-
PDF
- Split View
-
Views
-
Cite
Cite
Lei Wang, Jin-Kai Feng, Chong-De Lu, Jia-Yi Wu, Bin Zhou, Kang Wang, Xu-Biao Wei, Chao Liang, Hong-Kun Zhou, Jie Shi, Wei-Xing Guo, Wan Yee Lau, Mao-Lin Yan, Shu-Qun Cheng, Salvage Surgery for Initially Unresectable HCC With PVTT Converted by Locoregional Treatment Plus Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody, The Oncologist, Volume 29, Issue 8, August 2024, Pages e1041–e1050, https://doi.org/10.1093/oncolo/oyae032
Close - Share Icon Share
Abstract
This study aimed to compare the survival outcomes of patients with initially unresectable hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT) who underwent or did not undergo salvage surgery followed by a triple combination conversion treatment consisted of locoregional treatment (LRT), tyrosine kinase inhibitors (TKIs), and anti-PD-1 antibodies.
The data from 93 consecutive patients with initially unresectable HCC and PVTT across 4 medical centers were retrospectively reviewed. They were converted successfully by the triple combination treatment and underwent or did not undergo salvage resection. The baseline characteristics, conversion schemes, conversion treatment-related adverse events (CTRAEs), overall survival (OS), and progression-free survival (PFS) of the salvage surgery and non-surgery groups were compared. Multivariate Cox regression analysis was performed to identify independent risk factors for OS and PFS. Additionally, subgroup survival analysis was conducted by stratification of degree of tumor response and type of PVTT.
Of the 93 patients, 44 underwent salvage surgery, and 49 did not undergo salvage surgery. The OS and PFS of the salvage surgery and non-surgery groups were not significantly different (P = .370 and .334, respectively). The incidence and severity of CTRAEs of the 2 groups were also comparable. Subgroup analyses revealed that for patients with complete response (CR) or types III-IV PVTT, there was a trend toward better survival in patients who did not undergo salvage surgery. Multivariate analysis showed that baseline α-fetoprotein and best tumor response per mRECIST criteria were independent prognostic factors for OS and PFS.
For patients with initially unresectable HCC and PVTT who were successfully converted by the triple combination therapy, salvage liver resection may not be necessary, especially for the patients with CR or types III-IV PVTT.
For patients with initially unresectable hepatocellular carcinoma and portal vein tumor thrombus (PVTT) who were successfully converted by the triple combination therapy, salvage liver resection may not be necessary, especially for the patients with complete tumor response or types III-IV PVTT.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related mortality globally.1 More than 50% of patients with HCC are diagnosed at an advanced stage where they have already lost the opportunity for curative surgery, leading to a poor survival outcome.2 HCC tends to invade the portal vein branches and forms a portal vein tumor thrombus (PVTT). PVTT is the most prominent character and a poor prognostic factor of advanced HCC.3,4 Systemic therapy, such as tyrosine kinase inhibitors (TKIs) and anti-PD-1 antibodies, forms the cornerstone of treatment for advanced HCC.
Conversion therapy is aimed to downstage initially unresectable tumors and provide patients a chance to undergo curative resection. The conception of conversion therapy for HCC was proposed over 20 years ago.5,6 In the past, most conversion therapies for advanced HCC were locoregional treatment (LRT).7-9 Nevertheless, the conversion rate of LRT alone is only 9.5%–16.9%,10,11 and few patients can achieve tumor downstaging or remission following LRT alone. Recently, a series of studies have documented that locoregional and systemic combination therapy renders a better tumor response and a higher conversion rate in advanced HCC, which is attributable to a synergistic effect and various mechanisms of action.12-14 The conversion rate of locoregional and systemic combination treatment strategy for HCC was reported to range from 44.1% to 60%.14-16 However, there is limited knowledge about the necessity of salvage surgery after conversion therapy for patients with HCC with PVTT. It is still unknown whether it is necessary to maintain TKIs and/or anti-PD-1 antibodies treatment after conversion resection.
Therefore, there is an urgent need to conduct research to appraise the necessity of postoperative adjuvant treatment after conversion resection, because it has substantial clinical implications to inform surgeons of therapeutic decision-makings. In response to this demand, we performed a multicenter study, which investigated a cohort of 93 patients with initially unresectable HCC with PVTT who were successfully converted with a triple combination of TKI plus anti-PD-1 antibody and LRT (transarterial chemoembolization [TACE] and/or radiotherapy [RT]).
Materials and Methods
Patients
Consecutive patients with initially unresectable HCC with PVTT who were successfully converted by LRTs combined with TKIs and anti-PD-1 antibodies between June 2019 and October 2021, across 4 tertiary hospitals, Eastern Hepatobiliary Surgery Hospital, Fujian Provincial Hospital, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, and The First Hospital of Jiaxing, were included. The diagnosis of HCC conformed to the American Association for the Study of Liver Diseases and Chinese Guidelines for Diagnosis and Treatment of Primary Liver Cancer.17,18 The diagnosis of PVTT was based on typical radiological features on imaging studies or histological examinations.19 Cheng’s classification was used to classify PVTT into 4 types according to the extent of PVTT in the portal vein: type I, tumor thrombus in the segmental branches of the main portal vein (MPV) or above; type II, tumor thrombus extending to the right or left portal vein; type III, tumor thrombus extending to the MPV; and type IV, tumor thrombus extending to the MPV and superior mesenteric vein.20
At each center, the general condition of patients, tumor burden, and resectability were collectively assessed by a multidisciplinary treatment team, which consisted of liver surgeons, hepatologists, oncologists, radiologists, interventional radiologists, and pathologists. Patients were considered unresectable if they had low liver functional reserve of future liver remnant (FLR), risk for incomplete resection margin, or tumor thrombus extension into MPV or superior mesenteric vein (Supplementary Table S1).
This study protocol was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki and approved by the Clinical Research Ethics Committees of all participating institutions (Approval number: EHBHKY2022-H028-P001). Written informed consent was obtained before commencing the conversion therapy regimen and prior to salvage surgery.
Triple Combination Treatment Modalities
LRT was composed of TACE and/or RT. Super-selective TACE was performed through the right femoral artery using the Seldinger technique. Hepatic arteriography was performed to determine the number, size, and location of lesions, tumor feeding arteries, and anatomic variations. After the blood supply branch of the tumor was identified, a chemotherapeutic solution of pirarubicin mixed with iodized oil (less than 30 mL) was slowly injected into the super-selective tumor artery via a microcatheter, until the tumor lesion was well deposited with iodized oil and the blood flow was slowed down. Subsequently, the feeding arteries were selectively embolized using gelatin sponge particles until the blood flow was completely stagnant.
