-
PDF
- Split View
-
Views
-
Cite
Cite
Jaydira Del Rivero, Maureen Edgerly, Jean Ward, Ravi A. Madan, Sanjeeve Balasubramaniam, Tito Fojo, Ann W. Gramza, Phase I/II Trial of Vandetanib and Bortezomib in Adults with Locally Advanced or Metastatic Medullary Thyroid Cancer, The Oncologist, Volume 24, Issue 1, January 2019, Pages 16–e14, https://doi.org/10.1634/theoncologist.2018-0452
Close - Share Icon Share
Abstract
Vandetanib at a dose of 300 mg orally every day plus bortezomib 1.3 mg/m2 intravenously on days 1, 4, 8, and 11 could be administered safely.
Assessing outcomes in 17 patients with medullary thyroid cancer, investigators considered the combination to be more difficult to administer than single‐agent vandetanib and that achieving better outcomes was unlikely. Consequently, a planned phase II study was terminated early.
The proto‐oncogene RET (REarranged during Transfection) has a critical role in the pathogenesis of medullary thyroid cancer (MTC). Vandetanib (V), a multitargeted tyrosine kinase inhibitor approved for the treatment of MTC, is thought to inhibit RET in MTC. Supported by preclinical studies demonstrating that bortezomib (B) administration lowered RET mRNA and protein levels, we conducted a phase I study in advanced solid tumors of vandetanib in combination with bortezomib. The goal was to establish an RP2D (recommended phase II dose) for the combination of vandetanib plus bortezomib, a regimen envisioned as a dual strategy for targeting RET in MTC.
Patients with advanced solid tumors were treated with escalating doses of bortezomib or vandetanib to assess the safety and tolerability of daily oral vandetanib and intravenous (IV) bortezomib administered on days 1, 4, 8, and 11 of a 28‐day cycle. Intrapatient dose escalation was allowed.
Twenty‐two patients were enrolled and received escalating mg/m2 bortezomib and mg vandetanib (number of patients) at initial doses of 1 and 100 (3), 1.3 and 100 (6), 1.3 and 200 (6), and 1.3 and 300 (7), respectively. Patients received a median of four cycles of bortezomib/vandetanib (range: 1–10), with 13 patients escalating to 1.3/200 and 10 to 1.3/300. G3 toxicities occurring in more than one patient included hypertension (24%), fatigue (19%), thrombocytopenia (10%), diarrhea (10%), and arthralgia (10%). There were no drug‐related G4/5 toxicities. There was one dose‐limiting toxicity, G3 thrombocytopenia, at bortezomib/vandetanib doses of 1.3/200 in cycle 2 that resolved without intervention. Four patients with a diagnosis of MTC (27%) had a partial response (PR).
The MTD of the combination was established as bortezomib, 1.3 mg/m2 IV days 1, 4, 8, and 11 with vandetanib 300 mg p.o. daily. RECIST responses were observed in patients with a diagnosis of MTC.
Abstract
经验教训
• 口服凡德他尼,每日剂量为 300 mg,并在第 1?4?8 和 11 天静脉注射硼替佐米 1.3 mg/m2,可安全给药?
• 在评估 17 例甲状腺髓样癌患者的预后时,研究员认为联合用药比凡德他尼单药更难给药,而且未必会取得更好的疗效?因此,计划中的 II 期研究提前终止?
摘要
背景?原癌基因 RET(在转染过程中重排)在甲状腺髓样癌 (MTC) 的发病机理中具有重要作用?凡德他尼 (V) 是一种获批用于治疗 MTC 的多靶点酪氨酸激酶抑制剂,其被认为可抑制 MTC 中的 RET?在证明服用硼替佐米 (B) 可降低 RET mRNA 和蛋白质水平的临床前研究的支持下,我们对采用凡德他尼联合硼替佐米治疗晚期实体肿瘤进行了 I 期研究?目的是建立凡德他尼联合硼替佐米的 RP2D (推荐 II 期剂量)方案,该方案预期为 MTC 中靶向 RET 的双重策略?
方法?晚期实体肿瘤患者接受逐步加大的硼替佐米或凡德他尼剂量治疗,以评估每日口服凡德他尼和在 28 天周期内第 1?4?8 和 11 天静脉注射 (IV) 硼替佐米的安全性和耐受性?允许住院期间增加剂量?
