-
PDF
- Split View
-
Views
-
Cite
Cite
Rafael Adrián Pacheco‐Orozco, Lorena Montealegre‐Páez, Federico Cayol, Héctor Martínez‐Gregorio, Javier Oliver, Cecilia Frecha, Felipe Vaca‐Paniagua, Sandra Perdomo, AR‐V7 as a Biomarker for Resistance to Treatment with Abiraterone/Enzalutamide in Three Latin American Countries: A Hypothetical Cost‐Saving Analysis, The Oncologist, Volume 25, Issue 12, December 2020, Pages e1990–e1995, https://doi.org/10.1634/theoncologist.2020-0043
Close - Share Icon Share
Abstract
Prostate cancer is the most incident and one of the deadliest male cancers in Latin America. Treatment for patients with metastatic castration‐resistant prostate cancer (mCRPC) includes androgen receptor signaling inhibitors such as abiraterone and enzalutamide, for which androgen receptor splice variant 7 (AR‐V7) has emerged as a biomarker for primary resistance. Our study sought to analyze the potential economic impact of the use of AR‐V7 detection as a treatment indicator in patients with mCRPC in three Latin American countries.
A hypothetical cost prediction model for the use of noninvasive circulating tumor cell–based AR‐V7 testing as a treatment indicator for patients eligible for treatment with abiraterone/enzalutamide was conducted using available information on treatment and testing costs from Mexico, Argentina, and Colombia.
At an estimated prevalence of AR‐V7 positivity of 20%, the use of upfront AR‐V7 genetic testing resulted in annual net savings of $9,801,669.97, $6,390,055.75, and $3,096,780.91 in Mexico, Argentina, and Colombia, respectively. A direct relationship between AR‐V7 positivity prevalence and net savings was found.
The use of a noninvasive AR‐V7 detection assay as a treatment indicator tool in patients eligible for treatment with abiraterone or enzalutamide in Latin America could be a cost‐effective approach for the management of these patients. Additional efforts are needed to accurately determine the incidence of castration‐resistant prostate cancer cases and the prevalence of AR‐V7 positivity in Latin America in order to predict the potential economic benefit of its clinical use.
In Latin America, prostate cancer is the most frequently diagnosed cancer in men, and the burden of this disease is expected to double in this region by 2030. Noninvasive detection of androgen receptor splice variant 7 (AR‐V7) is being currently validated as a predictive biomarker for benefit with androgen receptor signaling inhibitor therapy in patients with metastatic castration‐resistant prostate cancer (mCRPC). This hypothetical cost‐saving analysis shows that AR‐V7 testing in peripheral blood of patients with CRPC eligible for treatment with abiraterone or enzalutamide might represent a cost‐effective strategy to select patients who will benefit from AR‐axis–directed treatment in three Latin American countries.
Introduction
Prostate cancer is the fourth most incident cancer in the world, with approximately 1.3 million new cases per year. It is the second most incident cancer among men and ranks as the fifth leading cause of cancer death [1]. In Latin America, it is the most frequently diagnosed cancer in men and has an age‐standardized incidence rate of 60.4 per 100,000 with a mortality rate of 14 per 100,000 [1]. The burden of disease is expected to double in this region by 2030 owing to an increase in the aged male population [2, 3].
The standard treatment of advanced prostate cancer is androgen‐deprivation therapy (ADT) [4, 5], which significantly reduces metastatic disease indicators, bone pain, and prostatic‐specific antigen (PSA) levels in most patients [4]. However, ADT is not considered to be curative as it suppresses disease symptoms and indicators for approximately 2–3 years and only 5%–10% of the patients survive more than 10 years [4]. Patients with recurrent disease are considered to have castration‐resistant prostate cancer (CRPC). Therapeutic options in this subgroup of patients include taxane chemotherapy, androgen receptor (AR)‐axis–targeted therapy, targeted alpha therapy (Radium‐223), and Sipuleucel T [2].
