-
PDF
- Split View
-
Views
-
Cite
Cite
Ricarda Merten, Oliver Ott, Marlen Haderlein, Simone Bertz, Arndt Hartmann, Bernd Wullich, Bastian Keck, Reinhard Kühn, Claus Michael Rödel, Christian Weiss, Christine Gall, Wolfgang Uter, Rainer Fietkau, Long‐Term Experience of Chemoradiotherapy Combined with Deep Regional Hyperthermia for Organ Preservation in High‐Risk Bladder Cancer (Ta, Tis, T1, T2), The Oncologist, Volume 24, Issue 12, December 2019, Pages e1341–e1350, https://doi.org/10.1634/theoncologist.2018-0280
Close - Share Icon Share
Abstract
The aim of this study was to evaluate the efficacy and safety of chemoradiotherapy (RCT) combined with regional deep hyperthermia (RHT) of high‐risk bladder cancer after transurethral resection of bladder tumor (TUR‐BT).
Between 1982 and 2016, 369 patients with pTa, pTis, pT1, and pT2 cN0–1 cM0 bladder cancer were treated with a multimodal treatment after TUR‐BT. All patients received radiotherapy (RT) of the bladder and regional lymph nodes. RCT was administered to 215 patients, RCT + RHT was administered to 79 patients, and RT was used in 75 patients. Treatment response was evaluated 4–6 weeks after treatment with TUR‐BT.
Complete response (CR) overall was 83% (290/351), and in treatment groups was RT 68% (45/66), RCT 86% (178/208), and RCT + RHT 87% (67/77). CR was significantly improved by concurrent RCT compared with RT (odds ratio [OR], 2.32; 95% confidence interval [CI], 1.05–5.12; p = .037), less influenced by hyperthermia (OR, 2.56; 95% CI, 0.88–8.00; p = .092). Overall survival (OS) after RCT was superior to RT (hazard ratio [HR], 0.7; 95% CI, 0.50–0.99; p = .045). Five‐year OS from unadjusted Kaplan‐Meier estimates was RCT 64% versus RT 45%. Additional RHT increased 5‐year OS to 87% (HR, 0.32; 95% CI, 0.18–0.58; p = .0001). RCT + RHT compared with RCT showed a significantly better bladder‐preservation rate (HR, 0.13; 95% CI, 0.03–0.56; p = .006). Median follow‐up was 71 months. The median number of RHT sessions was five.
The multimodal treatment consisted of a maximal TUR‐BT followed by RT; concomitant platinum‐based chemotherapy combined with RHT in patients with high‐grade bladder cancer improves local control, bladder‐preservation rate, and OS. It offers a promising alternative to surgical therapies like radical cystectomy.
Radical cystectomy with appropriate lymph node dissection has long represented the standard of care for muscle‐invasive bladder cancer in medically fit patients, despite many centers reporting excellent long‐term results for bladder preserving strategies. This retrospective analysis compares different therapeutic modalities in bladder‐preservation therapy. The results of this study show that multimodal treatment consisting of maximal transurethral resection of bladder tumor followed by radiotherapy, concomitant platinum‐based chemotherapy combined with regional deep hyperthermia in patients with Ta, Tis, T1–2 bladder carcinomas improves local control, bladder‐preservation rate, and survival. More importantly, these findings offer a promising alternative to surgical therapies like radical cystectomy. The authors hope that, in the future, closer collaboration between urologists and radiotherapists will further improve treatments and therapies for the benefit of patients.
Introduction
Bladder cancer is among the most common malignant tumors worldwide and the second leading urological tumor after prostate cancer.
Radical cystectomy with appropriate lymph node dissection has long represented the standard of care for muscle‐invasive bladder cancer in medically fit patients, despite many centers reporting excellent long‐term results for bladder preserving strategies. Cystectomy is associated with postoperative and perioperative complications of varying severity in up to 64% of patients and mortality rates ranging from 1.5% to 2.7% 1,2.
Today, a vast amount of clinical experience from single‐institution trials as well as prospective clinical trials on primary organ preserving treatment is available [3–6]. Multimodality organ‐preservation treatment has become the treatment of choice for many tumor entities [7–9]. Nevertheless, the issue of conservative treatments versus cystectomy for bladder cancer remains controversial. With the improvements in life expectancy combined with the high prevalence of concomitant diseases, and with increasing expectations concerning quality of life, the option of multimodal bladder‐conserving therapy is gaining importance.
A major prerequisite for success is a well‐established interdisciplinary team that coordinates the multimodal treatment for patients with bladder cancer. At our hospital, we have offered patients of all age groups the option of multimodal bladder‐preservation treatment since 1982 [4,10–14]. In the early days, all patients were treated with radiotherapy alone. The treatment was intensified in the nineties through the addition of concurrent chemotherapy and later through further addition of regional deep hyperthermia after transurethral resection [4,10,12]. The aim of this retrospective analysis was to report the effect of different therapeutic modalities on the frequency of bladder‐preservation, disease‐free survival (DFS), and overall survival.
Subjects, Materials, and Methods
Patients
From May 1982 to January 2016, 664 patients underwent bladder‐preservation treatment at our department. A retrospective review of these patients with respect to tumor characteristics demonstrated a considerable decrease of advanced tumor stages T3–4 over the last few years. Therefore, the patient population for analysis was reduced to patients with high‐risk superficial (Ta, Tis, T1) and muscle‐invasive (T2) bladder cancer. Among these, 372 of 664 patients had histologically confirmed pTa, pTis, or pT1–pT2 bladder cancer and no distant metastases or other concomitant malignancy and therefore were included. Three of 372 patients were treated with radiotherapy (RT) + regional deep hyperthermia (RHT) and thus excluded from analysis. In total, 297 of 369 men and 72 of 369 women constitute the analysis set (ratio 4:1; median age, 67 years; range, 32–91). For detailed information about clinical patient characteristics, see Table 1 and Figure 1.