Regarding RT technique, external beam radiotherapy was administered. The planned total radiation dose was 18 Gy, delivered in a fraction of 3 Gy using 6 megavolts X-ray with a linear accelerator (Elekta Synergy), at a rate of 5 fractions per week. Before each treatment course, patients underwent a megavoltage cone-beam computed tomography (CT) and were positioned with automated image registration with the application of the image guided radiotherapy (IGRT) system (Elekta Synergy). Considering the physiological movements due to respiratory cycle, the respiratory gating technique was used to minimize the radiation dose delivered to surrounding healthy tissues. The RT regimen was in compliance with that used in our previous research.4,21
Treatment with TKIs and anti-PD-1 antibodies was initiated within 1 week after LRT, based on patients’ liver function recovery. The TKIs used included sorafenib (400 mg orally twice daily) and lenvatinib (8 mg for bodyweight < 60 kg or 12 mg for bodyweight ≥ 60 kg, orally once daily).22,23The anti-PD-1 antibodies used included nivolumab (240 mg q2w),24 pembrolizumab (200 mg q3w),25 camrelizumab (200 mg q2w),26 tislelizumab (200 mg q3w),27 toripalimab (3 mg/kg bodyweight q2w),28 sintilimab (200 mg q3w),29 and penpulimab (200 mg q3w).30 The selection of anti-PD-1 antibody drugs was based on the most recent efficacy and safety data, patient economic status, and drug availability at each center. During conversion therapy, adverse events (AEs) were closely monitored. Specifically, if a patient experienced any grade 3 or higher AEs, the drug dosages were reduced until symptoms were alleviated.
Treatment Following Successful Conversion
After conversion therapy, the 93 patients were successfully downstaged and reached the resectability criteria (Supplementary Table S2): (1) sufficient FLR (≥ 40% for cirrhotic patients and ≥ 30% for non-cirrhotic patients); (2) adequate resection margin (R0 liver resection) is attainable; (3) complete or partial response (CR or PR) of intrahepatic lesions maintained for at least 2 months; (4) types III-IV PVTT were downstaged to type I or II PVTT on contrast-enhanced imaging; (5) no serious AEs following conversion therapy; and (6) no contraindications for liver resection (eg, good general condition, Child-Pugh score ≤ B7, and absence of portal hypertension).11
The decisions to undergo salvage surgery or not after conversion treatment depended on patients. For patients who did not undergo salvage surgery, TKIs and/or anti-PD-1 antibodies were continuously used for at least 6 months. Patients scheduled for salvage liver resection were required to discontinue TKIs for at least 1 week, anti-PD-1 antibodies for at least 2 weeks, and LRTs for at least 4 weeks. The extent of hepatectomy depended on tumor number and size, as well as the degree of liver cirrhosis of the patients. The surgical approaches, including laparoscopic and open surgery, were determined through shared decision-making between the surgeons and patients. Following salvage surgery, it was recommended to undergo postoperative adjuvant treatment by using all or part of the systemic conversion therapy drugs for at least 6 months.11
Definitions of Study Endpoints and Follow-up
The primary endpoints were overall survival (OS) and progression-free survival (PFS). OS was defined as the interval between the date of initiation of conversion therapy and the patient’s death or last follow-up. PFS was defined as the period between the date of salvage surgery and the date of HCC recurrence or last follow-up for the surgery group, and the period between the date of downstaging after conversion therapy and the date of tumor progression or last follow-up for the non-surgery group. The secondary endpoints were conversion treatment-related AEs (CTRAEs) for both groups, and perioperative complications and 90-day mortality for the salvage surgery group. Conversion treatment-related AEs (CTRAEs) and postoperative complications were categorized using the Clavien-Dindo classification.31 Patients were closely monitored after conversion or salvage surgery every 2-3 months during the first 2 years, followed by every 6 months thereafter. The follow-up surveillance items included serum tumor markers, abdominal ultrasound, and contrast-enhanced CT or MRI. This study was censored on June 30, 2023.
Statistical Analysis
Categorical variables were presented as frequencies and percentages, and compared using the chi-square test or Fisher’s exact test, where appropriate. Continuous variables were expressed as median (interquartile range [IQR]) or mean (standard deviation [SD]), and compared using the Mann-Whitney U test or Student’s t test. The Kaplan-Meier method was used to estimate OS and PFS. The log-rank test was used to compare survival differences between different groups. To identify independent prognostic factors, univariate and multivariate Cox regression analyses were conducted. In the multivariate Cox regression analysis, variables with P values < .1 in the univariate analysis were incorporated using the backward selection LR method. All statistical tests were 2-tailed, and a P-value <.05 was considered statistically significant. The statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software (version 24, IBM, Chicago, IL, USA).
Results
Patient Characteristics
Ninety-three patients with initially unresectable HCC with PVTT who underwent (n = 44) or did not undergo salvage resection (n = 49) after triple combination conversion treatment were included (Supplementary Fig. S1). The patients had a mean age of 54.5 ± 9.3 years, with the majority of patients being male (92.5%); 44.1% of patients had baseline AFP ≥ 400 ng/mL, and 74.2% of patients had PIVKA-II ≥ 400 mAU/mL. The mean maximum tumor diameter was 10.1 ± 3.2 cm, and multiple tumors were observed in 43.0% of patients. Forty-two patients (45.2%) had type II PVTT, and the remaining 51 patients (54.8%) had types III-IV PVTT (Table 1). As shown in Table 1, there were no marked differences in baseline characteristics between the surgery and non-surgery groups. Of the 44 patients who underwent salvage surgery, 26 (59.1%) received postoperative adjuvant treatment, with 17 (38.6%) receiving a combination of TKI and anti-PD-1 antibody, 7 (15.9%) receiving TKI alone, and 2 (4.5%) receiving anti-PD-1 antibody alone.