In patients with metastatic CRPC (mCRPC), AR remains active and drives disease progression, making it a suitable target for androgen receptor signaling inhibitors (ARSis) such as abiraterone and enzalutamide. Both ARSis have shown improvements in survival outcomes for patients with mCRPC [6]. Recently, the splicing variant of the androgen receptor called androgen receptor splice variant 7 (AR‐V7) was identified as a potential biomarker associated with resistance to treatment with abiraterone and enzalutamide [7, 8]. In addition, the presence of nuclear‐specific AR‐V7 splice variant has been shown to correlate with an improvement in survival outcomes in patients treated with taxane chemotherapy as opposed to ARSis; hence, it has been validated as a predictive factor for response to treatment in patients with mCRPC [9–11]. These results were recently confirmed by a large multicenter prospective trial, the PROPHECY study [12]. However, it is still necessary to confirm AR‐V7 splice variant utility for clinical decision making.
Detection of AR‐V7 splice variant can be achieved through liquid biopsy, an emergent technique able to detect circulating tumor cells or tumor‐associated nucleic acids in peripheral fluids [5]. Different kinds of liquid biopsy–based approaches have been validated for the detection of AR‐V7 in peripheral blood, including nuclear detection of the variant based on circulant tumor cells assays [10, 13], exosome detection, and circulating free RNA in plasma [6].
Regardless of the potential benefits of using treatment indicator biomarkers for prostate cancer, their clinical implementation faces great challenges in Latin America. Some of the barriers include difficulty of access to new technologies and medicines, key structural and organizational limitations of health systems, unequal distribution of resources destined for treatment and research in cancer, and lack of knowledge about new technologies and therapeutic possibilities among the different health system actors [2, 14].
Enhancement of access to primary care is the top priority for health care in Latin America, but accessibility to high‐cost medications and implementation of new technologies need addressing as well [15]. Therefore, the optimal allocation of resources is critical for the improvement of cancer care and research in the region.
The aim of our study was to conduct a hypothetical cost‐saving analysis in order to evaluate the potential use of a noninvasive AR‐V7 splice variant assay as an indicator of treatment resistance in patients with castration‐resistant prostate cancer in three Latin American countries.
Materials and Methods
Cost‐Prediction Model
A hypothetical prediction model for total treatment costs of castration‐resistant prostate cancer was designed with and without the upfront use of AR‐V7 detection assays (Fig. 1). Mexico, Argentina, and Colombia were selected as representative countries of the Latin American region because they have similar health system regimes and share common challenges in their medical attention procedures [16].
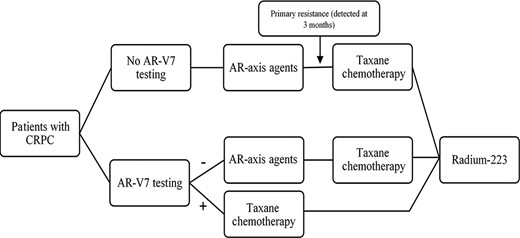
Proposed clinical treatment algorithm for metastatic CRPC with and without the use of AR‐V7 testing as a resistance biomarker in three Latin American countries. Abbreviations: AR, androgen receptor; AR‐V7, androgen receptor splice variant 7; CPRC, castration‐resistant prostate cancer.
The clinical management algorithm for CRPC was adapted from the recommendations in Sade et al. [2], which are based on the guidelines by the European Society of Medical Oncology, the National Comprehensive Cancer Network (NCCN), the American Urological Association, and the American Society of Clinical Oncology and applied to the Latin American context. Abiraterone acetate dose was defined as 1,000 mg p.o. daily and enzalutamide dose was defined as 160 mg daily, as per current standards [17]. Our model considered the difference in cost of the first 3 months of treatment. Under this scenario, AR‐V7‐positive patients do not benefit from first‐line ARSi therapy and primary resistance is detected during the first imaging and PSA control, which is recommended to be performed every 3–6 months [17].
Consolidated Data on CRPC Cases and Treatment Costs
Incidence of prostate cancer in the three countries was obtained from the estimates of GLOBOCAN 2018 [18]. It has been reported that 20%–25% of prostate cancer cases progress to metastatic disease and become castration resistant [19]. Therefore, we estimated that 25% of total incidence cases constitute patients eligible for treatment with ARSis. Out of the total number of ARSi‐eligible patients, the prevalence of AR‐V7 positivity was assumed to be 20% in our main analysis, in agreement with the existing literature on primary resistance to AR‐axis agents [19–21]. Because of the lack of data on prevalence of AR‐V7 positivity in Latin America, a secondary analysis was performed in which cost‐saving was calculated assuming different prevalence rates (5%, 15%, 30%) in AR‐V7 positivity (supplemental online Table 1). The total number of estimated AR‐V7‐positive patients detected by the assay were defined as avoided cases.