| Characteristics . | RT . | RCT . | RCT + RHT . | p value . |
|---|---|---|---|---|
| Number of patients, n (%) | 75 (20) | 215 (58) | 79 (21) | |
| Sex, n (%) | .217 | |||
| Male | 55 (74) | 177 (82) | 65 (82) | |
| Female | 20 (27) | 38 (18) | 14 (18) | |
| Age at initial diagnosis, median (range), yr | 75 (40–91) | 66 (32–87) | 67 (38–88) | <.001 |
| Median follow‐up (months) | 40 | 80 | 72 | .002 |
| Median follow‐up (years) | 3 | 7 | 6 | .011 |
| Recurrent tumor, n (%) | .539 | |||
| Primary tumor | 62 (83) | 165 (76) | 60 (76) | |
| Recurrent tumor | 13 (17) | 49 (23) | 19 (24) | |
| Not reported | 1 (1) | |||
| Median numbers of TUR‐BT bevor treatment (range) | 1 (1–7) | 2 (1–20) | 2 (1–10) | .042 |
| Histologic subtypes, n (%) | .724 | |||
| Urothelial carcinoma | 70 (93) | 204 (95) | 76 (96) | |
| Squamous cell carcinoma | 5 (2) | 1 (1) | ||
| Urothelial + squamous cell carcinoma | 3 (4) | 5 (2) | ||
| Others | 2 (3) | 1 (1) | 2 (23) | |
| Multifocal growth pattern, n (%) | .232 | |||
| Unifocal | 42 (56) | 107 (50) | 45 (57) | |
| Multifocal | 28 (37) | 102 (47) | 29 (37) | |
| Not reported | 5 (7) | 6 (3) | 5 (6) | |
| Associated carcinoma in situ, n (%) | .190 | |||
| Cis | 12 (16) | 61 (28) | 22 (28) | |
| No cis | 53 (71) | 142 (66) | 57 (72) | |
| Not reported | 10 (13) | 12 (6) | ||
| Median numbers of RHT (range) | 5 (1–10) | |||
| Pathological T‐category (initial TUR‐BT), n (%) | .004 | |||
| Ta/Tis | 3 (4) | 5 (2) | 7 (9) | |
| T1 | 20 (27) | 95 (44) | 38 (48) | |
| T2 | 52 (69) | 115 (54) | 34 (43) | |
| Clinical lymph node classification, n (%) | .301 | |||
| N0 | 70 (94) | 183 (85) | 75 (94) | |
| N+ | 1 (1) | 10 (5) | 2 (3) | |
| Not reported | 4 (5) | 22 (10) | 2 (3) | |
| Lymphovascular invasion, n (%) | .296 | |||
| L0 | 38 (51) | 116 (54) | 22 (28) | |
| L1 | 24 (32) | 53 (25) | 10 (13) | |
| L2 | 4 (5) | 7 (3) | ||
| Not reported | 9 (12) | 39 (18) | 47 (59) | |
| Grading, n (%) | <.001 | |||
| G1/2 | 39 (52) | 75 (35) | 12 (15) | |
| G3/4 | 36 (48) | 138 (64) | 67 (85) | |
| Not reported | 2 (1) | |||
| Resection margin after initial TUR‐BT, n (%) | <.001 | |||
| R0 | 25 (34) | 91 (42) | 50 (63) | |
| R1 | 16 (21) | 77 (36) | 15 (19) | |
| R2 | 30 (40) | 27 (13) | 2 (3) | |
| Not reported | 4 (5) | 20 (9) | 12 (15) |
| Characteristics . | RT . | RCT . | RCT + RHT . | p value . |
|---|---|---|---|---|
| Number of patients, n (%) | 75 (20) | 215 (58) | 79 (21) | |
| Sex, n (%) | .217 | |||
| Male | 55 (74) | 177 (82) | 65 (82) | |
| Female | 20 (27) | 38 (18) | 14 (18) | |
| Age at initial diagnosis, median (range), yr | 75 (40–91) | 66 (32–87) | 67 (38–88) | <.001 |
| Median follow‐up (months) | 40 | 80 | 72 | .002 |
| Median follow‐up (years) | 3 | 7 | 6 | .011 |
| Recurrent tumor, n (%) | .539 | |||
| Primary tumor | 62 (83) | 165 (76) | 60 (76) | |
| Recurrent tumor | 13 (17) | 49 (23) | 19 (24) | |
| Not reported | 1 (1) | |||
| Median numbers of TUR‐BT bevor treatment (range) | 1 (1–7) | 2 (1–20) | 2 (1–10) | .042 |
| Histologic subtypes, n (%) | .724 | |||
| Urothelial carcinoma | 70 (93) | 204 (95) | 76 (96) | |
| Squamous cell carcinoma | 5 (2) | 1 (1) | ||
| Urothelial + squamous cell carcinoma | 3 (4) | 5 (2) | ||
| Others | 2 (3) | 1 (1) | 2 (23) | |
| Multifocal growth pattern, n (%) | .232 | |||
| Unifocal | 42 (56) | 107 (50) | 45 (57) | |
| Multifocal | 28 (37) | 102 (47) | 29 (37) | |
| Not reported | 5 (7) | 6 (3) | 5 (6) | |
| Associated carcinoma in situ, n (%) | .190 | |||
| Cis | 12 (16) | 61 (28) | 22 (28) | |
| No cis | 53 (71) | 142 (66) | 57 (72) | |
| Not reported | 10 (13) | 12 (6) | ||
| Median numbers of RHT (range) | 5 (1–10) | |||
| Pathological T‐category (initial TUR‐BT), n (%) | .004 | |||
| Ta/Tis | 3 (4) | 5 (2) | 7 (9) | |
| T1 | 20 (27) | 95 (44) | 38 (48) | |
| T2 | 52 (69) | 115 (54) | 34 (43) | |
| Clinical lymph node classification, n (%) | .301 | |||
| N0 | 70 (94) | 183 (85) | 75 (94) | |
| N+ | 1 (1) | 10 (5) | 2 (3) | |
| Not reported | 4 (5) | 22 (10) | 2 (3) | |
| Lymphovascular invasion, n (%) | .296 | |||
| L0 | 38 (51) | 116 (54) | 22 (28) | |
| L1 | 24 (32) | 53 (25) | 10 (13) | |
| L2 | 4 (5) | 7 (3) | ||
| Not reported | 9 (12) | 39 (18) | 47 (59) | |
| Grading, n (%) | <.001 | |||
| G1/2 | 39 (52) | 75 (35) | 12 (15) | |
| G3/4 | 36 (48) | 138 (64) | 67 (85) | |
| Not reported | 2 (1) | |||
| Resection margin after initial TUR‐BT, n (%) | <.001 | |||
| R0 | 25 (34) | 91 (42) | 50 (63) | |
| R1 | 16 (21) | 77 (36) | 15 (19) | |
| R2 | 30 (40) | 27 (13) | 2 (3) | |
| Not reported | 4 (5) | 20 (9) | 12 (15) |
Abbreviations: Cis, carcinoma in situ; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy; TUR‐BT, transurethral resection of bladder tumor.
