-analysis) of over 100 randomized clinical trials revealed that adjuvant chemotherapy improves disease-free survival (DFS) and overall survival (OS) in patients with early-stage breast cancer (Table 2). At 10 years, the risk of recurrence was reduced by 22%-37% and the risk of death by 14%-27%. The improvement in OS associated with adjuvant chemotherapy was even more significant at 10 years than at five years, both in premenopausal and postmenopausal patients. Ovarian ablation resulted in improved survival in premenopausal women, with a risk reduction similar to that obtained after combination chemotherapy [20]. Tamoxifen was effective in patients older than 50 years whose tumors were estrogen-receptor (ER)-positive.
| Adjuvant Therapy . | Age <50 years . | Age >50 years . | Reference . | ||
|---|---|---|---|---|---|
| . | Recurrence . | Death . | Recurrence . | Death . | . |
| Combination chemotherapy versus no treatment | 37% SD 5 | 27% SD 6 | 22% SD 4 | 14% SD 5 | [19] |
| Recurrence* | Death* | ||||
| Tamoxifen** versus no treatment | 46% SD 4 | 22% SD 5 | [21] | ||
| Chemotherapy plus Tamoxifen** versus chemotherapy | 52% SD 8 | 47% SD 9 | [21] | ||
| Adjuvant Therapy . | Age <50 years . | Age >50 years . | Reference . | ||
|---|---|---|---|---|---|
| . | Recurrence . | Death . | Recurrence . | Death . | . |
| Combination chemotherapy versus no treatment | 37% SD 5 | 27% SD 6 | 22% SD 4 | 14% SD 5 | [19] |
| Recurrence* | Death* | ||||
| Tamoxifen** versus no treatment | 46% SD 4 | 22% SD 5 | [21] | ||
| Chemotherapy plus Tamoxifen** versus chemotherapy | 52% SD 8 | 47% SD 9 | [21] | ||
All data are presented as proportional risk reduction (percent and standard deviation [SD]).
*Regardless of age
**Tamoxifen 20 mg orally per day for five years (ER-positive patients only).
| Adjuvant Therapy . | Age <50 years . | Age >50 years . | Reference . | ||
|---|---|---|---|---|---|
| . | Recurrence . | Death . | Recurrence . | Death . | . |
| Combination chemotherapy versus no treatment | 37% SD 5 | 27% SD 6 | 22% SD 4 | 14% SD 5 | [19] |
| Recurrence* | Death* | ||||
| Tamoxifen** versus no treatment | 46% SD 4 | 22% SD 5 | [21] | ||
| Chemotherapy plus Tamoxifen** versus chemotherapy | 52% SD 8 | 47% SD 9 | [21] | ||
| Adjuvant Therapy . | Age <50 years . | Age >50 years . | Reference . | ||
|---|---|---|---|---|---|
| . | Recurrence . | Death . | Recurrence . | Death . | . |
| Combination chemotherapy versus no treatment | 37% SD 5 | 27% SD 6 | 22% SD 4 | 14% SD 5 | [19] |
| Recurrence* | Death* | ||||
| Tamoxifen** versus no treatment | 46% SD 4 | 22% SD 5 | [21] | ||
| Chemotherapy plus Tamoxifen** versus chemotherapy | 52% SD 8 | 47% SD 9 | [21] | ||
All data are presented as proportional risk reduction (percent and standard deviation [SD]).
*Regardless of age
**Tamoxifen 20 mg orally per day for five years (ER-positive patients only).
Patients with ER-poor tumors had marginal benefit from tamoxifen. Although the relative risk reduction with these interventions was the same for all patients, the absolute benefit was more prominent in patients at the highest risk of relapse. A third meta-analysis based on 37,000 patients treated in 55 randomized clinical trials confirmed that tamoxifen can reduce the risk of recurrence and the risk of death for women older than 50 but it also showed that, in contrast to earlier reports, it has the same benefits for those younger than 50 [21].
Adjuvant Chemotherapy for Node-Positive Breast Cancer
The most important prognostic factor for patients with breast cancer is the number of lymph nodes containing malignant cells. As a group, patients with positive lymph nodes have a 70% risk for relapse at 10 years. The risk is higher (about 90%) for patients with more than ten positive lymph nodes. According to the Oxford Meta-Analysis, adjuvant chemotherapy can reduce the risk of recurrence from breast cancer by 37% in premenopausal patients and by 22% in postmenopausal patients. Independent randomized clinical trials, as well as the Oxford overview, showed that adjuvant chemotherapy is effective in node-positive premenopausal and postmenopausal patients, regardless of the hormonal status of the tumor [19, 22] (Table 1). In addition to chemotherapy, postmenopausal patients with tumors rich in hormone receptors gain an extra 15%-20% risk reduction by adding long-term tamoxifen [23]. A third meta-analysis completed in 1995 [21] showed that women with ER-positive tumors benefit from tamoxifen regardless of age, menopausal status, or concurrent chemotherapy.
Adjuvant Chemotherapy for Node-Negative Breast Cancer
Adjuvant chemotherapy also improves survival in patients with node-negative breast cancer. Approximately 30% of these patients relapse and die of metastatic breast cancer. Chemotherapy has been shown to reduce this risk by 14%-27% depending on the patient's age [19]. However, since most patients with node-negative breast cancer are cured with local treatments, the patient's risk must be estimated as accurately as possible prior to initiation of cytotoxic therapy. Adjuvant chemotherapy is currently recommended to patients with moderately or poorly differentiated invasive tumors larger than 1 or 2 cm or negative hormone receptors [24]. The risk for node-negative patients with well-differentiated tumors less than 1 cm in size is 10% or less [25], and these patients should not receive adjuvant chemotherapy. The treatment of postmenopausal women with node-negative, ER-rich tumors is evolving. According to the 1998 Oxford Meta-Analysis [21], five years of tamoxifen therapy results in a 42% proportional reduction in the risk of relapse and a 22% proportional reduction in the risk of death. The addition of tamoxifen to adjuvant chemotherapy decreased the risk of relapse by an additional 52%, although the number of patients analyzed was small. The 1998 Oxford Meta-Analysis indicates that the absolute reduction in the risk of death with tamoxifen plus chemotherapy over tamoxifen alone is 6%. A large randomized trial (NSABP B-20) showed that a short course of chemotherapy combined with long-term tamoxifen is superior to tamoxifen alone [26]. In this study, the proportional reduction in the risk of recurrence was 35% and the reduction in the risk of death was 30%-36% for chemotherapy plus tamoxifen over tamoxifen alone. The absolute improvement in survival from the addition of chemotherapy to tamoxifen was 4%. This is of similar magnitude to the 6% benefit in absolute mortality reduction using chemotherapy plus tamoxifen over tamoxifen alone observed in the 1998 Oxford Meta-Analysis. Since the addition of chemotherapy to tamoxifen provides a real but small benefit, the decision to give chemotherapy to postmenopausal women with node-negative, ER-positive tumors should be individualized. The presence of concurrent medical conditions may preclude the use of chemotherapy in this setting.