Market prices of abiraterone and enzalutamide were obtained in U.S. Dollars (USD) in the three Latin American countries. In Colombia, prices were retrieved from the health ministry webpage [22]. In Argentina, prices were obtained from the National Drugs Vademecum of the Administación Nacional de Medicamentos, Alimentos y Tecnología Médica [23]. Finally, in México, prices were taken from the Instituto Mexicano del Seguro Social database [24]. When different commercial brands were found in the same national database, the highest value for each drug was used for our analysis. Treatment cost was defined as the cost of abiraterone or enzalutamide at its usual dose for 3 months (until the first PSA control is performed).
Listed prices of commercially available AR‐V7 assays in peripheral blood samples were requested to certified commercial distributors in the three countries. Costs associated with possible side effects derived from the use of the drug were not considered.
Results
AR‐V7 Detection Assay
We found two validated and commercially available assays designed for the detection of AR‐V7, Epic Sciences’ (San Diego, USA) oncotypeDX AR‐V7 Nucleus Detect and Qiagen's (Venlo, Netherlands) AdnaTest Prostate Cancer Panel AR‐V7. Out of the two, only the latter is being marketed to individual diagnostic laboratories for use within a given health care system and is commercially available in Argentina, Colombia, and Mexico. Qiagen's AdnaTest Prostate Cancer Panel AR‐V7 was initially developed by the Johns Hopkins University and has a cost of 225 USD per reaction in the U.S. market. The reported specificity of the AdnaTest Prostate Cancer Panel AR‐V7 detection is more than 90% [25] and has undergone further analytic and clinical validation in the past 3 years [26, 27]. Commercialization of this kit in Latin American countries is mediated by Qiagen's commercial and distributer partners in each country, and prices are usually higher owing to importation fees and taxes. For instance, in Colombia, the price of the kit is 18,711,560 COP (Colombian Pesos; 5,690 USD, or 474 USD per reaction*). In México, the price is 67,778 MXN (Mexican Pesos; 3,606 USD, or 300 USD per reaction*). Finally, in Argentina, the kit is sold for 7,730 USD, or 644 USD per reaction. The cost calculation for each reaction does not include the cost of laboratory equipment and supplies or qualified staff salaries. *Exchange rates by January 15, 2020.
Cost‐Saving Analysis of CRPC Treatment
The estimated number of incident prostate cancer cases in 2018 was 25,409, 11,600, and 12,712 for Mexico, Argentina, and Colombia, respectively. Assuming that 25% of patients develop metastatic disease and resistance to castration, a total of 6,352, 2,900, and 3,178 patients would be eligible for treatment with abiraterone/enzalutamide in each country. The single‐dose cost was $102.08, $158.20, and $80.48 USD for abiraterone and $127.15, $263.40, and $106.48 for enzalutamide in Mexico, Argentina, and Colombia, respectively.
Patients with CRPC who do not undergo AR‐V7 testing are usually treated with AR‐axis agents, with very little benefit for AR‐V7‐positive patients, and are then changed to taxane chemotherapy upon detection of resistance (Fig. 1). AR‐V7 testing, on the other hand, may allow selection of patients who will benefit from each of the treatment options. CRPC treatment cost without AR‐V7 testing represents treatment costs for all eligible patients, whereas cost including AR‐V7 testing represents treatment costs for the estimated AR‐V7‐negative patients plus the cost of testing all ARSi‐eligible patients. For Mexico, cost without AR‐V7 testing was $58,359,391.20 whereas cost with AR‐V7 testing was $48,596,501.72, giving a net saving of 16.7%. Similarly, for Argentina, the cost without AR‐V7 testing was $41,290,200.00 whereas the cost with AR‐V7 testing was $34,900,144.25, representing net savings of 15.5%. Finally, in Colombia, the cost without testing was $23,018,889.60 whereas the cost with testing was $19,922,108.69, giving a total cost reduction of 13.5% (Table 1).