| Characteristic . | Total (n = 93) n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| Age (years) | 54.5 ± 9.3 | 54.5 ± 10.6 | 54.5 ± 8.0 | .978 |
| Sex (male/female) | 86 (92.5)/7 (7.5) | 41 (93.2)/3 (6.8) | 45 (91.8)/4 (8.2) | 1.000 |
| Etiology (HBV/HCV/non-viral) | 84(90.3)/2(2.2)/7(7.5) | 38 (86.4)/1 (2.3)/5 (11.4) | 46 (93.9)/1 (2.0)/2 (4.1) | .410 |
| ECOG performance status (0/1) | 52 (55.9)/41 (44.1) | 29 (65.9)/15 (34.1) | 23 (46.9)/26 (53.1) | .066 |
| Liver cirrhosis (present/absent) | 68 (73.1)/25 (26.9) | 29 (63.6)/15 (36.4) | 39 (81.6)/10 (18.4) | .137 |
| TBil (µmol/L) | 16.1 (12.1-21.7) | 16.4 (11.7-23.4) | 15.8 (12.5-20.6) | .814 |
| ALB (g/L) | 40.0 ± 4.5 | 40.5 ± 4.9 | 39.6 ± 4.1 | .319 |
| ALT (U/L) | 41 (28-64) | 45 (25.8-66.3) | 39 (28-62) | .749 |
| AST (U/L) | 48 (31-70) | 44 (26.5-71.0) | 49 (34-71) | .579 |
| PLT (*109/L) | 156 (118–192) | 157.5 (126-232.5) | 140 (108.5-181.5) | .074 |
| HGB (g/L) | 141.2 ± 22.7 | 145.1 ± 22.8 | 137.7 ± 22.2 | .118 |
| PT (s) | 12.2 (11.5-13.1) | 12.0 (11.4-13.2) | 12.2 (11.6-12.9) | .923 |
| Child-Pugh class (A/B) | 82 (88.2)/11 (11.8) | 39 (88.6)/5 (11.4) | 43 (87.8)/6 (12.2) | .895 |
| Baseline AFP (<400/≥400 ng/mL) | 52 (55.9)/41 (44.1) | 22 (50.0)/ 22 (50.0) | 30 (61.2)/19 (38.8) | .276 |
| Baseline PIVKA-II (<400/≥400 mAU/mL) | 24 (25.8)/69 (74.2) | 12 (27.3)/32 (72.7) | 12 (24.5)/37 (75.5) | .759 |
| Tumor number (single/multiple) | 53 (57.0)/40 (43.0) | 27 (61.4)/17 (38.6) | 26 (53.1)/23 (46.9) | .419 |
| Tumor diameter (cm) | 10.1 ± 3.2 | 10.4 ± 3.2 | 9.9 ± 3.2 | .490 |
| Tumor diameter (<10/≥10 cm) | 47 (50.5)/46 (49.5) | 19 (43.2)/25 (56.8) | 28 (57.1)/21 (42.9) | .179 |
| PVTT Cheng’s type (II/III-IV) | 42 (45.2)/51 (54.8) | 22 (50.0)/22 (50.0) | 20 (40.8)/29 (59.2) | .374 |
| Adjuvant therapy following salvage surgery | — | 26 (59.1)/18 (40.9) | — | — |
| Characteristic . | Total (n = 93) n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| Age (years) | 54.5 ± 9.3 | 54.5 ± 10.6 | 54.5 ± 8.0 | .978 |
| Sex (male/female) | 86 (92.5)/7 (7.5) | 41 (93.2)/3 (6.8) | 45 (91.8)/4 (8.2) | 1.000 |
| Etiology (HBV/HCV/non-viral) | 84(90.3)/2(2.2)/7(7.5) | 38 (86.4)/1 (2.3)/5 (11.4) | 46 (93.9)/1 (2.0)/2 (4.1) | .410 |
| ECOG performance status (0/1) | 52 (55.9)/41 (44.1) | 29 (65.9)/15 (34.1) | 23 (46.9)/26 (53.1) | .066 |
| Liver cirrhosis (present/absent) | 68 (73.1)/25 (26.9) | 29 (63.6)/15 (36.4) | 39 (81.6)/10 (18.4) | .137 |
| TBil (µmol/L) | 16.1 (12.1-21.7) | 16.4 (11.7-23.4) | 15.8 (12.5-20.6) | .814 |
| ALB (g/L) | 40.0 ± 4.5 | 40.5 ± 4.9 | 39.6 ± 4.1 | .319 |
| ALT (U/L) | 41 (28-64) | 45 (25.8-66.3) | 39 (28-62) | .749 |
| AST (U/L) | 48 (31-70) | 44 (26.5-71.0) | 49 (34-71) | .579 |
| PLT (*109/L) | 156 (118–192) | 157.5 (126-232.5) | 140 (108.5-181.5) | .074 |
| HGB (g/L) | 141.2 ± 22.7 | 145.1 ± 22.8 | 137.7 ± 22.2 | .118 |
| PT (s) | 12.2 (11.5-13.1) | 12.0 (11.4-13.2) | 12.2 (11.6-12.9) | .923 |
| Child-Pugh class (A/B) | 82 (88.2)/11 (11.8) | 39 (88.6)/5 (11.4) | 43 (87.8)/6 (12.2) | .895 |
| Baseline AFP (<400/≥400 ng/mL) | 52 (55.9)/41 (44.1) | 22 (50.0)/ 22 (50.0) | 30 (61.2)/19 (38.8) | .276 |
| Baseline PIVKA-II (<400/≥400 mAU/mL) | 24 (25.8)/69 (74.2) | 12 (27.3)/32 (72.7) | 12 (24.5)/37 (75.5) | .759 |
| Tumor number (single/multiple) | 53 (57.0)/40 (43.0) | 27 (61.4)/17 (38.6) | 26 (53.1)/23 (46.9) | .419 |
| Tumor diameter (cm) | 10.1 ± 3.2 | 10.4 ± 3.2 | 9.9 ± 3.2 | .490 |
| Tumor diameter (<10/≥10 cm) | 47 (50.5)/46 (49.5) | 19 (43.2)/25 (56.8) | 28 (57.1)/21 (42.9) | .179 |
| PVTT Cheng’s type (II/III-IV) | 42 (45.2)/51 (54.8) | 22 (50.0)/22 (50.0) | 20 (40.8)/29 (59.2) | .374 |
| Adjuvant therapy following salvage surgery | — | 26 (59.1)/18 (40.9) | — | — |
Abbreviations: AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECOG, eastern cooperative oncology group; HBV, hepatitis B virus; HCV, hepatitis C virus; HGB, hemoglobin; PIVKA-II, protein induced by vitamin K absence or antagonist II; PLT, platelet; PT, prothrombin time; PVTT, portal vein tumor thrombus; TBil, total bilirubin.
| Characteristic . | Total (n = 93) n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| Age (years) | 54.5 ± 9.3 | 54.5 ± 10.6 | 54.5 ± 8.0 | .978 |
| Sex (male/female) | 86 (92.5)/7 (7.5) | 41 (93.2)/3 (6.8) | 45 (91.8)/4 (8.2) | 1.000 |
| Etiology (HBV/HCV/non-viral) | 84(90.3)/2(2.2)/7(7.5) | 38 (86.4)/1 (2.3)/5 (11.4) | 46 (93.9)/1 (2.0)/2 (4.1) | .410 |
| ECOG performance status (0/1) | 52 (55.9)/41 (44.1) | 29 (65.9)/15 (34.1) | 23 (46.9)/26 (53.1) | .066 |
| Liver cirrhosis (present/absent) | 68 (73.1)/25 (26.9) | 29 (63.6)/15 (36.4) | 39 (81.6)/10 (18.4) | .137 |
| TBil (µmol/L) | 16.1 (12.1-21.7) | 16.4 (11.7-23.4) | 15.8 (12.5-20.6) | .814 |
| ALB (g/L) | 40.0 ± 4.5 | 40.5 ± 4.9 | 39.6 ± 4.1 | .319 |
| ALT (U/L) | 41 (28-64) | 45 (25.8-66.3) | 39 (28-62) | .749 |
| AST (U/L) | 48 (31-70) | 44 (26.5-71.0) | 49 (34-71) | .579 |
| PLT (*109/L) | 156 (118–192) | 157.5 (126-232.5) | 140 (108.5-181.5) | .074 |
| HGB (g/L) | 141.2 ± 22.7 | 145.1 ± 22.8 | 137.7 ± 22.2 | .118 |
| PT (s) | 12.2 (11.5-13.1) | 12.0 (11.4-13.2) | 12.2 (11.6-12.9) | .923 |
| Child-Pugh class (A/B) | 82 (88.2)/11 (11.8) | 39 (88.6)/5 (11.4) | 43 (87.8)/6 (12.2) | .895 |
| Baseline AFP (<400/≥400 ng/mL) | 52 (55.9)/41 (44.1) | 22 (50.0)/ 22 (50.0) | 30 (61.2)/19 (38.8) | .276 |
| Baseline PIVKA-II (<400/≥400 mAU/mL) | 24 (25.8)/69 (74.2) | 12 (27.3)/32 (72.7) | 12 (24.5)/37 (75.5) | .759 |
| Tumor number (single/multiple) | 53 (57.0)/40 (43.0) | 27 (61.4)/17 (38.6) | 26 (53.1)/23 (46.9) | .419 |