Use of Prognostic Factors in Node-Negative Breast Cancer
Since 70% of patients with node-negative breast cancer can be cured with local regional therapy, it is imperative to identify patients at high risk who may benefit from systemic adjuvant treatments. The most widely used prognostic factors for node-negative breast cancer are the size, histologic and nuclear grades, and hormonal status of the tumor (Table 3). In addition, some prognostic factors are also predictive of response to therapy. For example, the level of estrogen and progesterone receptors is the best predictive indicator of response to hormonal therapy [27] and should be assessed in every patient with breast cancer [28].
Although many other tumor markers have been identified in the past 15 years, the 1997 ASCO guidelines do not recommend using any of them to support treatment decisions for patients with node-negative breast cancer. One of the most controversial markers is the overexpression and/or amplification of the HER-2/neu gene, which has been associated with a poor prognosis in node-positive and node-negative patients in some studies. However, the majority of studies done have been retrospective analyses, have included a small number of patients, and have used a variety of reagents that are not well standardized. Recently, Andrulis and coworkers [29] reported a prospective study showing that amplification of the HER-2/neu gene is an independent predictor of poor DFS rates in patients with node-negative breast cancer. In this study, HER-2/neu amplification was independent of tumor size, histologic grade, and hormone receptor status.
Other prognostic markers are under investigation for patients with node-negative breast cancer, including the expression of epidermal growth factor receptor [30], expression of the Ki-67 antigen [31], mutations in tumor suppressor genes p53 [32, 33] and nm23 [34], expression of the invasion marker cathepsin [35], and serum markers CEA, CA 15-3, and CA 27.29 [36] (Table 3). The number of microvessels in breast primary tumors may be used as a marker of angiogenesis, and it has been associated with a poor prognosis [37]. Flow cytometry allows determination of the proportion of cancer cells in the S-phase of the cell cycle. A high S-phase fraction has been associated with a poor prognosis [38]. Unfortunately, there is no single prognostic indicator with enough power to stratify node-negative patients as high- or low-risk. Therefore, therapeutic decisions should be made using well-accepted prognostic and predictive indicators. Perhaps combining several prognostic factors will provide ways to influence treatment decisions. One such model is the Nottingham index, which incorporates the tumor size, lymph node status, and histologic grade to define subsets of patients with different prognoses [39]. Clark and coworkers [40, 41] proposed another model that includes the tumor size, the number of positive lymph nodes, the S-phase fraction and the ER status.
| I. Well-established . |
|---|
| ▴ Tumor size |
| ▴ Nuclear and histologic grades |
| ▴ Estrogen and progesterone receptor status |
| II Under investigation |
| ▴ S-phase fraction |
| ▴ Ploidy |
| ▴ Lymphatic invasion |
| ▴ Proliferation markers (Ki-67) |
| ▴ HER-2/neu |
| ▴ EGFR |
| ▴ p53 |
| ▴ nm23 |
| ▴ Cathepsin D |
| ▴ Microvessel count (MVC) |
| I. Well-established . |
|---|
| ▴ Tumor size |
| ▴ Nuclear and histologic grades |
| ▴ Estrogen and progesterone receptor status |
| II Under investigation |
| ▴ S-phase fraction |
| ▴ Ploidy |
| ▴ Lymphatic invasion |
| ▴ Proliferation markers (Ki-67) |
| ▴ HER-2/neu |
| ▴ EGFR |
| ▴ p53 |
| ▴ nm23 |
| ▴ Cathepsin D |
| ▴ Microvessel count (MVC) |
| I. Well-established . |
|---|
| ▴ Tumor size |
| ▴ Nuclear and histologic grades |
| ▴ Estrogen and progesterone receptor status |
| II Under investigation |
| ▴ S-phase fraction |
| ▴ Ploidy |
| ▴ Lymphatic invasion |
| ▴ Proliferation markers (Ki-67) |
| ▴ HER-2/neu |
| ▴ EGFR |
| ▴ p53 |
| ▴ nm23 |
| ▴ Cathepsin D |
| ▴ Microvessel count (MVC) |
| I. Well-established . |
|---|
| ▴ Tumor size |
| ▴ Nuclear and histologic grades |
| ▴ Estrogen and progesterone receptor status |
| II Under investigation |
| ▴ S-phase fraction |
| ▴ Ploidy |
| ▴ Lymphatic invasion |
| ▴ Proliferation markers (Ki-67) |
| ▴ HER-2/neu |
| ▴ EGFR |
| ▴ p53 |
| ▴ nm23 |
| ▴ Cathepsin D |
| ▴ Microvessel count (MVC) |
Role of Neoadjuvant Chemotherapy for Breast Cancer
As for adjuvant systemic therapy, the principal goals of neoadjuvant chemotherapy in breast cancer are to improve local control and to improve survival. Preoperative chemotherapy offers several additional advantages (Table 4). Inoperable tumors or tumors that are too large to be removed by lumpectomy can be effectively downstaged by use of neoadjuvant chemotherapy. A decrease in the extent of surgery leads to improved cosmetic results and the possibility of a reduction in local recurrences [42]. Another advantage of neoadjuvant therapy is the in vivo assessment of tumor sensitivity to chemotherapy, which allows optimization of available therapeutic agents. One disadvantage of neoadjuvant chemotherapy is that preoperative treatment causes a loss of information regarding lymph node status prior to systemic treatment. Although lymph node status is the most important prognostic indicator for patients with early-stage breast cancer, patients with tumors larger than 1 cm are currently offered postoperative systemic therapy regardless of lymph node involvement [43]. Furthermore, regional metastatic disease that involves axillary lymph nodes retains prognostic information after neoadjuvant chemotherapy [44].