| Characteristics . | RT . | RCT . | RCT + RHT . | p value . |
|---|---|---|---|---|
| Number of patients, n (%) | 75 (20) | 215 (58) | 79 (21) | |
| Sex, n (%) | .217 | |||
| Male | 55 (74) | 177 (82) | 65 (82) | |
| Female | 20 (27) | 38 (18) | 14 (18) | |
| Age at initial diagnosis, median (range), yr | 75 (40–91) | 66 (32–87) | 67 (38–88) | <.001 |
| Median follow‐up (months) | 40 | 80 | 72 | .002 |
| Median follow‐up (years) | 3 | 7 | 6 | .011 |
| Recurrent tumor, n (%) | .539 | |||
| Primary tumor | 62 (83) | 165 (76) | 60 (76) | |
| Recurrent tumor | 13 (17) | 49 (23) | 19 (24) | |
| Not reported | 1 (1) | |||
| Median numbers of TUR‐BT bevor treatment (range) | 1 (1–7) | 2 (1–20) | 2 (1–10) | .042 |
| Histologic subtypes, n (%) | .724 | |||
| Urothelial carcinoma | 70 (93) | 204 (95) | 76 (96) | |
| Squamous cell carcinoma | 5 (2) | 1 (1) | ||
| Urothelial + squamous cell carcinoma | 3 (4) | 5 (2) | ||
| Others | 2 (3) | 1 (1) | 2 (23) | |
| Multifocal growth pattern, n (%) | .232 | |||
| Unifocal | 42 (56) | 107 (50) | 45 (57) | |
| Multifocal | 28 (37) | 102 (47) | 29 (37) | |
| Not reported | 5 (7) | 6 (3) | 5 (6) | |
| Associated carcinoma in situ, n (%) | .190 | |||
| Cis | 12 (16) | 61 (28) | 22 (28) | |
| No cis | 53 (71) | 142 (66) | 57 (72) | |
| Not reported | 10 (13) | 12 (6) | ||
| Median numbers of RHT (range) | 5 (1–10) | |||
| Pathological T‐category (initial TUR‐BT), n (%) | .004 | |||
| Ta/Tis | 3 (4) | 5 (2) | 7 (9) | |
| T1 | 20 (27) | 95 (44) | 38 (48) | |
| T2 | 52 (69) | 115 (54) | 34 (43) | |
| Clinical lymph node classification, n (%) | .301 | |||
| N0 | 70 (94) | 183 (85) | 75 (94) | |
| N+ | 1 (1) | 10 (5) | 2 (3) | |
| Not reported | 4 (5) | 22 (10) | 2 (3) | |
| Lymphovascular invasion, n (%) | .296 | |||
| L0 | 38 (51) | 116 (54) | 22 (28) | |
| L1 | 24 (32) | 53 (25) | 10 (13) | |
| L2 | 4 (5) | 7 (3) | ||
| Not reported | 9 (12) | 39 (18) | 47 (59) | |
| Grading, n (%) | <.001 | |||
| G1/2 | 39 (52) | 75 (35) | 12 (15) | |
| G3/4 | 36 (48) | 138 (64) | 67 (85) | |
| Not reported | 2 (1) | |||
| Resection margin after initial TUR‐BT, n (%) | <.001 | |||
| R0 | 25 (34) | 91 (42) | 50 (63) | |
| R1 | 16 (21) | 77 (36) | 15 (19) | |
| R2 | 30 (40) | 27 (13) | 2 (3) | |
| Not reported | 4 (5) | 20 (9) | 12 (15) |
| Characteristics . | RT . | RCT . | RCT + RHT . | p value . |
|---|---|---|---|---|
| Number of patients, n (%) | 75 (20) | 215 (58) | 79 (21) | |
| Sex, n (%) | .217 | |||
| Male | 55 (74) | 177 (82) | 65 (82) | |
| Female | 20 (27) | 38 (18) | 14 (18) | |
| Age at initial diagnosis, median (range), yr | 75 (40–91) | 66 (32–87) | 67 (38–88) | <.001 |
| Median follow‐up (months) | 40 | 80 | 72 | .002 |
| Median follow‐up (years) | 3 | 7 | 6 | .011 |
| Recurrent tumor, n (%) | .539 | |||
| Primary tumor | 62 (83) | 165 (76) | 60 (76) | |
| Recurrent tumor | 13 (17) | 49 (23) | 19 (24) | |
| Not reported | 1 (1) | |||
| Median numbers of TUR‐BT bevor treatment (range) | 1 (1–7) | 2 (1–20) | 2 (1–10) | .042 |
| Histologic subtypes, n (%) | .724 | |||
| Urothelial carcinoma | 70 (93) | 204 (95) | 76 (96) | |
| Squamous cell carcinoma | 5 (2) | 1 (1) | ||
| Urothelial + squamous cell carcinoma | 3 (4) | 5 (2) | ||
| Others | 2 (3) | 1 (1) | 2 (23) | |
| Multifocal growth pattern, n (%) | .232 | |||
| Unifocal | 42 (56) | 107 (50) | 45 (57) | |
| Multifocal | 28 (37) | 102 (47) | 29 (37) | |
| Not reported | 5 (7) | 6 (3) | 5 (6) | |
| Associated carcinoma in situ, n (%) | .190 | |||
| Cis | 12 (16) | 61 (28) | 22 (28) | |
| No cis | 53 (71) | 142 (66) | 57 (72) | |
| Not reported | 10 (13) | 12 (6) | ||
| Median numbers of RHT (range) | 5 (1–10) | |||
| Pathological T‐category (initial TUR‐BT), n (%) | .004 | |||
| Ta/Tis | 3 (4) | 5 (2) | 7 (9) | |
| T1 | 20 (27) | 95 (44) | 38 (48) | |
| T2 | 52 (69) | 115 (54) | 34 (43) | |
| Clinical lymph node classification, n (%) | .301 | |||
| N0 | 70 (94) | 183 (85) | 75 (94) | |
| N+ | 1 (1) | 10 (5) | 2 (3) | |
| Not reported | 4 (5) | 22 (10) | 2 (3) | |
| Lymphovascular invasion, n (%) | .296 | |||
| L0 | 38 (51) | 116 (54) | 22 (28) | |
| L1 | 24 (32) | 53 (25) | 10 (13) | |
| L2 | 4 (5) | 7 (3) | ||
| Not reported | 9 (12) | 39 (18) | 47 (59) | |
| Grading, n (%) | <.001 | |||
| G1/2 | 39 (52) | 75 (35) | 12 (15) | |
| G3/4 | 36 (48) | 138 (64) | 67 (85) | |
| Not reported | 2 (1) | |||
| Resection margin after initial TUR‐BT, n (%) | <.001 | |||
| R0 | 25 (34) | 91 (42) | 50 (63) | |
| R1 | 16 (21) | 77 (36) | 15 (19) | |
| R2 | 30 (40) | 27 (13) | 2 (3) | |
| Not reported | 4 (5) | 20 (9) | 12 (15) |
Abbreviations: Cis, carcinoma in situ; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy; TUR‐BT, transurethral resection of bladder tumor.
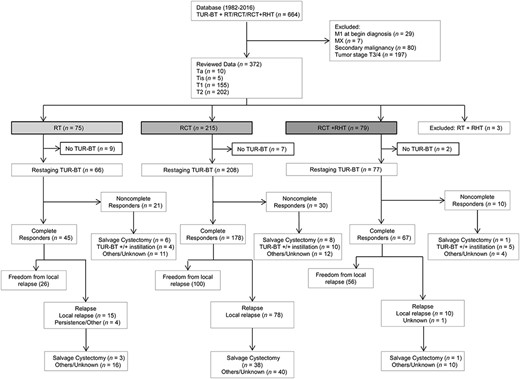
The flowchart of the trial according to the CONSORT statement.
Abbreviations: M, distant metastasis; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy; TUR‐BT, transurethral resection of bladder tumor.