| Tumor diameter (cm) | 10.1 ± 3.2 | 10.4 ± 3.2 | 9.9 ± 3.2 | .490 |
| Tumor diameter (<10/≥10 cm) | 47 (50.5)/46 (49.5) | 19 (43.2)/25 (56.8) | 28 (57.1)/21 (42.9) | .179 |
| PVTT Cheng’s type (II/III-IV) | 42 (45.2)/51 (54.8) | 22 (50.0)/22 (50.0) | 20 (40.8)/29 (59.2) | .374 |
| Adjuvant therapy following salvage surgery | — | 26 (59.1)/18 (40.9) | — | — |
| Characteristic . | Total (n = 93) n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| Age (years) | 54.5 ± 9.3 | 54.5 ± 10.6 | 54.5 ± 8.0 | .978 |
| Sex (male/female) | 86 (92.5)/7 (7.5) | 41 (93.2)/3 (6.8) | 45 (91.8)/4 (8.2) | 1.000 |
| Etiology (HBV/HCV/non-viral) | 84(90.3)/2(2.2)/7(7.5) | 38 (86.4)/1 (2.3)/5 (11.4) | 46 (93.9)/1 (2.0)/2 (4.1) | .410 |
| ECOG performance status (0/1) | 52 (55.9)/41 (44.1) | 29 (65.9)/15 (34.1) | 23 (46.9)/26 (53.1) | .066 |
| Liver cirrhosis (present/absent) | 68 (73.1)/25 (26.9) | 29 (63.6)/15 (36.4) | 39 (81.6)/10 (18.4) | .137 |
| TBil (µmol/L) | 16.1 (12.1-21.7) | 16.4 (11.7-23.4) | 15.8 (12.5-20.6) | .814 |
| ALB (g/L) | 40.0 ± 4.5 | 40.5 ± 4.9 | 39.6 ± 4.1 | .319 |
| ALT (U/L) | 41 (28-64) | 45 (25.8-66.3) | 39 (28-62) | .749 |
| AST (U/L) | 48 (31-70) | 44 (26.5-71.0) | 49 (34-71) | .579 |
| PLT (*109/L) | 156 (118–192) | 157.5 (126-232.5) | 140 (108.5-181.5) | .074 |
| HGB (g/L) | 141.2 ± 22.7 | 145.1 ± 22.8 | 137.7 ± 22.2 | .118 |
| PT (s) | 12.2 (11.5-13.1) | 12.0 (11.4-13.2) | 12.2 (11.6-12.9) | .923 |
| Child-Pugh class (A/B) | 82 (88.2)/11 (11.8) | 39 (88.6)/5 (11.4) | 43 (87.8)/6 (12.2) | .895 |
| Baseline AFP (<400/≥400 ng/mL) | 52 (55.9)/41 (44.1) | 22 (50.0)/ 22 (50.0) | 30 (61.2)/19 (38.8) | .276 |
| Baseline PIVKA-II (<400/≥400 mAU/mL) | 24 (25.8)/69 (74.2) | 12 (27.3)/32 (72.7) | 12 (24.5)/37 (75.5) | .759 |
| Tumor number (single/multiple) | 53 (57.0)/40 (43.0) | 27 (61.4)/17 (38.6) | 26 (53.1)/23 (46.9) | .419 |
| Tumor diameter (cm) | 10.1 ± 3.2 | 10.4 ± 3.2 | 9.9 ± 3.2 | .490 |
| Tumor diameter (<10/≥10 cm) | 47 (50.5)/46 (49.5) | 19 (43.2)/25 (56.8) | 28 (57.1)/21 (42.9) | .179 |
| PVTT Cheng’s type (II/III-IV) | 42 (45.2)/51 (54.8) | 22 (50.0)/22 (50.0) | 20 (40.8)/29 (59.2) | .374 |
| Adjuvant therapy following salvage surgery | — | 26 (59.1)/18 (40.9) | — | — |
Abbreviations: AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECOG, eastern cooperative oncology group; HBV, hepatitis B virus; HCV, hepatitis C virus; HGB, hemoglobin; PIVKA-II, protein induced by vitamin K absence or antagonist II; PLT, platelet; PT, prothrombin time; PVTT, portal vein tumor thrombus; TBil, total bilirubin.
Combination Conversion Therapy Procedure
All patients received at least one session of combination conversion therapy (Table 2). The TKIs included sorafenib (8, 8.6%) and lenvatinib (85, 91.4%). Among the anti-PD-1 antibodies administered, sintilimab was the most commonly used (55, 59.1%), followed by camrelizumab (25, 26.9%), pembrolizumab (7, 7.5%), toripalimab (3, 3.2%), nivolumab (1, 1.1%), tislelizumab (1, 1.1%), and penpulimab (1, 1.1%). The LRTs included TACE (54, 58.1%), radiotherapy (26, 27.9%), and TACE combined with radiotherapy (13, 14.0%). There were no significant differences in conversion therapy categories between the salvage surgery and non-surgery groups.
| Conversion therapy class . | Total (n = 93), n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| TKIs | ||||
| Sorafenib | 8 (8.6) | 3 (4.5) | 5 (10.2) | .259 |
| Lenvatinib | 85 (91.4) | 41 (90.9) | 44 (89.8) | |
| Anti-PD-1 antibodies | ||||
| Nivolumab | 1 (1.1) | 0 | 1 (2.0) | .070 |
| Pembrolizumab | 7 (7.5) | 3 (6.8) | 4 (8.2) | |
| Camrelizumab | 25 (26.9) | 16 (36.1) | 9 (18.3) | |
| Tislelizumab | 1 (1.1) | 1(2.3) | 0 | |
| Toripalimab | 3 (3.2) | 0 | 3 (6.1) | |
| Sintilimab | 55 (59.1) | 23 (52.3) | 32 (65.3) | |
| Penpulimab | 1 (1.1) | 1(2.3) | 0 | |
| Locoregional treatment | .446 | |||
| TACE | 54 (58.1) | 23 (52.3) | 31 (63.3) | |
| Radiotherapy | 26 (27.9) | 15 (34.1) | 11 (22.4) | |
| TACE + Radiotherapy | 13 (14.0) | 6 (13.6) | 7 (14.3) | |
| Conversion time, median (range), months | 3.3 (1.2-9.7) | 3.2 (1.2-7.5) | 3.7 (1.9-9.7) | .444 |
| Best tumor response per mRECIST criteria | ||||
| CR | 29 (31.2) | 16 (36.4) | 13 (26.5) | .307 |
| PR | 64 (68.8) | 28 (63.6) | 36 (73.5) |
| Conversion therapy class . | Total (n = 93), n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| TKIs | ||||
| Sorafenib | 8 (8.6) | 3 (4.5) | 5 (10.2) | .259 |
| Lenvatinib | 85 (91.4) | 41 (90.9) | 44 (89.8) | |
| Anti-PD-1 antibodies | ||||
| Nivolumab | 1 (1.1) | 0 | 1 (2.0) | .070 |
| Pembrolizumab | 7 (7.5) | 3 (6.8) | 4 (8.2) | |
| Camrelizumab | 25 (26.9) | 16 (36.1) | 9 (18.3) | |
| Tislelizumab | 1 (1.1) | 1(2.3) | 0 | |
| Toripalimab | 3 (3.2) | 0 | 3 (6.1) | |
| Sintilimab | 55 (59.1) | 23 (52.3) | 32 (65.3) | |
| Penpulimab | 1 (1.1) | 1(2.3) | 0 | |
| Locoregional treatment | .446 | |||
| TACE | 54 (58.1) | 23 (52.3) | 31 (63.3) | |
| Radiotherapy | 26 (27.9) | 15 (34.1) | 11 (22.4) | |
| TACE + Radiotherapy | 13 (14.0) | 6 (13.6) | 7 (14.3) | |
| Conversion time, median (range), months | 3.3 (1.2-9.7) | 3.2 (1.2-7.5) | 3.7 (1.9-9.7) | .444 |
| Best tumor response per mRECIST criteria | ||||
| CR | 29 (31.2) | 16 (36.4) | 13 (26.5) | .307 |
| PR | 64 (68.8) | 28 (63.6) | 36 (73.5) |
Abbreviations: CR, complete response; mRECIST, the modified Response Evaluation Criteria in Solid Tumors; PR, partial response; TACE, transarterial chemoembolization; TKIs, tyrosine kinase inhibitors.