| Advantages: . |
|---|
| Systemic |
| ▴ Potential decrease in de novo chemotherapy resistance |
| ▴ Assessment of tumor response to chemotherapy |
| Local |
| ▴ Increase in breast-conserving surgery |
| ▴ Improved cosmetic results |
| Disadvantages: |
| ▴ Loss of pre-chemotherapy information about axillary lymph node status |
| ▴ Loss of information about microscopic tumor size |
| ▴ Delayed local/regional therapy |
| ▴ Generalizing drug resistance |
| Advantages: . |
|---|
| Systemic |
| ▴ Potential decrease in de novo chemotherapy resistance |
| ▴ Assessment of tumor response to chemotherapy |
| Local |
| ▴ Increase in breast-conserving surgery |
| ▴ Improved cosmetic results |
| Disadvantages: |
| ▴ Loss of pre-chemotherapy information about axillary lymph node status |
| ▴ Loss of information about microscopic tumor size |
| ▴ Delayed local/regional therapy |
| ▴ Generalizing drug resistance |
| Advantages: . |
|---|
| Systemic |
| ▴ Potential decrease in de novo chemotherapy resistance |
| ▴ Assessment of tumor response to chemotherapy |
| Local |
| ▴ Increase in breast-conserving surgery |
| ▴ Improved cosmetic results |
| Disadvantages: |
| ▴ Loss of pre-chemotherapy information about axillary lymph node status |
| ▴ Loss of information about microscopic tumor size |
| ▴ Delayed local/regional therapy |
| ▴ Generalizing drug resistance |
| Advantages: . |
|---|
| Systemic |
| ▴ Potential decrease in de novo chemotherapy resistance |
| ▴ Assessment of tumor response to chemotherapy |
| Local |
| ▴ Increase in breast-conserving surgery |
| ▴ Improved cosmetic results |
| Disadvantages: |
| ▴ Loss of pre-chemotherapy information about axillary lymph node status |
| ▴ Loss of information about microscopic tumor size |
| ▴ Delayed local/regional therapy |
| ▴ Generalizing drug resistance |
Neoadjuvant Chemotherapy for LABC
Since 1974, all patients with LABC seen at MD Anderson have received neoadjuvant chemotherapy followed by surgery, adjuvant chemotherapy, and XRT (Fig. 1). Our first clinical trial included 174 patients with stage IIIA and IIIB breast cancer. All patients received three cycles of preoperative FAC chemotherapy (5-FU, doxorubicin [Adriamycin], cyclophosphamide). After neoadjuvant chemotherapy and surgery, the tumor was eradicated in all but nine patients. The 10-year DFS rates were 45% for patients with stage IIIA disease and 33% for patients with stage IIIB disease [45].
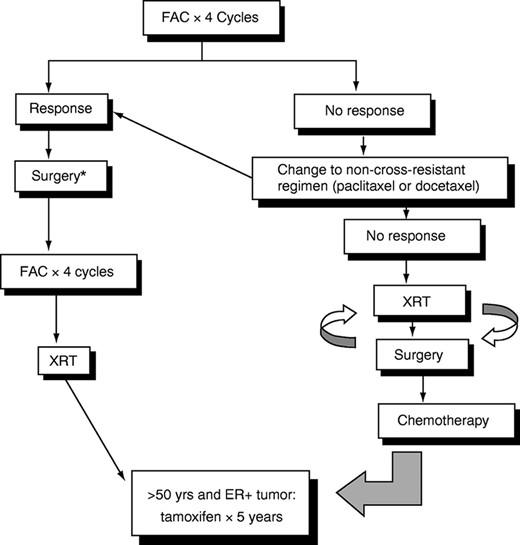
Multidisciplinary treatment of locally advanced breast cancer. *Lumpectomy followed by radiation or mastectomy, depending on response.
De Lena and colleagues [46] showed that administration of adjuvant chemotherapy after neoadjuvant chemotherapy and local treatment is more effective than neoadjuvant chemotherapy alone. However, the optimal duration of chemotherapy, both preoperatively and postoperatively, has not been clearly defined. Although the chemotherapy regimens have varied widely among studies, most clinical trials have used doxorubicin-containing regimens. Clinical response to neoadjuvant chemotherapy correlates with improved OS rates, with the best outcome associated with complete clinical and pathological response [47]. Another advantage of neoadjuvant chemotherapy is the possibility of breast-conserving surgery in selected patients with LABC [48]. Last, doxorubicin-based chemotherapy improves survival in patients with primary and metastatic breast cancer [14, 49, 50]. Therefore, neoadjuvant chemotherapy is the treatment of choice for patients with LABC.
Neoadjuvant Chemotherapy for Operable Tumors
In the 1980s, the use of neoadjuvant chemotherapy was extended to patients with operable tumors requiring mastectomy, in attempts to increase the number of breast-conserving procedures. The clinical considerations underlying the development of neoadjuvant chemotherapy were the following: A) the promising results obtained in patients with LABC in terms of DFS and OS; decreasing the tumor size leads to limited surgical treatment and improved cosmesis [48]; B) the ability of adjuvant chemotherapy to prolong OS in patients with early-stage breast cancer [19], and C) the identical OS associated with mastectomy or lumpectomy and then XRT [10]. Anderson and coworkers [51] reported a pathological complete response of 17% using neoadjuvant CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone) in patients with early-stage breast cancer. Jacquillat and colleagues [52] were among the first to use neoadjuvant chemotherapy followed by radiation therapy in early-stage breast cancer. In some cases, surgery was avoided altogether with acceptable local relapse rates. Bonadonna and coworkers [53] used neoadjuvant chemotherapy in treating 231 patients with operable breast tumors larger than 3 cm. At the time this study was conducted, the standard of care for these patients was a modified radical mastectomy. After three cycles of neoadjuvant chemotherapy, the tumor decreased in size to less than 3 cm in 89% of the patients, who then underwent breast-conserving surgery. In this clinical trial, only 11% of the patients required a mastectomy.