In addition, because of the fact of different prognostic relevance, special attention is given to the groups non‐muscle‐invasive bladder cancer (NMIBC) and muscle‐invasive bladder cancer (MIBC; see supplemental online Figs. 1 and 2).
Exclusion criteria for hyperthermia were metal implants, severe cardiovascular diseases, and a Karnofsky Performance Index >1.
Criteria for Toxicity
Adverse hematologic effects were analyzed according to National Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 [15]. In addition, the late effects of normal tissue‐subjective, objective, management, and analytic (LENT‐SOMA) grading system was used to examine nonhematologic toxicity [16].
Treatment
Multimodal treatment starts with transurethral resection of bladder tumor (TUR‐BT), including tumor mapping, with maximal resection of the gross tumor, if feasible. Residual tumor status was assessed through biopsies from all resection margins: R0 was classified as microscopically complete TUR‐BT, R1 indicated microscopic residual tumor, and R2 indicated macroscopic residual tumor. TNM category was assigned according to the 1987 edition of the TNM classification by the International Union Against Cancer and has been modified according to the 2010 TNM classification by the American Joint Committee on cancer 17,18. All patients had to have a bladder volume >300 ml.
RT was initiated 4–6 weeks after TUR‐BT using 15 MV photons from a linear accelerator and three‐dimensional conformal treatment planning. A four‐field box technique was usually used, and radiotherapy was administered in daily fractions of 1.8 Gy on 5 consecutive days each week. All patients were treated with an empty bladder. Clinical target volume included the whole bladder, the presacral, and the internal iliac lymph nodes. The median dose to the whole bladder and pelvic lymph nodes was 50.4 Gy (range, 16.2–55.8). In case of R0 resection after TUR‐BT, the whole bladder received a boost to 55.8 Gy with a 1.5–2.0 cm safety margin in all directions. For R1/2 resection, patients received a 9‐Gy boost to the whole bladder to a total dose of 59.4 Gy. Platinum‐based chemotherapy was delivered as standard chemotherapy and applied simultaneously in the first and fifth weeks for 5 days each week. In case of mild renal insufficiency, heart disease, or other concurrent diseases, other regimes were used. More detailed information on the chemotherapy regimen is shown in supplemental online Table 1.
Regional deep hyperthermia was performed once weekly with the BSD‐2000 3D/PC‐Hyperthermia System (BSD Medical Corporation, Salt Lake City, UT). At our hospital, either Sigma Eye or Sigma‐60 applicator was used to emit electromagnetic microwave irradiation (90–100 MHz) that surrounds the patient's pelvic region. The minimum planed number of hyperthermia treatments was five applications performed once weekly. Patients were treated while supine with a circular water pillow used to connect the antenna power to the patient. After patient positioning, four calibrated thermometry probes were located in the bladder cavity, rectum, anal fold, and mouth. Bladder temperature was measured with a thermistor inserted into the bladder via a Foley catheter. Throughout the entire treatment, cardiac function, blood pressure, and oxygen were constantly controlled. Furthermore, patients were requested to make it known if they experienced any unpleasant sensation suggestive of hot spots, such as burning sensations or pain. Adjustments of treatment settings were changes in power output per channel, frequency or phase settings, and placement of additional water boluses. Tolerances for power outputs were increased up to patients’ compliance. Intravesical and other temperatures spots were constantly measured every 10 seconds during RHT and for 15 minutes thereafter. Treatment continued for 60 minutes after bladder temperatures reached 41.5°C or for a maximum total duration of 90 minutes, whichever was shortest. With the measured intravesical temperatures, the cumulative equivalent minutes at 43°C were calculated as a value in order to describe the thermal dose applied to the bladder [13,19,20]. Radiation started within an interval of 60 minutes after RHT. Cystectomy was undertaken in nonresponder patients after TUR‐BT re‐evaluation. In case of persistent, extensive associated carcinoma in situ or recurrent tumor, additional treatments, such as re‐TUR‐BT (one or more) with or without topical chemoinstillation for superficial tumors or salvage cystectomy for muscle‐invasive tumors, were initiated.
Statistical Analysis
Statistical analysis was performed using SPSS v21.0 (SPSS; Chicago, IL) and R 3.3.3. [21].
Differences between continuous data on clinical patient characteristics were analyzed using ANOVA on nonmissing data. The Chi‐square test was used to determine statistical significance between categorical data.
The main characteristics available in the data base that are important, or have a potential effect on the outcome, are gender, age (defined as age at time of initial diagnosis), recurrent tumor, multifocal growth pattern, associated carcinoma in situ, histologic grading, tumor stage, lymph node classification, histologic subtypes, and resection margin.
Complete response determined by control TUR‐BT 4–6 weeks following completion of therapeutic modality was modeled by logistic regression. Overall survival (OS), DFS, and time to cystectomy were defined from the beginning of our treatment (determined as the date of initial TUR‐BT). DFS was defined as the absence of locoregional or distant disease recurrence and death from any cause. All patients with information on CR were considered (n = 351). Patients who did not have a complete response (CR) at control TUR‐BT counted as therapy failure and were entered as having an event at time 0. For OS and DFS, we used Cox models stratified by therapeutic modalities. Survival curves are shown for median values of covariates in the whole patient collective. Hazard ratios (HRs) to quantify the treatment effect are estimated by Cox models adjusted for therapeutic modality. Reported 5‐ and 10‐year rates are taken from Kaplan‐Meier estimates. In the multivariable models, the variables grading, tumor stage, and resection status have been included because the patient group has changed considerably over the past years. Further variables were selected for the multivariable logistic regression model for which p < .1573 (Akaike criterion) in univariate analysis adjusted for treatment. The multivariable Cox models contain all variables of the logistic model plus those variables for which p < .1573 in the respective univariate models stratified by treatment. Patients with missing values were excluded from the respective analyses (50 for logistic regression, 62 for OS, and 50 for DFS).
Because of a lack of sufficient data on performance of cystectomy following RT, especially in the first years of bladder‐preservation treatment in Erlangen (1982 to about 1990), the analysis of time to cystectomy was limited to 294 patients treated with chemoradiotherapy (RCT) or RCT + RHT. A total of 240 of 294 patients with nonmissing covariates were included. Time to cystectomy is regarded within a competing risk model, with death as competing endpoint. Hazards are estimated by censoring competing events stratified by therapeutic modality and adjusted for covariates predictive for OS or selected by Akaike criterion. Cumulative incidence curves for the endpoint cystectomy are shown for median covariate values.
Late treatment‐related toxicity is evaluated by Chi‐square tests for the RCT and RCT + RHT groups only because of the lack of missing blood parameters for patients treated with RT.
Results
Median follow‐up of all patients (n = 369) was 71 months, with a range from 5–336 months. Median follow‐up for patients with high‐risk superficial (Ta, Tis, T1) and patients with muscle‐invasive (T2) bladder cancer was 81 months and 61 months. All patients were treated with curative intent. The median number of hyperthermia treatments was 5 (range, 1–10). The median interval between the initial TUR‐BT and beginning of chemoradiotherapy was 42 days (range, 9–202). The median interval between the end of radiotherapy and control TUR‐BT was 46 days (range, 1–289). Median covariate values of the whole patient collective, used for multivariable models, are age = 67 years, tumor = primary, pT‐category = T2, clinical lymph node classification = N0, resection status = R1, and histologic grading = G3/4.