| . | México . | Argentina . | Colombia . |
|---|---|---|---|
| No. of patients eligible for treatment with ARSis | 6,352 | 2,900 | 3,178 |
| Assay costs | $300.52 | $644.13 | $474.20 |
| Number of cases avoideda | 1,270 | 580 | 636 |
| Cost per avoided case | $9,187.20 | $14,238.00 | $7,243.20 |
| Expenditures in ARSis avoided | $11,671,878.24 | $8,258,040.00 | $4,603,777.92 |
| AR‐V7 testing costs | $1,908,988.76 | $1,867,984.25 | $1,506,997.01 |
| Net savings | $9,801,669.97 | $6,390,055.75 | $3,096,780.91 |
| . | México . | Argentina . | Colombia . |
|---|---|---|---|
| No. of patients eligible for treatment with ARSis | 6,352 | 2,900 | 3,178 |
| Assay costs | $300.52 | $644.13 | $474.20 |
| Number of cases avoideda | 1,270 | 580 | 636 |
| Cost per avoided case | $9,187.20 | $14,238.00 | $7,243.20 |
| Expenditures in ARSis avoided | $11,671,878.24 | $8,258,040.00 | $4,603,777.92 |
| AR‐V7 testing costs | $1,908,988.76 | $1,867,984.25 | $1,506,997.01 |
| Net savings | $9,801,669.97 | $6,390,055.75 | $3,096,780.91 |
Step‐by‐step cost‐saving analysis (in U.S. Dollars) after introduction of AR‐V7 detection test in patients with mCRPC in three Latin American countries at an estimated prevalence of AR‐V7 positivity of 20%.
aAvoided case: Cases in which AR‐V7 positivity is detected and unnecessary expenditures in ARSi treatment are avoided.
Abbreviations: ARSi, androgen receptor signaling inhibitor; AR‐V7, androgen receptor splice variant 7; mCRPC, metastatic castration‐resistant prostate cancer.
| . | México . | Argentina . | Colombia . |
|---|---|---|---|
| No. of patients eligible for treatment with ARSis | 6,352 | 2,900 | 3,178 |
| Assay costs | $300.52 | $644.13 | $474.20 |
| Number of cases avoideda | 1,270 | 580 | 636 |
| Cost per avoided case | $9,187.20 | $14,238.00 | $7,243.20 |
| Expenditures in ARSis avoided | $11,671,878.24 | $8,258,040.00 | $4,603,777.92 |
| AR‐V7 testing costs | $1,908,988.76 | $1,867,984.25 | $1,506,997.01 |
| Net savings | $9,801,669.97 | $6,390,055.75 | $3,096,780.91 |
| . | México . | Argentina . | Colombia . |
|---|---|---|---|
| No. of patients eligible for treatment with ARSis | 6,352 | 2,900 | 3,178 |
| Assay costs | $300.52 | $644.13 | $474.20 |
| Number of cases avoideda | 1,270 | 580 | 636 |
| Cost per avoided case | $9,187.20 | $14,238.00 | $7,243.20 |
| Expenditures in ARSis avoided | $11,671,878.24 | $8,258,040.00 | $4,603,777.92 |
| AR‐V7 testing costs | $1,908,988.76 | $1,867,984.25 | $1,506,997.01 |
| Net savings | $9,801,669.97 | $6,390,055.75 | $3,096,780.91 |
Step‐by‐step cost‐saving analysis (in U.S. Dollars) after introduction of AR‐V7 detection test in patients with mCRPC in three Latin American countries at an estimated prevalence of AR‐V7 positivity of 20%.
aAvoided case: Cases in which AR‐V7 positivity is detected and unnecessary expenditures in ARSi treatment are avoided.
Abbreviations: ARSi, androgen receptor signaling inhibitor; AR‐V7, androgen receptor splice variant 7; mCRPC, metastatic castration‐resistant prostate cancer.
We performed an additional net saving estimation analysis in different scenarios for AR‐V7 positivity prevalence rates (5%, 15%, 30%), according to the percentage of prevalence recently reported in the literature [9, 10, 12, 13, 21, 26, 28, 29]. Overall, there is a direct relation between AR‐V7 positivity prevalence and net savings. In Mexico and Argentina, even the lowest (5%) AR‐V7 positivity prevalence would result in net savings of $1,008,980.80 USD and $196,525.75 USD, respectively. On the other hand, in Colombia, such a low prevalence would result in economic losses estimated at −$356,052.53 USD (Fig. 2; supplemental online Table 1). In Colombia, a breakeven point would be approximately reached at a prevalence of 6.5%, which means that any prevalence greater than this would result in cost savings.