Although it is clear that neoadjuvant chemotherapy can improve the patient's chances for breast-conserving surgery, none of the randomized clinical trials conducted to date have shown a clear survival advantage over the standard postoperative approach. Scholl and colleagues [54] reported an improvement in OS rate in patients undergoing neoadjuvant chemotherapy compared with those undergoing adjuvant chemotherapy. However, these authors found no difference in DFS rates, and the results are difficult to interpret. The largest clinical trial conducted to date is the NSABP B-18. In this study, 1,523 women with stage I or II breast cancer were randomized to receive four cycles of doxorubicin and cyclophosphamide (AC) either before or after resection of the primary tumor. Of the 759 women randomized to the neoadjuvant arm of the study, 80% underwent breast-conserving surgery. A significant downstaging of primary tumor and axillary lymph nodes was noted in patients who underwent neoadjuvant chemotherapy [55]. However, the five-year OS rate was identical for the two groups [56]. Longer follow-up and larger studies may be needed to definitively determine whether there is a higher rate of survival among patients who receive neoadjuvant chemotherapy versus those who receive adjuvant chemotherapy.
Conventional Chemotherapy Regimens Used in the Adjuvant and Neoadjuvant Settings
From the initial adjuvant clinical trials of adjuvant chemotherapy, it became apparent that multi-agent chemotherapy was superior to single-agent chemotherapy. Most of the chemotherapy trials considered in the 1992 Oxford Meta-Analysis used CMF-based regimens. All versions of CMF were roughly comparable in efficacy, and no clear advantage was noted for specific differences in routes of administration or scheduling. Six months of CMF was as effective as longer courses. Recent data indicate that “classical CMF” (oral cyclophosphamide on days 1 to 14 with i.v. methotrexate and fluorouracil on days 1 and 8, repeated every 28 days) results in better outcomes than the modified CMF (all three agents administered i.v. every 21 days) both in the metastatic and adjuvant settings [57].
Role of Anthracyclines in the Adjuvant and Neoadjuvant Settings
The doxorubicin-based chemotherapy regimens most commonly used are FAC (5-FU 500 mg/m2, doxorubicin 50 mg/m2, cyclophosphamide 500 mg/m2) or CAF (cyclophosphamide 600 mg/m2, doxorubicin 60 mg/m2, 5-FU 600 mg/m2) and AC (doxorubicin 60 mg/m2, cyclophosphamide 600 mg/m2). Randomized clinical trials indicate that anthracycline-containing regimens are superior to regimens that do not include anthracyclines 5176, 3470, 4243. Although several studies found no outcome differences between CMF and doxorubicin-based regimens, no trial showed CMF to be superior to anthracycline-containing therapy [3]. Fisher and coworkers showed that four cycles of AC are comparable with six months of CMF [58]. The AC regimen was chosen for further development by the NSABP because it is administered for a shorter duration and with minimal toxicity than CMF. Retrospective studies conducted at MD Anderson indicate a better outcome among patients treated with FAC than with control groups treated with non-doxorubicin regimen [59]. Although the required duration of systemic chemotherapy is not well established, six cycles of FAC or four cycles of AC are acceptable options. The risk for cardiac toxicity associated with anthracycline-based regimens in the adjuvant setting is minimal at the doses recommended, especially if doxorubicin is administered as a protracted i.v. infusion for 48 to 72 h [60]. One of the most important long-term complications associated with doxorubicin-based chemotherapy is the development of acute myelocytic leukemia (AML). In one study, the 10-year risk of AML was 1.5% for all patients treated, 2.5% for patients treated with radiotherapy-plus-chemotherapy, and 0.5% for the chemotherapy-only group [61]. Addition of BCG [62] or alpha-interferon [63] to FAC in the adjuvant setting did not improve DFS or OS.
High-Dose Chemotherapy for Early-Stage Breast Cancer
Dose intensity is defined as the amount of drug administered over a period of time (i.e., mg/m2/week). Correlation between chemotherapy dose intensity and response rates has been seen in patients with metastatic breast cancer [64]. Hryniuk and Levine [65] reported a similar relationship in the adjuvant setting. To test this hypothesis, the Cancer and Leukemia Group B (CALGB) conducted a large prospective study that compared three different dose levels of CAF in patients with node-positive breast cancer [66]. Patients randomized to the low-dose arm received half as much chemotherapy over four months as those in the high-dose arm. Patients in the intermediate-dose arm received the same total amount of chemotherapy (in total mg/m2) as those in the high-dose arm, but over six months rather than four months. Outcome for patients in the low-dose arm was significantly worse than that of patients in the other two arms. Although there was a trend favoring the high-dose arm, it was not statistically different from that of the intermediate-dose arm. These results suggest a linear dose-response relationship for doxorubicin-based regimens, although a threshold effect cannot be excluded. In this study, patients whose tumors overexpressed the HER-2/neu protein had an improved survival with higher dose of doxorubicin [66]. Other trials also have suggested that outcome is improved when full doses of chemotherapy are delivered without delay, preserving dose intensity [67]. By contrast, in NSABP protocols B-22 and B-25, intensification of cyclophosphamide over a fourfold range in the AC regimen did not improve DFS or OS rates [68], and in CALGB protocol 93-44, intensification of doxorubicin by 50% had no detectable effect on outcome [69].