Response
Complete response at control TUR‐BT was achieved by 290 of 351 patients (83%) which divide into 45/66 (68%), 178/208 (86%), and 67/77 (87%) patients treated with RT, RCT, and RCT + RHT, respectively. Adjusted CR rates predicted for median covariate values result to 72%, 86%, and 87% for RT, RCT, and RCT + RHT. The higher CR rate for RT arises mainly as the prediction is done for a better resection status than seen in the RT group.
In subgroup analysis, complete response at control TUR‐BT for patients with high‐risk superficial (Ta, Tis, T1) was reached in 146 of 166 (88%) patients, of whom 19 of 22 (86%) were treated with RT, 87 of 99 (88%) were treated with RCT, and 40 of 45 (89%) patients were treated with RCT + RHT, respectively. For patients with muscle‐invasive (T2) bladder cancer, the analysis showed a complete response rate of 78% (144/185). CR was achieved by 26 of 44 (59%), 91 of 109 (84%), and 27 of 32 (84%) patients for RT, RCT, and RCT + RHT.
Additionally, data analysis of complete remission for patients with tumor stage T2, R2 resection showed a CR rate of 38% (9/24), 67% (12/18), and 100% (1/1) for RT, RCT, and RCT + RHT. For patients with tumor stage T2, R1 resection showed a CR rate of 46% (6/13), 80% (36/45), and 70% (7/10) for RT, RCT, and RCT + RHT.
RCT was superior to RT in the multivariate analysis (OR, 2.32; 95% confidence interval [CI], 1.05–5.12; p = .037). Additional RHT could not increase the CR rate compared with RCT and was not statistically significant to RT (OR, 2.56; 95% CI, 0.88–8.00; p = .092; see Table 2).
Analysis of influence in patients and therapy‐related parameters on complete response, results of univariate and multivariate logistic regressions
| Variable and category . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| OR (95% CI) . | p value . | OR (95% CI) . | p value . | |
| Treatment | ||||
| RCT vs. RT | 2.77 (1.44–5.28) | .002 | 2.32 (1.05–5.12) | .037 |
| RCT + RHT vs. RT | 3.13 (1.38–7.52) | .008 | 2.56 (0.88–8.00) | .092 |
| Gender; female vs. male | 1.22 (0.60–2.66) | .597 | ||
| Age at diagnosis per yr | 0.97 (0.94–1.00) | .030 | 0.97 (0.94–1.01) | .122 |
| Recurrent tumor vs. primary tumor | 0.41 (0.22–0.77) | .005 | 0.34 (0.16–0.71) | .004 |
| Histologic subtypes; nonurothelial carcinoma vs. urothelial carcinoma | 0.75 (0.25–2.77) | .631 | ||
| Multifocal vs. unifocal growth pattern | 0.75 (0.41–1.36) | .340 | ||
| No cis vs. cis | 1.18 (0.60–2.25) | .612 | ||
| pT category; T2 vs. Ta/Tis/T1 | 0.95 (0.89–0.99) | .053 | 0.59 (0.29–1.17) | .134 |
| Clinical lymph node classification; N+ vs. N0 | 0.55 (0.16–2.59) | .394 | ||
| Resection status | ||||
| R1 vs. R0 | 0.57 (0.26–1.22) | .143 | 0.63 (0.29–1.40) | .255 |
| R2 vs. R0 | 0.20 (0.09–0.45) | <.001 | 0.24 (0.10–0.55) | <.001 |
| Histologic grading; G3/4 vs. G1/2 | 0.74 (0.39–1.37) | .348 | 0.68 (0.32–1.38) | .297 |
| Variable and category . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| OR (95% CI) . | p value . | OR (95% CI) . | p value . | |
| Treatment | ||||
| RCT vs. RT | 2.77 (1.44–5.28) | .002 | 2.32 (1.05–5.12) | .037 |
| RCT + RHT vs. RT | 3.13 (1.38–7.52) | .008 | 2.56 (0.88–8.00) | .092 |
| Gender; female vs. male | 1.22 (0.60–2.66) | .597 | ||
| Age at diagnosis per yr | 0.97 (0.94–1.00) | .030 | 0.97 (0.94–1.01) | .122 |
| Recurrent tumor vs. primary tumor | 0.41 (0.22–0.77) | .005 | 0.34 (0.16–0.71) | .004 |
| Histologic subtypes; nonurothelial carcinoma vs. urothelial carcinoma | 0.75 (0.25–2.77) | .631 | ||
| Multifocal vs. unifocal growth pattern | 0.75 (0.41–1.36) | .340 | ||
| No cis vs. cis | 1.18 (0.60–2.25) | .612 | ||
| pT category; T2 vs. Ta/Tis/T1 | 0.95 (0.89–0.99) | .053 | 0.59 (0.29–1.17) | .134 |
| Clinical lymph node classification; N+ vs. N0 | 0.55 (0.16–2.59) | .394 | ||
| Resection status | ||||
| R1 vs. R0 | 0.57 (0.26–1.22) | .143 | 0.63 (0.29–1.40) | .255 |
| R2 vs. R0 | 0.20 (0.09–0.45) | <.001 | 0.24 (0.10–0.55) | <.001 |
| Histologic grading; G3/4 vs. G1/2 | 0.74 (0.39–1.37) | .348 | 0.68 (0.32–1.38) | .297 |
Abbreviations: CI, confidence interval; cis, associated carcinoma in situ; OR, odds ratio; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy.