| Conversion therapy class . | Total (n = 93), n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| TKIs | ||||
| Sorafenib | 8 (8.6) | 3 (4.5) | 5 (10.2) | .259 |
| Lenvatinib | 85 (91.4) | 41 (90.9) | 44 (89.8) | |
| Anti-PD-1 antibodies | ||||
| Nivolumab | 1 (1.1) | 0 | 1 (2.0) | .070 |
| Pembrolizumab | 7 (7.5) | 3 (6.8) | 4 (8.2) | |
| Camrelizumab | 25 (26.9) | 16 (36.1) | 9 (18.3) | |
| Tislelizumab | 1 (1.1) | 1(2.3) | 0 | |
| Toripalimab | 3 (3.2) | 0 | 3 (6.1) | |
| Sintilimab | 55 (59.1) | 23 (52.3) | 32 (65.3) | |
| Penpulimab | 1 (1.1) | 1(2.3) | 0 | |
| Locoregional treatment | .446 | |||
| TACE | 54 (58.1) | 23 (52.3) | 31 (63.3) | |
| Radiotherapy | 26 (27.9) | 15 (34.1) | 11 (22.4) | |
| TACE + Radiotherapy | 13 (14.0) | 6 (13.6) | 7 (14.3) | |
| Conversion time, median (range), months | 3.3 (1.2-9.7) | 3.2 (1.2-7.5) | 3.7 (1.9-9.7) | .444 |
| Best tumor response per mRECIST criteria | ||||
| CR | 29 (31.2) | 16 (36.4) | 13 (26.5) | .307 |
| PR | 64 (68.8) | 28 (63.6) | 36 (73.5) |
| Conversion therapy class . | Total (n = 93), n (%) . | Patients who underwent hepatectomy (n = 44), n (%) . | Patients who did not undergo hepatectomy (n = 49), n (%) . | P-value . |
|---|---|---|---|---|
| TKIs | ||||
| Sorafenib | 8 (8.6) | 3 (4.5) | 5 (10.2) | .259 |
| Lenvatinib | 85 (91.4) | 41 (90.9) | 44 (89.8) | |
| Anti-PD-1 antibodies | ||||
| Nivolumab | 1 (1.1) | 0 | 1 (2.0) | .070 |
| Pembrolizumab | 7 (7.5) | 3 (6.8) | 4 (8.2) | |
| Camrelizumab | 25 (26.9) | 16 (36.1) | 9 (18.3) | |
| Tislelizumab | 1 (1.1) | 1(2.3) | 0 | |
| Toripalimab | 3 (3.2) | 0 | 3 (6.1) | |
| Sintilimab | 55 (59.1) | 23 (52.3) | 32 (65.3) | |
| Penpulimab | 1 (1.1) | 1(2.3) | 0 | |
| Locoregional treatment | .446 | |||
| TACE | 54 (58.1) | 23 (52.3) | 31 (63.3) | |
| Radiotherapy | 26 (27.9) | 15 (34.1) | 11 (22.4) | |
| TACE + Radiotherapy | 13 (14.0) | 6 (13.6) | 7 (14.3) | |
| Conversion time, median (range), months | 3.3 (1.2-9.7) | 3.2 (1.2-7.5) | 3.7 (1.9-9.7) | .444 |
| Best tumor response per mRECIST criteria | ||||
| CR | 29 (31.2) | 16 (36.4) | 13 (26.5) | .307 |
| PR | 64 (68.8) | 28 (63.6) | 36 (73.5) |
Abbreviations: CR, complete response; mRECIST, the modified Response Evaluation Criteria in Solid Tumors; PR, partial response; TACE, transarterial chemoembolization; TKIs, tyrosine kinase inhibitors.
The median time from the initiation of conversion treatment to successful downstaging was 3.3 months (range, 1.2-9.7 months) for the whole cohort. Sixty-four patients (68.8%) achieved partial response and 29 patients (31.2%) had complete response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). The conversion time and best tumor response rate were also comparable between the 2 groups (Table 2).
Survival of Patients Who Underwent or Did not Undergo Salvage Surgery
At the data cutoff on June 30, 2023, 45 patients (48.4%) had died, and 59 patients (63.4%) had disease recurrence or progression. The median OS for all 93 patients was 28.3 months (Supplementary Fig. S2A), and the median PFS for the entire cohort was 20.4 months (Supplementary Fig. S2B). After successful conversion therapy, 44 patients (47.3%) underwent salvage curative resection and 49 patients (52.7%) refused to undergo hepatectomy. The patients who underwent salvage surgery had similar OS (P = .370, Fig. 1A) and PFS (P = .334, Fig. 1B) compared with those who did not. The median OS and PFS for the salvage surgery group were 27.5 and 17.3 months, respectively. The median OS and PFS for the non-surgery group were 31.0 and 21.0 months, respectively (Fig. 1).
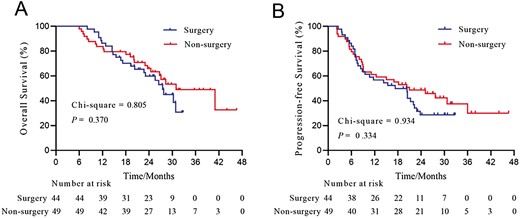
Overall survival (A) and progression-free survival (B) of patients with HCC and PVTT who underwent (n = 44) or did not undergo (n = 49) conversion surgery. Abbreviations: HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombus.
Subgroup Survival Analysis
Then, subgroup survival analysis was performed separately according to tumor treatment response (Fig. 2) and type of PVTT (Fig. 3). In patients who achieved PR, the OS (P = .859, Fig. 2A) and PFS (P = .641, Fig. 2B) of patients who underwent salvage surgery and those who did not were not significantly different. The median OS and PFS for the surgery group were 27.5 and 14.9 months, respectively; the median OS and PFS for the non-surgery group were 27.1 and 10.8 months, respectively. For patients who achieved CR, the survival curves revealed a trend toward better OS and PFS in patients who did not undergo surgery compared with those who underwent salvage surgery (for OS, P = .136, Fig. 2C; for PFS, P = 0.134, Fig. 2D). The median OS and PFS for the surgery group were not reached and 22.3 months, respectively; the median OS and PFS for the non-surgery group were both not reached.