Norton [70] introduced the concept of dose density, defined as the administration of chemotherapy over short periods of time (e.g., weekly or twice-a-week dosing instead of every three to four weeks). In this approach, chemotherapy agents are delivered at full dose (with or without growth factor support) in a sequential manner. Theoretically, this model would overcome the problem of regrowth of metastases between cycles. Bhardwaj and colleagues [71] showed that four cycles of CMFVP (CMF plus vincristine, and prednisone) followed by four cycles of doxorubicin were safe and effective as adjuvant therapy, with a 42-month DFS rate of 80%.
The use of high-dose chemotherapy (HDCT) with hematopoietic support is also being investigated in the adjuvant setting. Peters and coworkers [72] conducted a pilot study in which 102 patients with primary breast cancer involving 10 or more axillary lymph nodes received high-dose cyclophosphamide, cisplatin, and carmustine with autologous bone marrow support as consolidation after standard-dose CAF adjuvant chemotherapy. In this study, the 2.5-year DFS rate was 72%, suggesting a survival improvement compared to the survival seen in patients treated with conventional chemotherapy. Other phase II pilot studies have shown similarly encouraging results [73]. Two phase III studies comparing this strategy with standard-dose adjuvant chemotherapy have completed accrual. An Intergroup trial coordinated by the Eastern Cooperative Oncology Group compared six cycles of CAF alone with six cycles of CAF followed by HDCT consolidation. Another study, coordinated by the CALGB, used four cycles of CAF in all patients, followed by a randomization to either high-dose cisplatin, carmustine, and cyclophosphamide with hematopoietic support or to these same three agents at “conventional” dose levels.
HDCT is also being investigated as treatment for LABC. Two independent groups recently reported encouraging results using HDCT as primary therapy for patients with inflammatory carcinoma [74, 75]. Both trials have indicated that HDCT might produce higher DFS rates than standard-dose chemotherapy and provide the basis for prospective randomized clinical trials.
Clinical trials using HDCT must be analyzed with caution. One of the main limitations of phase II trials is the lack of appropriate control groups. In addition, HDCT trials require a more intensive evaluation for metastases and organ function than is routinely done before standard-dose chemotherapy. In one study, 27% of patients with 10 or more involved nodes at presentation who seemed to be free of metastases after “standard” screening were found to have metastatic disease when more intensive evaluation was done [76]. Garcia-Carbonero and colleagues [77] showed that meeting criteria for inclusion in HDCT trials was an independent indicator of good prognosis in patients with more than 10 positive lymph nodes who underwent conventional adjuvant chemotherapy. In this study, patients younger than 60 years of age with no significant concomitant disease and no progression during adjuvant treatment (HDCT criteria) had a better outcome than patients not meeting these criteria. Therefore, the role of HDCT with hematopoietic stem cell support can be determined only by prospective, randomized trials. Hortobagyi and coworkers [78] have recently reported the results of a randomized trial of HDCT consolidation for patients with more than 10 positive lymph nodes receiving standard-dose FAC. In this study, two courses of HDCT after eight cycles of FAC were not superior to eight cycles of FAC alone. The results of these trials will clarify the real contribution of HDCT. Until these issues are resolved, conventional regimens should be delivered at full dose and on time. At present, the routine use of HDCT cannot be recommended in the adjuvant setting.
Integration of New Cytotoxic Agents in the Adjuvant Setting
Drugs that demonstrate substantial antitumor activity in patients with metastatic breast cancer become candidates for testing in the adjuvant setting. These include the taxanes, vinorelbine, new antimetabolites, novel anthracyclines, and topoisomerase I inhibitors (Table 5).
| Mechanism of action . | Agent . |
|---|---|
| Microtubule inhibitors | Paclitaxel (taxol) |
| Docetaxel (taxotere) | |
| Vinorelbine (navelbine) | |
| Antimetabolites | Gemcitabine |
| Capecitabine | |
| UFT (oral 5-FU) | |
| Edatrexate | |
| Anthracyclines | Annamycin |
| Liposomal doxorubicin (doxil) | |
| Losoxantrone (CI-941) | |
| Topoisomerase I inhibitors | 9-Aminocamptothecin (9-AC) |
| CPT-11 (topotecan) | |
| Irinotecan |
| Mechanism of action . | Agent . |
|---|---|
| Microtubule inhibitors | Paclitaxel (taxol) |
| Docetaxel (taxotere) | |
| Vinorelbine (navelbine) | |
| Antimetabolites | Gemcitabine |
| Capecitabine | |
| UFT (oral 5-FU) | |
| Edatrexate | |
| Anthracyclines | Annamycin |
| Liposomal doxorubicin (doxil) | |
| Losoxantrone (CI-941) | |
| Topoisomerase I inhibitors | 9-Aminocamptothecin (9-AC) |
| CPT-11 (topotecan) | |
| Irinotecan |
| Mechanism of action . | Agent . |
|---|---|
| Microtubule inhibitors | Paclitaxel (taxol) |
| Docetaxel (taxotere) | |
| Vinorelbine (navelbine) | |
| Antimetabolites | Gemcitabine |
| Capecitabine | |
| UFT (oral 5-FU) | |
| Edatrexate | |
| Anthracyclines | Annamycin |
| Liposomal doxorubicin (doxil) | |
| Losoxantrone (CI-941) | |
| Topoisomerase I inhibitors | 9-Aminocamptothecin (9-AC) |
| CPT-11 (topotecan) | |
| Irinotecan |
| Mechanism of action . | Agent . |
|---|---|
| Microtubule inhibitors | Paclitaxel (taxol) |
| Docetaxel (taxotere) | |
| Vinorelbine (navelbine) | |
| Antimetabolites | Gemcitabine |
| Capecitabine | |
| UFT (oral 5-FU) | |
| Edatrexate | |
| Anthracyclines | Annamycin |
| Liposomal doxorubicin (doxil) | |
| Losoxantrone (CI-941) | |
| Topoisomerase I inhibitors | 9-Aminocamptothecin (9-AC) |
| CPT-11 (topotecan) | |
| Irinotecan |
Paclitaxel and Docetaxel
A recent development in the use of adjuvant chemotherapy for the treatment of breast cancer is the introduction of the taxanes paclitaxel (taxol) and docetaxel (docetaxel). Both drugs induce objective responses in a high proportion of patients with metastatic breast cancer, even in anthracycline-resistant patients [79, 80]. Trials of paclitaxel and docetaxel in the adjuvant setting, alone or in combination with other agents, are under way (Table 6). An ongoing Intergroup trial randomized 3,500 node-positive patients to four cycles of doxorubicin at 60, 75, or 90 mg/m2 plus cyclophosphamide at 600 mg/m2 and then to either paclitaxel at 175 mg/m2 administered over three hours every three weeks or no further treatment. Preliminary data indicate a DFS and a marginal OS improvement for the group receiving AC followed by paclitaxel, with a similar outcome for the three dose levels of doxorubicin [69]. Although these results are promising, they represent an interim analysis, and longer follow-up is needed to determine the real contribution and safety of paclitaxel in the adjuvant setting. In the NSABP B-28 study, patients are currently being randomized to paclitaxel or no further treatment after four cycles of AC. In an ongoing study at MD Anderson, paclitaxel as a single agent is being compared with FAC as neoadjuvant chemotherapy approaches for patients with stage II and III (operable) breast cancer [81].