Analysis of influence in patients and therapy‐related parameters on complete response, results of univariate and multivariate logistic regressions
| Variable and category . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| OR (95% CI) . | p value . | OR (95% CI) . | p value . | |
| Treatment | ||||
| RCT vs. RT | 2.77 (1.44–5.28) | .002 | 2.32 (1.05–5.12) | .037 |
| RCT + RHT vs. RT | 3.13 (1.38–7.52) | .008 | 2.56 (0.88–8.00) | .092 |
| Gender; female vs. male | 1.22 (0.60–2.66) | .597 | ||
| Age at diagnosis per yr | 0.97 (0.94–1.00) | .030 | 0.97 (0.94–1.01) | .122 |
| Recurrent tumor vs. primary tumor | 0.41 (0.22–0.77) | .005 | 0.34 (0.16–0.71) | .004 |
| Histologic subtypes; nonurothelial carcinoma vs. urothelial carcinoma | 0.75 (0.25–2.77) | .631 | ||
| Multifocal vs. unifocal growth pattern | 0.75 (0.41–1.36) | .340 | ||
| No cis vs. cis | 1.18 (0.60–2.25) | .612 | ||
| pT category; T2 vs. Ta/Tis/T1 | 0.95 (0.89–0.99) | .053 | 0.59 (0.29–1.17) | .134 |
| Clinical lymph node classification; N+ vs. N0 | 0.55 (0.16–2.59) | .394 | ||
| Resection status | ||||
| R1 vs. R0 | 0.57 (0.26–1.22) | .143 | 0.63 (0.29–1.40) | .255 |
| R2 vs. R0 | 0.20 (0.09–0.45) | <.001 | 0.24 (0.10–0.55) | <.001 |
| Histologic grading; G3/4 vs. G1/2 | 0.74 (0.39–1.37) | .348 | 0.68 (0.32–1.38) | .297 |
| Variable and category . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| OR (95% CI) . | p value . | OR (95% CI) . | p value . | |
| Treatment | ||||
| RCT vs. RT | 2.77 (1.44–5.28) | .002 | 2.32 (1.05–5.12) | .037 |
| RCT + RHT vs. RT | 3.13 (1.38–7.52) | .008 | 2.56 (0.88–8.00) | .092 |
| Gender; female vs. male | 1.22 (0.60–2.66) | .597 | ||
| Age at diagnosis per yr | 0.97 (0.94–1.00) | .030 | 0.97 (0.94–1.01) | .122 |
| Recurrent tumor vs. primary tumor | 0.41 (0.22–0.77) | .005 | 0.34 (0.16–0.71) | .004 |
| Histologic subtypes; nonurothelial carcinoma vs. urothelial carcinoma | 0.75 (0.25–2.77) | .631 | ||
| Multifocal vs. unifocal growth pattern | 0.75 (0.41–1.36) | .340 | ||
| No cis vs. cis | 1.18 (0.60–2.25) | .612 | ||
| pT category; T2 vs. Ta/Tis/T1 | 0.95 (0.89–0.99) | .053 | 0.59 (0.29–1.17) | .134 |
| Clinical lymph node classification; N+ vs. N0 | 0.55 (0.16–2.59) | .394 | ||
| Resection status | ||||
| R1 vs. R0 | 0.57 (0.26–1.22) | .143 | 0.63 (0.29–1.40) | .255 |
| R2 vs. R0 | 0.20 (0.09–0.45) | <.001 | 0.24 (0.10–0.55) | <.001 |
| Histologic grading; G3/4 vs. G1/2 | 0.74 (0.39–1.37) | .348 | 0.68 (0.32–1.38) | .297 |
Abbreviations: CI, confidence interval; cis, associated carcinoma in situ; OR, odds ratio; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy.
In the multivariable logistic regression, R2‐resection (OR, 0.24; 95% CI, 0.10–0.55; p < 0001) and primary tumor (OR, 0.34; 95% CI, 0.16–0.71; p = .004) were significantly associated with CR. The variable grading (p = .348) and pT category (p = .053) were included in the multivariable model because the patient group had changed considerably over the past years. None of these factors reached statistical significance.
Overall Survival
The 5‐ and 10‐year overall survival for RCT were 64% (95% CI, 58%–71%) and 39% (95% CI, 33%–47%) and for RT were 45% (95%CI, 35%–58%) and 19% (95% CI, 12%–30%); additional hyperthermia further increased the 5‐ and 10‐year OS to 87% (95% CI, 79%–95%) and 60% (95% CI, 47%–77%).
In the subgroup analysis, patients with NMIBC, the 5‐year overall survival for RCT was 75% (95% CI, 67%–84%) and for RT was 59% (95% CI, 42%–84%); additional hyperthermia further increased the 5‐year OS to 94% (95% CI, 87%–100%). For patients with MIBC, the 5‐year overall survival for RCT was 57% (95% CI, 48%–67%) and for RT was 43% (95% CI, 31%–61%); additional hyperthermia further increased the 5‐year OS to 77% (95% CI, 63%–94%).
In the multivariate analysis for the whole group, RCT was superior to RT (HR, 0.70; 95% CI, 0.50–0.99; p = .045); combined RHT increased OS (HR, 0.32; 95% CI, 0.18–0.58; p = .0001) as well (see Table 3). Figure 2 show OS curves adjusted for median patient characteristics stratified by therapeutic modality, Please note that adjustment achieves comparability of treatment effects but affects the curves which thus deviate from 5‐ and 10‐year rates from Kaplan‐Meier estimates. Furthermore, HRs and p values in Table 3 for treatment effects are given for an adjusted Cox model and are not stratified by treatment and therefore do not directly correspond to differences in plotted survival curves.
Analysis of influence in patients and therapy‐related parameters to overall survival, results of univariate and multivariate Cox regressions stratified by therapeutic modality (unless otherwise stated)
Results for Cox regression adjusted for therapeutic modality.
Abbreviations: CI, confidence interval; cis, associated carcinoma in situ; HR, hazard ratio; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy.
Analysis of influence in patients and therapy‐related parameters to overall survival, results of univariate and multivariate Cox regressions stratified by therapeutic modality (unless otherwise stated)
Results for Cox regression adjusted for therapeutic modality.
Abbreviations: CI, confidence interval; cis, associated carcinoma in situ; HR, hazard ratio; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy.
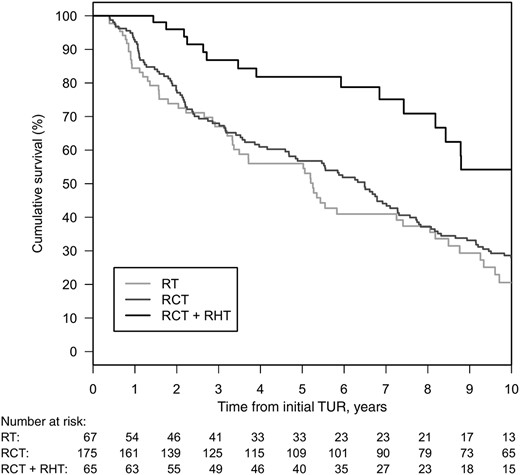
Overall survival adjusted for median patient characteristics stratified by therapeutic modality.
Abbreviations: RT, radiotherapy; RCT, chemoradiotherapy; RHT, regional deep hyperthermia; TUR, transurethral resection.
Redefinition of the reference treatment group in multivariate analysis showed a significantly better OS through the addition of hyperthermia to chemoradiotherapy (RCT + RHT vs. RCT: HR, 0.46; 95% CI, 0.27–0.78, p = .004).
Disease‐Free Survival
Cumulative estimated DFS for RCT (40%; 95% CI, 34%–47% and 24%; 95% CI, 18%–30%) was superior to RT (20%; 95% CI, 12%–32% and 6%; 95% CI, 2%–16%) for 5‐ and 10‐year as well as in multivariate analysis (HR, 0.70; 95% CI, 0.50–0.98; p = .039), respectively. Additional hyperthermia further increased the 5‐ and 10‐year DFS to 66% (95% CI, 55%–78%) and 46% (95% CI, 34%–62%) in multivariate analysis (HR, 0.39; 95% CI, 0.24–0.64; p = .0001; see supplemental online Fig. S3 and supplemental online Table 2).