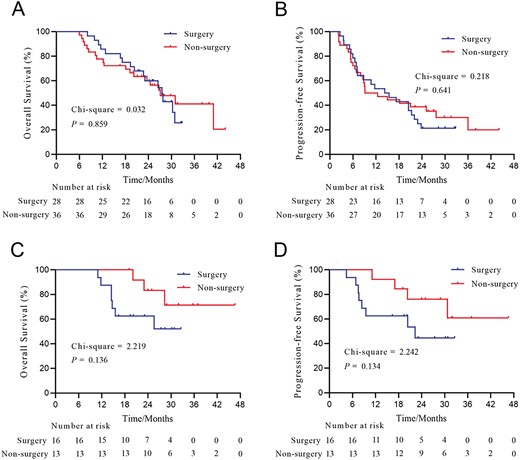
Overall survival and progression-free survival of patients with HCC and PVTT who underwent or did not undergo conversion surgery stratified by the degree of tumor response. (A) OS of patients who achieved PR; (B) PFS of patients who achieved PR; (C) OS of patients who achieved CR; (D) PFS of patients who achieved CR. Abbreviations: CR, complete response; HCC, hepatocellular carcinoma; OS, overall survival; PFS, progression-free survival; PR, partial response; PVTT, portal vein tumor thrombus.
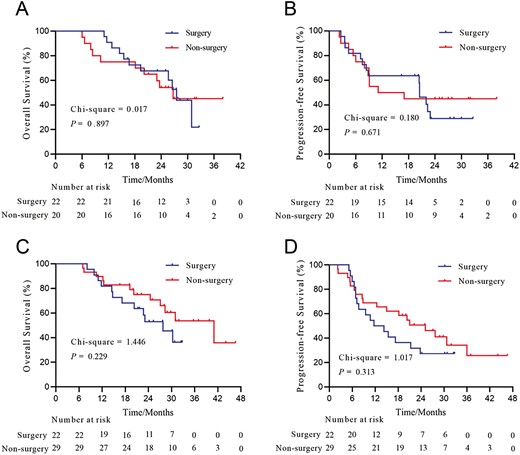
Overall survival and progression-free survival of patients with HCC and PVTT who underwent or did not undergo conversion surgery stratified by the type of PVTT. (A) OS of patients who had type II PVTT; (B) PFS of patients who had type II PVTT; (C) OS of patients who had types III-IV PVTT; (D) PFS of patients who types III-IV PVTT. Abbreviations: HCC, hepatocellular carcinoma; OS, overall survival; PFS, progression-free survival; PVTT, portal vein tumor thrombus.
In patients with type II PVTT, the OS (P = .897, Fig. 3A) and PFS (P = .671, Fig. 3B) of patients who underwent salvage surgery and those who did not were comparable. In the type II PVTT subgroup, the median OS and PFS for the surgery group were 27.5 and 20.5 months, respectively; the median OS and PFS for the non-surgery group were 26.7 and 14.1 months, respectively. For patients with types III–IV PVTT, the survival curves illustrated a trend toward better OS and PFS in patients who did not undergo surgery compared with those who underwent salvage surgery (for OS, P = .229, Fig. 3C; for PFS, P = .313, Fig. 3D). The median OS and PFS for the surgery group were 27.8 and 13.0 months, respectively; the median OS and PFS for the non-surgery group were 41.0 and 25.1 months, respectively.
Independent Prognostic Factors of OS and PFS
Baseline serum AFP (adjusted HR = 2.22, 95% CI 1.21-4.09, P = .010) and best tumor response per mRECIST criteria (adjusted HR = 2.48, 95% CI 1.14-5.35, P = .021) were independent prognostic factors for OS. Baseline serum AFP (adjusted HR = 2.08, 95% CI 1.23-3.50, P = .006) and best tumor response per mRECIST criteria (adjusted HR = 3.06, 95% CI 1.58-5.95, P = .001) were also independent prognostic factors for PFS (Table 3).
Univariate and multivariate analysis of factors associated with OS and PFS.
| Characteristics . | Overall survival . | Progression-free survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Age, ≥55 vs. <55, years | 1.31 (0.72-2.37) | .374 | 1.17 (0.70-1.95) | .556 | ||||
| Sex, male vs. female | 1.33 (0.41-4.34) | .635 | 1.03 (0.41-2.58) | .955 | ||||
| Child-Pugh class, B vs. A | 2.75 (1.26-6.00) | .011 | 2.08 (1.02-4.27) | .045 | ||||
| TBil, ≥17.1 vs. <17.1, µmol/L | 1.50 (0.83-2.71) | .179 | 1.27 (0.76-2.12) | .367 | ||||
| ALB, ≥40 vs. <40, g/L | 0.70 (0.39-1.27) | .242 | 0.97 (0.58–1.62) | .913 | ||||
| ALT, ≥40 vs. <40, U/L | 1.40 (0.77-2.53) | .268 | 1.37 (0.82-2.30) | .229 | ||||
| Tumor diameter, ≥10 vs. <10, cm | 1.86 (1.02-3.40) | .044 | 1.57 (0.93-2.63) | .091 | ||||
| Tumor number, multiple vs. single | 1.43 (0.80-2.57) | .230 | 1.30 (0.78-2.18) | .314 | ||||
| PVTT Cheng’s type, III–IV vs. II | 1.54 (0.84-2.82) | .167 | 1.79 (1.04-3.06) | .034 | ||||
| Baseline AFP, ≥400 vs. <400, ng/mL | 2.39 (1.30-4.40) | .005 | 2.22 (1.21-4.09) | .010 | 2.19 (1.30-3.70) | .003 | 2.08 (1.23-3.50) | .006 |
| Baseline PIVKA-II, ≥400 vs. <400, mAU/mL | 1.43 (0.75-2.74) | .277 | 1.38 (0.78-2.43) | .263 | ||||
| Best tumor response per mRECIST criteria, PR vs. CR | 2.67 (1.24-5.76) | .012 | 2.48 (1.14-5.35) | .021 | 3.19 (1.64-6.19) | .001 | 3.06 (1.58-5.95) | .001 |
| Salvage surgery, yes vs. no | 1.31 (0.72-2.38) | .371 | 1.29 (0.77-2.16) | .337 | ||||
| Characteristics . | Overall survival . | Progression-free survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Age, ≥55 vs. <55, years | 1.31 (0.72-2.37) | .374 | 1.17 (0.70-1.95) | .556 | ||||
| Sex, male vs. female | 1.33 (0.41-4.34) | .635 | 1.03 (0.41-2.58) | .955 | ||||
| Child-Pugh class, B vs. A | 2.75 (1.26-6.00) | .011 | 2.08 (1.02-4.27) | .045 | ||||
| TBil, ≥17.1 vs. <17.1, µmol/L | 1.50 (0.83-2.71) | .179 | 1.27 (0.76-2.12) | .367 | ||||
| ALB, ≥40 vs. <40, g/L | 0.70 (0.39-1.27) | .242 | 0.97 (0.58–1.62) | .913 | ||||
| ALT, ≥40 vs. <40, U/L | 1.40 (0.77-2.53) | .268 | 1.37 (0.82-2.30) | .229 | ||||
| Tumor diameter, ≥10 vs. <10, cm | 1.86 (1.02-3.40) | .044 | 1.57 (0.93-2.63) | .091 | ||||
| Tumor number, multiple vs. single | 1.43 (0.80-2.57) | .230 | 1.30 (0.78-2.18) | .314 | ||||
| PVTT Cheng’s type, III–IV vs. II | 1.54 (0.84-2.82) | .167 | 1.79 (1.04-3.06) | .034 | ||||
| Baseline AFP, ≥400 vs. <400, ng/mL | 2.39 (1.30-4.40) | .005 | 2.22 (1.21-4.09) | .010 | 2.19 (1.30-3.70) | .003 | 2.08 (1.23-3.50) | .006 |
| Baseline PIVKA-II, ≥400 vs. <400, mAU/mL | 1.43 (0.75-2.74) | .277 | 1.38 (0.78-2.43) | .263 | ||||
| Best tumor response per mRECIST criteria, PR vs. CR | 2.67 (1.24-5.76) | .012 | 2.48 (1.14-5.35) | .021 | 3.19 (1.64-6.19) | .001 | 3.06 (1.58-5.95) | .001 |
| Salvage surgery, yes vs. no | 1.31 (0.72-2.38) | .371 | 1.29 (0.77-2.16) | .337 | ||||
Abbreviations: AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; CR, complete response; mRECIST, the modified Response Evaluation Criteria in Solid Tumors; OS, overall survival; PFS, progression-free survival; PIVKA-II, protein induced by vitamin K absence or antagonist II; PR, partial response; PVTT, portal vein tumor thrombus; TBil, total bilirubin.