结果?有 22 名患者参与试验,逐渐加大的以 mg/m2 为单位的硼替佐米和以 mg 为单位的凡德他尼给药剂量(患者数量),初始剂量数据分别为 1 和 100 (3)?1.3 和 100 (6)?1.3 和 200 (6),以及 1.3 和 300 (7)?患者接受平均四个周期的硼替佐米/凡德他尼(范围是:1‐10)治疗,其中 13 名患者的剂量逐渐加大至 1.3/200,10 名患者逐渐加大至 1.3/300?超过一名患者出现的 G3 毒性,包括高血压 (24%)?疲劳 (19%)?血小板减少 (10%)?腹泻 (10%) 和关节痛 (10%)?未出现与药物相关的 G4/5 毒性?在第 2 个周期内,当硼替佐米/凡德他尼的剂量为 1.3/200 时,出现 G3 血小板减少,此为一种剂量限制性毒性,无需干预即可消除?有 4 例确诊为 MTC (27%) 的患者出现部分缓解 (PR)?
结论?联合用药的 MTD(最大耐受剂量)设定为第 1?4?8 和 11 天硼替佐米 1.3 mg/m2 IV,同时每天口服凡德他尼300 mg?确诊为 MTC 的一例患者观察到 RECIST(实体瘤疗效评价标准)缓解?
Discussion
Activating point mutations in RET kinase are present in nearly all hereditary MTC and at least 50% of sporadic MTC. RET has therefore been considered an attractive therapeutic target in MTC. Vandetanib, an oral inhibitor of vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor, and RET, was approved by the U.S. Food and Drug Administration (FDA) in April 2011 after a phase III trial demonstrated an improvement in median progression‐free survival (PFS) compared with placebo (hazard ratio 0.45; 95% confidence interval [CI] 0.30–0.69; p < .001) and an overall response rate of 45% in patients with metastatic MTC. Cabozantinib, a tyrosine kinase inhibitor of hepatocyte growth factor receptor, VEGFR‐2, and RET, has also demonstrated clinical activity in patients with MTC, albeit at a dose far in excess of that tolerable by patients. A phase III trial comparing cabozantinib at a starting dose of 140 mg reported a median PFS of 11.2 months for cabozantinib versus 4.0 months for placebo (hazard ratio 0.28; 95% CI 0.19–0.40; p < .001), a result that subsequently led to its approval by FDA in 2012 in patients with progressive metastatic MTC. However, toxicity limits its use and we lack evidence of its efficacy at lower doses and thus cannot know with certainty its benefit.
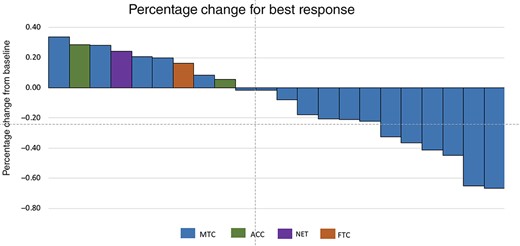
RECIST response. Each patient's percentage represents the best response by RECIST criteria. Abbreviations: ACC, adrenal cortical carcinoma; FTC, follicular thyroid cancer; NET, neuroendocrine tumors; MTC, medullary thyroid cancer.
Preclinical studies demonstrating bortezomib administration reduced both RET mRNA and protein levels prompted this clinical trial to determine the optimal dose of bortezomib in combination with vandetanib. The ultimate goal was to use these two drugs in combination in a strategy targeting both the levels and the activity of the RET proto‐oncogene. This study was designed to evaluate the safety and tolerability of combined daily oral vandetanib and on a days 1, 4, 8, and 11 every 28 days schedule to establish the optimal doses (recommend phase II doses) of the drug combination in adults with locally advanced or metastatic cancer, including MTC.
Twenty‐two patients with advanced or metastastic cancer were enrolled, 17 of whom had a diagnosis of MTC. Four dose levels were explored, with patients receiving initial doses of bortezomib/vandetanib (mg/m2 B/mg V) of 1/100 (3), 1.3/100 (6), 1.3/200 (6), and 1.3/300 (7). The MTD of the combination was established as oral vandetanib at a daily dose of 300 mg with bortezomib 1.3 mg/m2 administered intravenously on days 1, 4, 8, and 11 every 28 days.
Vandetanib and cabozantinib are approved agents for the treatment of patients with progressive metastatic medullary thyroid carcinoma who are ineligible for surgery and who have disease that is growing or causing symptoms. Unfortunately, intrinsic or acquired resistance limit their effectiveness, and efforts are ongoing to seek new treatment options. This trial testing the combination of bortezomib and vandetanib established bortezomib 1.3 mg/m2 administered intravenously on days 1, 4, 8, and 11 with oral vandetanib at a daily dose of 300 mg as the RP2D. Although the original plan called for a phase II study in patients with MTC using the RP2D, only one patient enrolled in the phase II portion, after which the study was terminated. The reason for study termination was the feeling that the activity of the combination was comparable to single‐agent vandetanib but more difficult to tolerate and that prolonged administration would not be possible.