| Research group . | Trial design . | ||
|---|---|---|---|
| Paclitaxel trials . | |||
| Intergroup | AC (60/600) | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| AC (75/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| AC (90/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| Intergroup (4-9 + LN) | A→Paclitaxel→C | versus | AC × 4→HDCT |
| CALGB | A→Paclitaxel→C | versus | AC × 4→Paclitaxel |
| NSABP B-28 | AC (60/600) × 4 | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| Milan NCI | A→CMF | versus | A + Paclitaxel→CMF |
| MDACC | FAC × 8 | versus | Paclitaxel × 4→FAC × 4 |
| Docetaxel trials | |||
| Canadian group | FAC × 6 | versus | Docetaxel/AC (TAC) |
| Canadian group | AC × 6 | versus | Adriamycin/Docetaxel × 6 |
| NSABP B-27 | Surgery | ||
| AC (60/600) × 4 | ← | Docetaxel→Surgery | |
| Surgery→Docetaxel | |||
| Research group . | Trial design . | ||
|---|---|---|---|
| Paclitaxel trials . | |||
| Intergroup | AC (60/600) | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| AC (75/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| AC (90/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| Intergroup (4-9 + LN) | A→Paclitaxel→C | versus | AC × 4→HDCT |
| CALGB | A→Paclitaxel→C | versus | AC × 4→Paclitaxel |
| NSABP B-28 | AC (60/600) × 4 | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| Milan NCI | A→CMF | versus | A + Paclitaxel→CMF |
| MDACC | FAC × 8 | versus | Paclitaxel × 4→FAC × 4 |
| Docetaxel trials | |||
| Canadian group | FAC × 6 | versus | Docetaxel/AC (TAC) |
| Canadian group | AC × 6 | versus | Adriamycin/Docetaxel × 6 |
| NSABP B-27 | Surgery | ||
| AC (60/600) × 4 | ← | Docetaxel→Surgery | |
| Surgery→Docetaxel | |||
| Research group . | Trial design . | ||
|---|---|---|---|
| Paclitaxel trials . | |||
| Intergroup | AC (60/600) | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| AC (75/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| AC (90/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| Intergroup (4-9 + LN) | A→Paclitaxel→C | versus | AC × 4→HDCT |
| CALGB | A→Paclitaxel→C | versus | AC × 4→Paclitaxel |
| NSABP B-28 | AC (60/600) × 4 | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| Milan NCI | A→CMF | versus | A + Paclitaxel→CMF |
| MDACC | FAC × 8 | versus | Paclitaxel × 4→FAC × 4 |
| Docetaxel trials | |||
| Canadian group | FAC × 6 | versus | Docetaxel/AC (TAC) |
| Canadian group | AC × 6 | versus | Adriamycin/Docetaxel × 6 |
| NSABP B-27 | Surgery | ||
| AC (60/600) × 4 | ← | Docetaxel→Surgery | |
| Surgery→Docetaxel | |||
| Research group . | Trial design . | ||
|---|---|---|---|
| Paclitaxel trials . | |||
| Intergroup | AC (60/600) | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| AC (75/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| AC (90/600) | < | Paclitaxel × 4 | |
| No Paclitaxel | |||
| Intergroup (4-9 + LN) | A→Paclitaxel→C | versus | AC × 4→HDCT |
| CALGB | A→Paclitaxel→C | versus | AC × 4→Paclitaxel |
| NSABP B-28 | AC (60/600) × 4 | < | Paclitaxel × 4 |
| No Paclitaxel | |||
| Milan NCI | A→CMF | versus | A + Paclitaxel→CMF |
| MDACC | FAC × 8 | versus | Paclitaxel × 4→FAC × 4 |
| Docetaxel trials | |||
| Canadian group | FAC × 6 | versus | Docetaxel/AC (TAC) |
| Canadian group | AC × 6 | versus | Adriamycin/Docetaxel × 6 |
| NSABP B-27 | Surgery | ||
| AC (60/600) × 4 | ← | Docetaxel→Surgery | |
| Surgery→Docetaxel | |||
Sequential paclitaxel-based combinations are being explored in the adjuvant setting. Hudis and coworkers developed a dose-dense regimen in which three cycles of doxorubicin (90 mg/m2), three cycles of paclitaxel (250 mg/m2/24 h) and three cycles of cyclophosphamide (3,000 mg/m2) are administered sequentially (A→T→C) at 14-day intervals with G-CSF support. Preliminary results have shown an 81% DFS rate at three years [82]. Based on these encouraging results, an Intergroup randomized trial was launched for patients with four to nine positive nodes. In this study, patients are randomized to the above dose-dense sequential regimen or to four cycles of 80 mg/m2 doxorubicin plus 600 mg/m2 cyclophosphamide, followed by a single cycle of HDCT and autologous peripheral blood stem cell rescue. Another trial, led by the CALGB, is randomizing patients to A→T→C versus AC followed by paclitaxel administered on a three-week schedule. Gianni and colleagues are randomizing patients with high-risk breast cancer to doxorubicin plus paclitaxel (AT) followed by CMF or to the same regimen without paclitaxel.