The P values in bold denote statistical significance.
Univariate and multivariate analysis of factors associated with OS and PFS.
| Characteristics . | Overall survival . | Progression-free survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Age, ≥55 vs. <55, years | 1.31 (0.72-2.37) | .374 | 1.17 (0.70-1.95) | .556 | ||||
| Sex, male vs. female | 1.33 (0.41-4.34) | .635 | 1.03 (0.41-2.58) | .955 | ||||
| Child-Pugh class, B vs. A | 2.75 (1.26-6.00) | .011 | 2.08 (1.02-4.27) | .045 | ||||
| TBil, ≥17.1 vs. <17.1, µmol/L | 1.50 (0.83-2.71) | .179 | 1.27 (0.76-2.12) | .367 | ||||
| ALB, ≥40 vs. <40, g/L | 0.70 (0.39-1.27) | .242 | 0.97 (0.58–1.62) | .913 | ||||
| ALT, ≥40 vs. <40, U/L | 1.40 (0.77-2.53) | .268 | 1.37 (0.82-2.30) | .229 | ||||
| Tumor diameter, ≥10 vs. <10, cm | 1.86 (1.02-3.40) | .044 | 1.57 (0.93-2.63) | .091 | ||||
| Tumor number, multiple vs. single | 1.43 (0.80-2.57) | .230 | 1.30 (0.78-2.18) | .314 | ||||
| PVTT Cheng’s type, III–IV vs. II | 1.54 (0.84-2.82) | .167 | 1.79 (1.04-3.06) | .034 | ||||
| Baseline AFP, ≥400 vs. <400, ng/mL | 2.39 (1.30-4.40) | .005 | 2.22 (1.21-4.09) | .010 | 2.19 (1.30-3.70) | .003 | 2.08 (1.23-3.50) | .006 |
| Baseline PIVKA-II, ≥400 vs. <400, mAU/mL | 1.43 (0.75-2.74) | .277 | 1.38 (0.78-2.43) | .263 | ||||
| Best tumor response per mRECIST criteria, PR vs. CR | 2.67 (1.24-5.76) | .012 | 2.48 (1.14-5.35) | .021 | 3.19 (1.64-6.19) | .001 | 3.06 (1.58-5.95) | .001 |
| Salvage surgery, yes vs. no | 1.31 (0.72-2.38) | .371 | 1.29 (0.77-2.16) | .337 | ||||
| Characteristics . | Overall survival . | Progression-free survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Age, ≥55 vs. <55, years | 1.31 (0.72-2.37) | .374 | 1.17 (0.70-1.95) | .556 | ||||
| Sex, male vs. female | 1.33 (0.41-4.34) | .635 | 1.03 (0.41-2.58) | .955 | ||||
| Child-Pugh class, B vs. A | 2.75 (1.26-6.00) | .011 | 2.08 (1.02-4.27) | .045 | ||||
| TBil, ≥17.1 vs. <17.1, µmol/L | 1.50 (0.83-2.71) | .179 | 1.27 (0.76-2.12) | .367 | ||||
| ALB, ≥40 vs. <40, g/L | 0.70 (0.39-1.27) | .242 | 0.97 (0.58–1.62) | .913 | ||||
| ALT, ≥40 vs. <40, U/L | 1.40 (0.77-2.53) | .268 | 1.37 (0.82-2.30) | .229 | ||||
| Tumor diameter, ≥10 vs. <10, cm | 1.86 (1.02-3.40) | .044 | 1.57 (0.93-2.63) | .091 | ||||
| Tumor number, multiple vs. single | 1.43 (0.80-2.57) | .230 | 1.30 (0.78-2.18) | .314 | ||||
| PVTT Cheng’s type, III–IV vs. II | 1.54 (0.84-2.82) | .167 | 1.79 (1.04-3.06) | .034 | ||||
| Baseline AFP, ≥400 vs. <400, ng/mL | 2.39 (1.30-4.40) | .005 | 2.22 (1.21-4.09) | .010 | 2.19 (1.30-3.70) | .003 | 2.08 (1.23-3.50) | .006 |
| Baseline PIVKA-II, ≥400 vs. <400, mAU/mL | 1.43 (0.75-2.74) | .277 | 1.38 (0.78-2.43) | .263 | ||||
| Best tumor response per mRECIST criteria, PR vs. CR | 2.67 (1.24-5.76) | .012 | 2.48 (1.14-5.35) | .021 | 3.19 (1.64-6.19) | .001 | 3.06 (1.58-5.95) | .001 |
| Salvage surgery, yes vs. no | 1.31 (0.72-2.38) | .371 | 1.29 (0.77-2.16) | .337 | ||||
Abbreviations: AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; CR, complete response; mRECIST, the modified Response Evaluation Criteria in Solid Tumors; OS, overall survival; PFS, progression-free survival; PIVKA-II, protein induced by vitamin K absence or antagonist II; PR, partial response; PVTT, portal vein tumor thrombus; TBil, total bilirubin.
The P values in bold denote statistical significance.
CTRAEs in Patients Who Underwent or Did Not Undergo Salvage Surgery
As shown in Supplementary Tables S3andS4, the total incidence of CTRAEs in the surgery and non-surgery groups were 77.3% and 81.6%, respectively (P = .603). The incidence of grades III-IV CTRAEs was slightly higher in the non-surgery group than the surgery group (12.2% vs. 9.1%, P = .744).
Survival Comparison in Patients Who Underwent Salvage Surgery
Of the 44 patients who underwent salvage surgery, 26 continued to use TKI and/or anti-PD-1 antibody (group I), and 18 discontinued these systemic drugs (group II). As shown in Supplementary Fig. S3, the OS (P = .025, Supplementary Fig. S3A) and recurrence-free survival (RFS) (P = .015, Supplementary Fig. S3B) of the group I were significantly improved than group II.