In addition to this analysis, DFS for patients with NMIBC for RCT was 46% (95% CI, 37%–57%) and for RT was 14% (95% CI, 5%–39%); additional hyperthermia further increased the 5‐year DFS to 70% (95% CI, 57%–86%). For patients with MIBC, the 5‐year DFS for RCT was 34% (95% CI, 26%–44%) and for RT was 23% (95% CI, 13%–39%); additional hyperthermia further increased the 5‐year DFS to 60% (95% CI, 45%–81%).
Patients with R1 or R2 resections or recurrent tumors had a significantly higher risk of experiencing an event.
Additionally, tumor stage and grading were included because of the selection strategy after p value. In addition, the multivariate analysis with redefined reference treatment showed a significantly longer period of DFS through the addition of hyperthermia to chemoradiotherapy (RCT + RHT vs. RCT: HR, 0.56; 95% CI, 0.37–0.85; p = .006). In multivariate analysis, age (p < .0001), primary tumor (p = .005), and resection status (R2 vs. R0, p = .002) were significant predictors for extended DFS.
Cystectomy‐Free Survival
Overall, 55 patients who received RCT or RCT + RHT had to be treated with cystectomy following bladder conserving treatment. Most of these cases were due to tumor progression, with very few patients requiring a cystectomy due to therapeutic side effects (in particular bladder shrinking).
Comparing the treatment group RCT + RHT to RCT showed a significantly better 5‐ and 10‐year bladder‐preservation rate (96% vs. 82% and 96% vs. 78%, see Fig. 3; Cumulative incidence = 100% bladder‐preservation rate); this was also shown in multivariate analysis (HR, 0.13; 95% CI, 0.03–0.56; p = .006) compared with RCT.
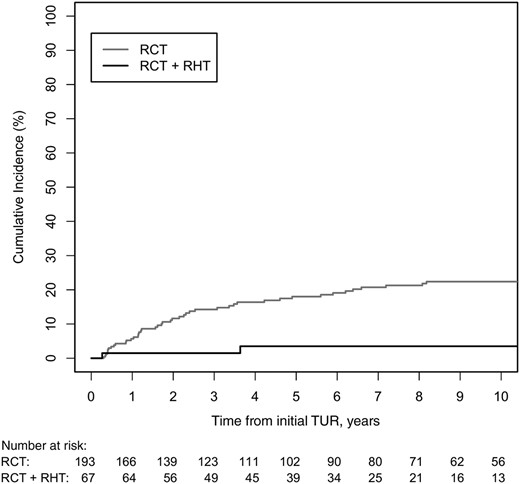
Cumulative incidence for competing event cystectomy in patients after multimodal bladder‐preserving treatment, stratified by the two different therapeutic modalities.
Abbreviations: RCT, chemoradiotherapy; RHT, regional deep hyperthermia; RT, radiotherapy; TUR, transurethral resection.
In the multivariate analysis, only resection status (R2 vs. R0; HR, 2.57; 95% CI, 1.05–6.30; p = .039) reached statistical significance with respect to the risk of cystectomy (see Table 4).
Analysis of influence in patients and therapy‐related parameters to cystectomy‐free survival, results of univariate and multivariate Cox regressions for hazard of cystectomy stratified by therapeutic modality (unless otherwise stated)
Results for Cox regression adjusted for therapeutic modality. This hazard ratio has the reference RCT and is therefore not comparable to the other hazard ratios for disease‐free survival and overall survival with reference radiotherapy.
Abbreviations: CI, confidence interval; cis, associated carcinoma in situ; HR, hazard ratio; RCT, chemoradiotherapy; RHT, regional deep hyperthermia.
Analysis of influence in patients and therapy‐related parameters to cystectomy‐free survival, results of univariate and multivariate Cox regressions for hazard of cystectomy stratified by therapeutic modality (unless otherwise stated)
Results for Cox regression adjusted for therapeutic modality. This hazard ratio has the reference RCT and is therefore not comparable to the other hazard ratios for disease‐free survival and overall survival with reference radiotherapy.
Abbreviations: CI, confidence interval; cis, associated carcinoma in situ; HR, hazard ratio; RCT, chemoradiotherapy; RHT, regional deep hyperthermia.
Acute Treatment‐Related Toxicities
In patients treated with RCT or RCT + RHT, some mild to moderate treatment‐related side effects (grades 1–2) were observed. These included transient cystitis, enteritis, and nausea and were managed by symptomatic treatment. Grade 3 acute toxicity occurred as leucopenia in 25% (54/215) for RCT and 11% (9/79) for RCT + RHT and as thrombocytopenia in 6% (12/215) for RCT and 3% (2/79) for RCT + RHT. Grade 4 toxicity was especially limited to hematological side effects (i.e., leucopenia) in 2% (5/215) for RCT and 3% (2/79) for RCT + RHT and as thrombocytopenia in 2% (4/215) for RCT and 1% (1/79) for RCT + RHT. Grade 3 bladder toxicities was reported in 6% (13/215) for RCT and 5% (4/79) for RCT + RHT.
A total of 1.3% (1/79) of patients from the RCT + RHT group and 1.8% (4/215) from the RCT group had grade 4 bladder toxicities. Life‐threatening bleeding, infections, or treatment‐related deaths were not seen (see supplemental online Table 3).
Late Treatment‐Related Toxicity
No side effects (grade 0) were seen in 61% (130/215) of patients for RCT and 32% (25/79) for RCT + RHT. Mild dysuria, nocturia, urgency, proctitis, and diarrhea (grade 2) were recorded in 2%–13% for RCT and 2%–22% for RCT + RHT.
In 14 of 215 patients for RCT and 17 of 79 for RCT + RHT, late toxicity was not documented. A total of 1.3% (1/79) of patients from the RCT + RHT group and 2.3% (5/215) from the RCT group underwent cystectomy because of a shrinking bladder. Reduced bladder capacity (100–200 ml) with <2‐hour intervals of micturition was seen in 10% (22/215) for RCT and 15% (12/79) for RCT + RHT. Five patients experienced late grade 4 gastrointestinal toxicity and required surgical intervention (see supplemental online Table 4).
Discussion
The present retrospective analysis compares different therapeutic modalities in bladder‐preservation therapy, implemented in the 1980s and 1990s in Erlangen 10,11. To our knowledge this is the largest investigation comparing various methods of bladder preservation. Concomitant chemotherapy in addition to radiotherapy significantly improved OS, the rate of CR, and extended DFS in patients with bladder cancer [22].
The current follow‐up evaluation of our database was confined to the subgroup of Ta, Tis, and T1–2 bladder carcinomas. Addition of hyperthermia to chemoradiotherapy does not seem to increase CR but leads to a significant reduction in salvage cystectomy, improving OS and DFS.
One of the aims of this retrospective study is to show the historical development of multimodal bladder‐preservation therapy. In our multivariate analysis, the complete response for NMIBC versus MIBC was not significantly different but reached borderline significance with p = .053. This might be explained by the different complete remission rates for MIBC in the radiotherapy only group and shows the need for concomitant chemotherapy, especially in the group of patient with T2 bladder cancer.