Two Canadian trials are evaluating the efficacy of docetaxel in the adjuvant setting. One of the trials is randomizing patients to FAC or TAC (docetaxel, doxorubicin and cyclophosphamide). The second trial is comparing the AC combination versus TA (docetaxel and doxorubicin). The NSABP B-27 trial is evaluating the role of adding docetaxel to AC in the neoadjuvant or adjuvant settings [83]. In this study, patients receive four cycles of neoadjuvant AC chemotherapy either alone (Group I) or four cycles of AC followed by four cycles of docetaxel in the neoadjuvant (Group II) or adjuvant (Group III) settings.
Vinorelbine
Vinorelbine (navelbine) is a novel vinca alkaloid that has shown significant activity against breast cancer. In previously untreated patients with metastatic breast cancer, the response rate is 45%. In patients previously treated with doxorubicin-based chemotherapy and/or taxanes, the response rate is 20%-30% [84]. Ongoing clinical trials are testing the efficacy of vinorelbine in combination with other drugs in the adjuvant and neoadjuvant setting [85].
Molecular Predictors of Response to Adjuvant and Neoadjuvant Chemotherapy
Molecular abnormalities present in the primary tumor may aid in selecting the optimal adjuvant therapy for patients with breast cancer. Chang and colleagues [86] showed that pretreatment p53 and HER-2/neu (also known as erbB-2) overexpression predict clinical response to neoadjuvant chemotherapy and improved OS. Retrospective data from large adjuvant chemotherapy trials have revealed an association between HER-2/neu overexpression and resistance to chemotherapy. Gusterson and colleagues [87] showed that women with early-stage breast cancer undergoing CMF chemotherapy in the adjuvant setting did not benefit if their tumors overexpressed the HER-2/neu protein. The CALGB 8541 trial evaluated CAF dose-intensity in the adjuvant setting. In this study, patients whose tumors overexpressed HER-2/neu had an improved survival with higher (full) doses of CAF chemotherapy [66]. These data suggest that HER-2/neu-mediated drug resistance may be overcome by full doses of doxorubicin.
The role of HER-2/neu overexpression and response to paclitaxel is controversial. Retrospective data from Memorial Sloan-Kettering Cancer Center [88] indicate increased sensitivity to paclitaxel in patients with metastases and HER-2/neu overexpressing tumors. However, randomized clinical trials showed a poor response to paclitaxel (14%) for this patient population and improved response rates and survival for patients treated with paclitaxel in combination with an anti-HER-2 antibody (herceptin) [89]. Experimental in vivo data indicate that HER-2/neu overexpressing tumors are resistant to paclitaxel-induced apoptosis [90], supporting the results observed in randomized clinical trials of Herceptin with or without paclitaxel.
Apoptosis is one of the most important mechanisms of chemotherapy-induced cytotoxicity. Ellis and coworkers [91] showed that doxorubicin-based neoadjuvant chemotherapy can induce apoptosis in primary tumors from patients undergoing doxorubicin-based primary chemotherapy. Anthracyclines induce apoptosis through the P53 protein and experimental tumors with a mutated (inactive) p53 gene are resistant to anthracyclines. Berruti and colleagues [92] reported an association between wild-type P53 protein and response to neoadjuvant doxorubicin-based chemotherapy, suggesting that an intact P53 may be required for chemotherapy-induced cytotoxicity. In contrast, the taxanes induce cell death in a P53-independent manner. In one study, paclitaxel was more effective for patients whose tumors overexpressed the P53 protein [93]. The Bcl-2 protein is another important molecule in the control of apoptosis. Bcl-2 expression has been associated with ER positive status and low proliferation [94]. Archer and colleagues [95] showed that Bcl-2-negative patients were more likely to respond to anthracycline-based chemotherapy than Bcl-2-positive patients. However, the role of Bcl-2 in drug sensitivity is not well defined. Another gene undergoing evaluation is the multi-drug-resistance gene (mdr-1). The MDR-1 protein is located at the cell membrane and functions as a pump that detoxifies the cell from noxious agents. Overexpression of the MDR-1 protein has been associated with resistance to anthracycline- and taxane-based chemotherapy in vitro. However, the clinical value of MDR-1 expression in breast cancer has not been clearly defined [96].
Integration of Non-Cytotoxic Agents for Breast Cancer
Our understanding of cancer at the molecular level is providing innumerable targets for therapeutic intervention, including growth factor receptors and their ligands, intracellular signal transduction molecules, cell-cycle regulatory proteins, and transcription factors (Table 7). Novel antihormonal agents include new antiestrogens, selective estrogen response modulators, aromatase inhibitors and luteinizing hormone-releasing hormone (LH-RH) agonists. Indirect strategies include angiogenesis inhibitors (e.g., TNP-470), agents that interfere with invasion (e.g., marimastat), modulators of drug resistance (e.g., antisense therapy to bcl-2), and inhibitors of osteoclastic activity in patients with bone metastasis (e.g., bisphosphonates). Immunotherapy approaches include the induction of antibody-dependent cell-mediated cytotoxicity by bispecific monoclonal antibodies and vaccine therapy.