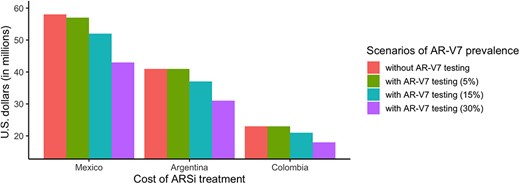
Comparison of costs of ARSi treatment in three scenarios of AR‐V7 prevalence. Abbreviations: ARSi, androgen receptor signaling inhibitor; AR‐V7, androgen receptor splice variant 7.
Discussion
Prostate cancer is the most common male cancer in Latin America and one of the top causes of cancer mortality in many of these countries [2]. The development and implementation of diagnostic and therapeutic tests based on the detection of new molecular biomarkers in peripheral blood for prostate cancer has the potential to improve patient management. In particular, the use of noninvasive AR‐V7 testing in patients with CRPC has shown to be a good candidate biomarker to improve clinical outcome and potentially lower unnecessary costs for the health care system.
Our results show that the use of upfront AR‐V7 testing in peripheral blood of patients with CRPC eligible for treatment with abiraterone or enzalutamide might represent a cost‐effective strategy to select patients who will benefit from AR‐axis–directed treatment in three Latin American countries.
A similar study carried out by Markowski and collegues demonstrated that AR‐V7 testing represents an economic benefit as long as the prevalence of AR‐V7 positivity in the studied population exceeds 5%. In their study, assuming a cost per reaction of 1,000 USD and a prevalence of AR‐V7 positivity of 30%, upfront testing would result in a net cost savings of 150 million USD per year [30]. Concordantly, in our study, AR‐V7 testing cost–benefit was also achieved when AR‐V7 positivity prevalence was greater than 5% and net savings ranged from $3,096,780.91 to $9,762,889.48 USD annually.
Currently, detection of AR‐V7 has been validated as a predictive biomarker for benefit with ARSi therapy. A recent study showed that 53% of patients with CRPC that were tested for AR‐V7 underwent a change in medical treatment and those patients whose treatment was modified accordingly were more prone to achieve a significant reduction of PSA than those whose treatment had remained the same [26]. A propensity score analysis also showed the potential benefit in therapeutic decision making of AR‐V7 testing in real‐world patients with mCRPC [31]. However, randomized clinical trials evaluating clinical outcomes in patients with AR‐V7‐driven treatment choices versus patients treated without initial AR‐V7 testing are still missing [28]. No information regarding increased prognosis related to AR‐V7 testing is available in Latin America. Therefore, the real‐world clinical utility of this testing in Latin American patients with CRPC must first be assessed before it is implemented in routine. Once validated as a therapeutic decision‐making biomarker, noninvasive detection of AR‐V7 could support the better use of the already limited resources destined for cancer care in the three analyzed countries.
Treatment of advanced prostate cancer is constantly evolving, and therefore, additional considerations should be considered for future cost–benefit analysis. Emerging recommendations include early use of AR‐axis–directed agents in hormone‐sensitive prostate cancer, as well as changes in the sequence of existing agents [32]. Also, recent evidence has suggested that abiraterone at a dose of 250 mg plus a low‐fat meal is noninferior to the standard dose of 1,000 mg during fasting [33]. This reduced dose was included as an alternative recommendation in the NCCN clinical practice guidelines for prostate cancer [17]. However, there is still debate over the bioavailability and benefits of low‐dose versus standard regimens [34–36]. Assuming that efficacy is similar, widespread use of a low‐dose abiraterone regimen would result in dramatic cost savings and greater population coverage of this treatment.
In Latin America, cancer incidence and cancer control programs show marked contrasts [37]. In many health systems, government funding is scarce and the economic impact on users is significant, which results in biased allocation of resources, underinvestment in equipment and infrastructure, and inequities in cancer care among groups [15]. This scenario makes Latin America generally unprepared to face the rise in cancer incidence and reflects on the disproportionally high mortality rates compared with other regions [15]. There is scarce information on the amount of economic resources spent in cancer care and control in Latin America [15, 37], but it is clear that it is not sufficient, considering the evidence on underfunded health systems and the relatively low priority given to cancer care [37]. One estimate suggests that the total economic burden of cancer care in Latin America is around 4 billion USD; however, the mean medical expenditure per patient is only $7.92 USD [15]. It is then fundamental that every expense allocated to cancer care is used wisely and efficiently.