Perioperative Outcomes After Salvage Surgery
The median interval from the commencement of conversion therapy to salvage surgery was 3.2 months (range, 1.2-7.5 months). As shown in Supplementary Table S5, 4 patients (9.1%) underwent laparoscopic surgery. Thirty-one patients (70.5%) underwent major hepatectomy. The median operative time was 180 minutes, and the median intraoperative blood loss was 300 mL, with 16 patients (36.4%) requiring intraoperative blood transfusion. The median postoperative hospital stay was 9 days. Fifteen patients (34.1%) experienced postoperative complications, of whom 3 patients (6.8%) experienced Clavien-Dindo grade IIIb or higher complications, including posthepatectomy liver failure (PHLF, n = 1), biliary fistula (n = 1), and gastrointestinal bleeding (n = 1). The 90-day mortality rate was 2.3% (1/44). This patient underwent left hemihepatectomy for HCC with tumor thrombus located at the left branch of the portal vein, but unfortunately died of delayed portal vein thrombosis 2.6 months after surgery. Furthermore, postoperative pathological examination reported microvascular invasion in 14 patients (31.8%) (Supplementary Table S5).
Discussions
The long-term prognosis of patients with advanced HCC is poor.32 The typical feature of advanced HCC is macrovascular invasion, especially portal vein tumor thrombus (PVTT). The presence of PVTT has been confirmed as one of the most significant risk factors of poor prognosis in HCC. According to Barcelona Clinic Liver Cancer (BCLC) guidelines, HCC associated with PVTT is not indicated for surgical resection, and systemic treatment is recommended.33 However, some Asian studies and consensus recommend liver resection be safely performed in highly selected patients with resectable HCC and PVTT.3,20,34
For patients with initially unresectable HCC and PVTT, many investigators in Asian countries have attempted the strategy of conversion resection.7,9,35 In real-world clinical practice, locoregional and systemic combination therapy is increasingly applied in advanced HCC due to its promising objective response rates. There is a mountain of clinical evidence supporting the use of locoregional and systemic combination regimens as a conversion strategy for unresectable advanced HCC.14,36,37
Nonetheless, the question remains as to whether salvage surgery is necessary for patients with initially unresectable HCC and PVTT who meet resectability criteria after successful conversion therapy by LRT, TKI, and anti-PD-1 antibody. To the best of our knowledge, this study represents the largest cohort of patients with HCC and PVTT who were converted by locoregional and systemic combination treatment and underwent or did not undergo salvage surgery, which will offer useful information regarding this promising conversion modality.
In our study, of the 93 patients with initially unresectable HCC and PVTT, 44 patients underwent salvage hepatectomy, and the other 49 patients did not undergo salvage liver resection. Our study found that the OS and PFS were not significantly different between the salvage surgery and non-surgery groups. This result was contrary to that reported by Zhu et al,38 which showed that patients with initially unresectable HCC who underwent salvage resection following conversion therapy had markedly longer OS compared with those who did not (median OS: not reached vs. 15.9 months, P < .001). The discrepancy may be attributable to differences in disease stage distribution. 43.6% (44/101) of patients had macrovascular invasion in the study by Zhu et al,38 whereas all of our patients had macrovascular invasion.
Subgroup survival analyses uncovered that for patients with complete response or with types III-IV PVTT, there was a trend toward better OS and PFS in patients who did not undergo surgery compared with those who underwent salvage surgery. Multivariate analysis showed that baseline serum AFP and best tumor response per mRECIST criteria were independent prognostic factors, which highlights the importance to attain CR when conversion therapy and lower baseline AFP before conversion treatment.
Furthermore, we found that the incidence and severity of CTRAEs between the salvage surgery and non-surgery groups were similar. It is noteworthy that the majority of complications were minor and controllable with appropriate medical interventions. Among these CTRAEs, the 4 most common CTRAEs were fatigue (38.6% and 32.7%), hypertension (29.5% and 26.5%), skin rash (20.5% and 16.3%), and capillary hyperplasia (11.4% and 10.2%) in both the surgery and non-surgery groups.
In addition, by analyzing the perioperative outcomes of patients who underwent conversion resection, we found that the safety profile of salvage surgery in these patients is acceptable. Although postoperative morbidity was observed in 34.1% of patients, the major postoperative complications only occurred in 3 (6.8%) patients. Additionally, our study reported a postoperative 90-day mortality rate of 2.3%, with only one patient died of delayed portal vein thrombosis. This mortality event reminded us to optimize perioperative care, refine surgical techniques, and recognize high-risk patients before performing salvage surgery. Furthermore, we found that adjuvant therapy by maintenance usage of TKIs and/or anti-PD-1 antibodies was beneficial for the improvement of survival in patients who underwent salvage surgery following conversion therapy (P = .025 for OS and .015 for RFS). This encouraging result emphasizes the importance to maintain systemic drugs (TKIs and/or anti-PD-1 antibodies) as postoperative adjuvant treatment.
Our study has several limitations. First, selection bias is inherent to this study due to its retrospective nature. Second, although multicenter data are used, the sample size is still not large enough. Third, the heterogeneity of conversion regimens and various kinds of locoregional and systemic treatment may limit the generalizability of our study results. Fourth, clinical tumor response, rather than pathological tumor response, is applied in this study, because pathological examination is not routinely conducted in patients not undergoing surgery. Finally, this study is conducted in China, where HBV-related HCC is prevalent; thus, whether the results can be extrapolated to patients with HCC with other etiologies remains unknown. Therefore, further research, especially prospective randomized controlled trials with uniform conversion regimen, is necessary to better evaluate the effect of salvage surgery in these patients.
Conclusion
For patients with initially unresectable HCC and PVTT who are successfully converted by a triple combination therapy consisted of LRT (TACE and/or RT), TKI, and anti-PD-1 antibodies, salvage surgery may not be necessary, especially for the patients with complete tumor response or types III-IV PVTT. For the patients who underwent salvage surgery following conversion therapy, adjuvant therapy using TKI alone or in combination with anti-PD-1 antibody is beneficial for postoperative survival.
Funding
This work was supported by the Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1004A), the Key Project of the National Natural Science Foundation of China (81730097), the National Natural Science Foundation of China (82072618), the National Key Research and Development Program of China (2022YFC2503700, 2022YFC2503705), and Shanghai Municipal Health Commission (2023ZZ02005).
Ethics Approval and Consent to Participate
The study protocol was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki and approved by the Clinical Research Ethics Committee (Approval number: EHBHKY2022-H028-P001). Written informed consent was obtained before commencing the conversion therapy regimen and prior to salvage surgery.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: L.W., J.-K.F., W.Y.L., M.-L.Y., S.-Q.C. Provision of study material or patients: L.W., C.-D.L., J.-Y.W., B.Z., K.W., C.L., H.-K.Z., J.S., W.-X.G. Collection and/or assembly of data: L.W., J.-K.F., C.-D.L., J.-Y.W. Data analysis and interpretation: L.W., J.-K.F., C.-D.L., J.-Y.W. Manuscript writing: final approval of manuscript: All authors.
Data Availability
The data that support the findings of our study are available from the corresponding author upon reasonable request.
References
Author notes
Lei Wang, Jin-Kai Feng, Chong-De Lu and Jia-Yi Wu Contributed equally.