| Target/mechanism of action . | Strategy or agent . |
|---|---|
| HER-2/neu | Herceptin (rhuHER2 mAb) |
| Bispecific mAb e23(Fv)PE | |
| E1A gene therapy | |
| E75 peptide vaccine | |
| EGFR | C225 mAb |
| Protein kinase C inhibitors | Bryostatin |
| Miltefosine | |
| Bcl-2 | Antisense therapy |
| Angiogenesis inhibitors | TNP-470 |
| Neovastat | |
| Angiostatin | |
| Endostatin | |
| CM101 (endotoxin) | |
| Interferon alpha | |
| IL-12 | |
| RPF4 | |
| Thalidomide | |
| Matrix metalloproteinase inhibitors | Marimastat |
| Bisphosphonates | Pamidronate |
| Clodronate | |
| Zoledronate | |
| Ibandronate | |
| Antiestrogens | Toremifene |
| Faslodex | |
| Selective estrogen response modulators | Raloxifene |
| Aromatase inhibitors | Anastrazole |
| Letrozole | |
| LH-RH agonists | Leuprolide |
| Goserelin |
| Target/mechanism of action . | Strategy or agent . |
|---|---|
| HER-2/neu | Herceptin (rhuHER2 mAb) |
| Bispecific mAb e23(Fv)PE | |
| E1A gene therapy | |
| E75 peptide vaccine | |
| EGFR | C225 mAb |
| Protein kinase C inhibitors | Bryostatin |
| Miltefosine | |
| Bcl-2 | Antisense therapy |
| Angiogenesis inhibitors | TNP-470 |
| Neovastat | |
| Angiostatin | |
| Endostatin | |
| CM101 (endotoxin) | |
| Interferon alpha | |
| IL-12 | |
| RPF4 | |
| Thalidomide | |
| Matrix metalloproteinase inhibitors | Marimastat |
| Bisphosphonates | Pamidronate |
| Clodronate | |
| Zoledronate | |
| Ibandronate | |
| Antiestrogens | Toremifene |
| Faslodex | |
| Selective estrogen response modulators | Raloxifene |
| Aromatase inhibitors | Anastrazole |
| Letrozole | |
| LH-RH agonists | Leuprolide |
| Goserelin |
| Target/mechanism of action . | Strategy or agent . |
|---|---|
| HER-2/neu | Herceptin (rhuHER2 mAb) |
| Bispecific mAb e23(Fv)PE | |
| E1A gene therapy | |
| E75 peptide vaccine | |
| EGFR | C225 mAb |
| Protein kinase C inhibitors | Bryostatin |
| Miltefosine | |
| Bcl-2 | Antisense therapy |
| Angiogenesis inhibitors | TNP-470 |
| Neovastat | |
| Angiostatin | |
| Endostatin | |
| CM101 (endotoxin) | |
| Interferon alpha | |
| IL-12 | |
| RPF4 | |
| Thalidomide | |
| Matrix metalloproteinase inhibitors | Marimastat |
| Bisphosphonates | Pamidronate |
| Clodronate | |
| Zoledronate | |
| Ibandronate | |
| Antiestrogens | Toremifene |
| Faslodex | |
| Selective estrogen response modulators | Raloxifene |
| Aromatase inhibitors | Anastrazole |
| Letrozole | |
| LH-RH agonists | Leuprolide |
| Goserelin |
| Target/mechanism of action . | Strategy or agent . |
|---|---|
| HER-2/neu | Herceptin (rhuHER2 mAb) |
| Bispecific mAb e23(Fv)PE | |
| E1A gene therapy | |
| E75 peptide vaccine | |
| EGFR | C225 mAb |
| Protein kinase C inhibitors | Bryostatin |
| Miltefosine | |
| Bcl-2 | Antisense therapy |
| Angiogenesis inhibitors | TNP-470 |
| Neovastat | |
| Angiostatin | |
| Endostatin | |
| CM101 (endotoxin) | |
| Interferon alpha | |
| IL-12 | |
| RPF4 | |
| Thalidomide | |
| Matrix metalloproteinase inhibitors | Marimastat |
| Bisphosphonates | Pamidronate |
| Clodronate | |
| Zoledronate | |
| Ibandronate | |
| Antiestrogens | Toremifene |
| Faslodex | |
| Selective estrogen response modulators | Raloxifene |
| Aromatase inhibitors | Anastrazole |
| Letrozole | |
| LH-RH agonists | Leuprolide |
| Goserelin |
Baselga and colleagues [97] reported a phase II clinical trial of rhuMAbHER2 (herceptin), a humanized anti-HER-2/neu monoclonal antibody. In this study, 45 patients with metastatic breast cancer refractory to multiple chemotherapies and hormone manipulations received weekly rhuMAb HER2 antibody therapy. The response rate in this heavily pre-treated group was 11%. In addition, 37% of patients had stable disease after multiple doses. The results of this study showed that binding a tumor-associated antigen (HER2) with a specific monoclonal antibody may result in objective clinical responses in patients with breast cancer. Slamon and coworkers [89] reported improved DFS and time to progression for the combination of herceptin with paclitaxel or AC versus chemotherapy (or antibody) alone in patients with metastatic breast cancer.
Current gene therapy trials for patients with breast cancer are setting the tone for future development. Ueno and colleagues [98] reported an E1A gene therapy trial for patients with HER-2/neu overexpressing tumors. In this study, patients with malignant pleural effusions or ascites received liposomal E1A gene infusions into the pleural or peritoneal cavity. Preliminary data indicate that HER2/neu expression was downregulated, confirming prior in vitro and in vivo data.
Although this is an era of tremendous excitement, clinical investigators are faced with new challenges [99]. Current methodology was developed to assess safety and efficacy of cytotoxic agents and may not apply to the clinical development of targeted biological therapy. One of the most important endpoints of early clinical trials is identification of the optimal biological dose, which is likely to be different from the maximum tolerated dose. In terms of efficacy, noncytotoxic agents may not induce objective tumor responses by current criteria, and therefore surrogate biological markers are needed. Nemunaitis and colleagues [100] have recently reported a method for assessing the biologic effects of marimastat, a metalloproteinase inhibitor in patients with advanced solid tumors. These authors were able to determine the optimal dose for subsequent randomized trials using a panel of serum tumor markers (CEA, CA-125, and CA19-9). In the future, it is likely that novel biological therapy will be used in combination with conventional chemotherapy.
Conclusion
In summary, chemotherapy will continue to play an important role in the management of patients with primary breast cancer. Future clinical trials will clarify the role of HDCT for patients at high risk. Molecular markers of response may assist in drug selection in the adjuvant setting. Development of novel therapeutic agents continues, based on expanded biological understanding of tumor development and progression.
Acknowledgments
We thankVickie Williams for editing assistance.