In addition, our results demonstrate the inequalities of liquid biopsy–based assay costs in the regional market. Prices for the AR‐V7 AdnaTest Kit greatly vary between countries, with Argentina having the higher retail price (7,730 USD) and México the lowest (3,533 USD). This is probably due to the recent economic crisis Argentina has experienced [38] and the additional costs of indirect commercial distributors in the region. Compared with the U.S. retail price, where this kit is sold by Qiagen for 2,784 USD [39], prices in Latin America can be twice or even three times as expensive, which just adds to an already long list of burdens for implementation of health care innovation in the region. Nevertheless, even with high testing costs, the economic benefit of avoiding unnecessary treatment with AR‐axis–directed targets is predicted to be considerable in all three countries.
A limitation of our study is that it only accounts for AR‐V7‐induced resistance to abiraterone and enzalutamide, disregarding any other possible mechanisms of resistance, such as AR and CYP17 upregulation, AR point mutations, glucocorticoid receptor upregulation, and other AR splice variants [20]. Additionally, it is difficult to assess the real incidence of castration‐resistant prostate cancer cases in Latin American countries because of the lack of accurate and updated epidemiological information. In Colombia, there are only four high‐quality population‐based cancer registries (PBCRs) included in the latest Cancer Incidence in Five Continents report; Argentina has five, and Mexico has none [40]. Moreover, information on cancer incidence by stage is often limited owing to the difficulties of PBCRs in collecting these data from medical records [41]. In addition, the prevalence of AR‐V7 positivity in Latin America is unknown, which restricts a more comprehensive cost‐effective analysis of noninvasive AR‐V7 testing in CRPC. This highlights the imperative need for prostate cancer molecular epidemiology studies in Latin America to improve treatment selection and clinical outcomes and to instrument a more adequate use of the limited resources available in the Latin American region for cancer care and control.
Conclusion
Prostate cancer is the most frequently diagnosed cancer in Latin American men, and mCRPC remains a lethal disease with high economic burden. The use of noninvasive AR‐V7 detection assays has the potential of significantly decreasing the costs associated with treatment of mCRPC in Argentina, Colombia, and Mexico when AR‐V7 positivity prevalence was greater than 5%. However, further research is needed to accurately determine the incidence of castration‐resistant prostate cancer cases and the prevalence of AR‐V7 positivity in Latin America to further evaluate the role of AR‐V7‐induced resistance to treatment with abiraterone and enzalutamide in the region.
Acknowledgments
This work was undertaken during the tenure of a medical social service of Rafael Adrián Pacheco Orozco at El Bosque University. This work was supported by the Faculty of Medicine and the Research Unit of Universidad El Bosque.
Author Contributions
Conception/design: Rafael Adrián Pacheco‐Orozco, Federico Cayol, Javier Oliver, Felipe Vaca‐Paniagua, Sandra Perdomo
Provision of study material or patients: Federico Cayol, Javier Oliver, Cecilia Frecha, Felipe Vaca‐Paniagua, Sandra Perdomo
Collection and/or assembly of data: Rafael Adrián Pacheco‐Orozco, Lorena Montealegre‐Páez, Federico Cayol, Héctor Martínez‐Gregorio, Javier Oliver, Felipe Vaca‐Paniagua, Sandra Perdomo
Data analysis and interpretation: Rafael Adrián Pacheco‐Orozco, Federico Cayol, Javier Oliver, Felipe Vaca‐Paniagua, Sandra Perdomo
Manuscript writing: Rafael Adrián Pacheco‐Orozco, Lorena Montealegre‐Páez, Federico Cayol, Héctor Martínez‐Gregorio, Javier Oliver, Cecilia Frecha, Felipe Vaca‐Paniagua, Sandra Perdomo
Final approval of manuscript: Rafael Adrián Pacheco‐Orozco, Lorena Montealegre‐Páez, Federico Cayol, Héctor Martínez‐Gregorio, Javier Oliver, Cecilia Frecha, Felipe Vaca‐Paniagua, Sandra Perdomo
Disclosures
The authors indicated no financial relationships.
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