Furthermore, in the RCT and RCT plus HT group, there was no significant difference in complete remission regarding MIBC versus NMIBC, but intensification of treatment (addition of chemotherapy ± hyperthermia) improved OS and DFS.
Regarding the small number of patients with R2 status who were treated with hyperthermia, data are difficult to interpret between different treatment groups, but we would conclude that tumors in the RCT group were not more advanced because of less aggressive TUR‐BTs.
Our analysis intends to contribute to the discussion of when bladder‐preservation therapy in high‐risk bladder cancer is possible. Cystectomy, with or without neoadjuvant therapy, as well as various bladder‐preservation therapies are proven effective for the treatment of bladder cancer [4,10,12,23]. Nevertheless, a direct comparison between bladder‐preservation modalities and radical cystectomy is difficult because of the lack of sufficiently sized randomized studies. For this reason, the use of retrospective data collections such as ours is necessary for comparison between different therapeutic modalities.
Bladder‐preservation strategies are often considered to be inferior to cystectomy, and radical cystectomy with pelvic lymphadenectomy is nowadays considered the “gold standard” treatment for muscle‐invasive bladder cancer. Recurrence‐free survival of 73% and a 5‐year survival rate of 62%–68% after radical cystectomy for all tumor stages are described in the literature 24,25. In high‐risk tumor stages, 5‐year OS ranges from 65% to 82% 26,27. Also, in a subgroup analysis of pT2 bladder cancer, subsequent recurrence‐free survival of 86% in lymph node (LN)‐negative versus 38% in LN‐positive patients and 5‐year cancer‐specific survival of 85% versus 60% are demonstrated [28].
According to the literature, only 10%–30% of patients with bladder cancer are treated with a multimodal therapy concept for organ preservation [29,30,31]. Several meta‐analyses summarized data from previous trials of bladder preserving therapy [3,5,32]. A recent meta‐analysis of combined chemoradiotherapy by Arcangeli et al. reported 78% CR, 28% muscle infiltrating recurrence, and 21% salvage cystectomy in patients with all tumor stages of bladder cancer [32]. The 5‐year OS was 56%. These results are in line with our findings for concurrent chemoradiotherapy, but, of note, in our analysis, only Ta, Tis, T1‐T2 tumor stages were included.
With the addition of hyperthermia, the 5‐year salvage cystectomy rate could be improved by 14%, and the 5‐year OS rate was 87% (i.e. higher than in most series with cystectomy). It is well known from the literature that hyperthermia is able to improve the efficacy of radio‐ and chemotherapy in many different tumor entities [25–27,33,34]. Synergy effects also result from the spatial interaction between chemotherapy and hyperthermia because most cytostatic drugs work better in areas with good blood flow, and hyperthermia is more effective in hypoxic tumor areas [25].
There are only few data available on hyperthermia in patients with bladder cancer [35]. A randomized study by Colombo et al. showed a reduction of local recurrence in a 2‐year follow‐up in patients with (Ta‐T1) high‐risk bladder cancer. The recurrence rate was significantly lower in the chemotherapy group with intravesical hyperthermia (17.1%), as compared with the Mitomycin C alone group (57.5%) [36]. This method has not been taken up widely because of a high rate of side effects 37,38.
Wittlinger et al., in 2009, reported the first data from Erlangen on hyperthermia in combination with chemoradiotherapy. As expected, the high rate of complete remission of 96% could not be maintained [13].
The Dutch Deep HT Group presented a randomized study for radiotherapy and hyperthermia in pelvic tumors, including bladder carcinomas. This trial has shown improved local tumor control without an increase in OS [39]. One explanation for the improved on DFS in our study is that hyperthermia activates the immune system through cell membrane changes 40,41. Another aspect is the effective heating of urine without much heat loss.
Evidently, our retrospective analysis has some limitations. As a result, the main risk factors are not homogeneously distributed among treatment groups. For example, more patients with T2 tumor stage were treated with RT, whereas more patients with poorly differentiated bladder cancer G3/4 were treated with RCT or RCT + RHT. Moreover, our analysis includes a time period during which patients did not receive uniform staging and during which combined chemoradiotherapy were not standard procedure. The development of technology in the past years has led to dose escalation, reduction of toxicity because of optimization of patient positioning, and improved protection of normal tissues [42]. Furthermore, research has led to major advances in clinical medicine with the use of new molecular targets and the developments of modern chemotherapy regimens. Finally, we excluded patients with T3–4 tumors from the analysis, because bladder‐preserving treatment of patients with this tumor stage has declined significantly in recent years.
RCT with or without combined RHT was well tolerated. However, it should be mentioned that the late side effects for bladder function were assessed retrospectively from patient records. Therefore, micturition disorders or function diagnostics that are not documented cannot be taken into account, leading to an underestimation of late side effects in this analysis.
The median age of patients with multimodal treatment was higher than that of patients undergoing radical cystectomy alone. However, many publications on bladder preserving therapy have shown similar results with respect to OS and DFS [43]. This is partly due to the fact that radical cystectomy is associated with increased risks and postoperative complications in elderly patients. Therefore, we can confirm that radiotherapy with or without hyperthermia could also be performed in elderly patients and improves the potential to spare the native bladder.
Conclusion
The multimodal treatment concept for bladder preservation is a safe and effective therapy with excellent CR rates and overall survival and is associated with acceptable toxicity. It offers a promising alternative to surgical therapies like radical cystectomy. It should therefore be an important objective to closely collaborate with urologists to improve treatments and therapies for the benefit of patients.
Acknowledgments
The present work was performed in partial fulfillment of the requirements for obtaining the “Dr. med.” degree.
Author Contributions
Conception/design: Ricarda Merten, Oliver Ott, Reinhard Kühn, Christian Weiss, Rainer Fietkau
Collection and/or assembly of data: Oliver Ott, Simone Bertz, Arndt Hartmann, Reinhard Kühn, Claus Rödel, Christian Weiss
Data analysis and interpretation: Ricarda Merten, Oliver Ott, Bernd Wullich, Claus Rödel, Christine Gall, Wolfgang Uter, Rainer Fietkau
Manuscript writing: Ricarda Merten, Oliver Ott, Marlen Haderlein, Simone Bertz, Arndt Hartmann, Bernd Wullich, Bastian Keck, Reinhard Kühn, Claus Rödel, Rainer Fietkau
Final approval of manuscript: Ricarda Merten, Oliver Ott, Marlen Haderlein, Simone Bertz, Arndt Hartmann, Bernd Wullich, Bastian Keck, Reinhard Kühn, Claus Rödel, Christian Weiss, Christine Gall, Wolfgang Uter, Rainer Fietkau
Disclosures
Simone Bertz: Roche (H), Merck Sharpe & Dohme (Other‐Sponsored PD‐L1 Training); Oliver Ott: Elekta Rainer Fietkau (H), Merck, AstraZeneca (RF), Novocure (ET), SKB (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
Contributed equally.
Disclosures of potential conflicts of interest may be found at the end of this article.