Trial Information
- Disease
Thyroid cancer – medullary
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase I
- Type of Study ‐ 2
Phase I/II
- Primary Endpoint
Safety
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
The activity of vandetanib plus bortezomib in adults with MTC using RECIST 1.1.
- Investigator's Analysis
Level of activity did not meet planned endpoint
Drug Information
- Drug 1
- Generic/Working Name
Vandetanib (CAPRELSA; ZD6474)
- Trade Name
Vandetanib
- Company Name
AstraZeneca/Sanofi Genzyme
- Drug Type
Small molecule
- Drug Class
VEGF
- Dose
100–300 mg per flat dose
- Route
p.o.
- Schedule of Administration
Administered daily
- Drug 2
- Generic/Working Name
Bortezomib (Velcade, PS‐341)
- Trade Name
Bortezomib
- Company Name
- Drug Type
Small molecule
- Drug Class
Other: 26S proteasome inhibitor
- Dose
1.0–1.3 mg/m2
- Route
IV
- Schedule of Administration
Days 1, 4, 8, and 11 of a 28‐day cycle
| Dose level . | Dose of drug: vandetanib (CAPRELSA; ZD6474) . | Dose of drug: bortezomib (Velcade, PS‐341) . | Number enrolled . | Number evaluable for toxicity . |
|---|---|---|---|---|
| 1 | 100 mg daily | 1 mg/m2 days 1, 4, 8, and 11 | 3 | 3 |
| 2 | 100 mg daily | 1.3 mg/m2 days 1, 4, 8, and 11 | 6 | 9 |
| 3 | 200 mg daily | 1.3 mg/m2 days 1, 4, 8, and 11 | 6 | 13 |
| 4 | 300 mg daily | 1.3 mg/m2 days 1, 4, 8, and 11 | 7 | 7 |
| Dose level . | Dose of drug: vandetanib (CAPRELSA; ZD6474) . | Dose of drug: bortezomib (Velcade, PS‐341) . | Number enrolled . | Number evaluable for toxicity . |
|---|---|---|---|---|
| 1 | 100 mg daily | 1 mg/m2 days 1, 4, 8, and 11 | 3 | 3 |
| 2 | 100 mg daily | 1.3 mg/m2 days 1, 4, 8, and 11 | 6 | 9 |
| 3 | 200 mg daily | 1.3 mg/m2 days 1, 4, 8, and 11 | 6 | 13 |
| 4 | 300 mg daily | 1.3 mg/m2 days 1, 4, 8, and 11 | 7 | 7 |
Patient Characteristics
- Number of Patients, Male
14
- Number of Patients, Female
8
- Stage
Metastatic or advanced solid tumors with focus on hereditary or sporadic, locally advanced or metastatic MTC
- Cancer Types or Histologic Subtypes
Medullary thyroid cancer, 19
Adrenocortical cancer, 2
Neuroendocrine tumor (not otherwise specified), 1
Primary Assessment Method
- Title
Total patient population
- Number of Patients Screened
22
- Number of Patients Enrolled
22
- Number of Patients Evaluable for Toxicity
21
- Number of Patients Evaluated for Efficacy
22
- Response Assessment CR
n = 0
- Response Assessment PR
n = 6
- Response Assessment SD
n = 11
- Response Assessment PD
n = 5
- Response Assessment OTHER
n = 0
Adverse Events
| All Cycles . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Alanine aminotransferase increased | 0% | 65% | 35% | 0% | 0% | 0% | 100% |
| Aspartate aminotransferase increased | 0% | 65% | 35% | 0% | 0% | 0% | 100% |
| Platelet count decreased | 0% | 82% | 6% | 12% | 0% | 0% | 100% |
| Rash maculo‐papular | 0% | 27% | 73% | 0% | 0% | 0% | 100% |
| Hypertension | 0% | 43% | 21% | 36% | 0% | 0% | 100% |
| Fatigue | 0% | 23% | 46% | 31% | 0% | 0% | 100% |
| White blood cell decreased | 0% | 55% | 45% | 0% | 0% | 0% | 100% |
| Electrocardiogram QT corrected interval prolonged | 0% | 28% | 67% | 6% | 0% | 0% | 101% |
| Peripheral sensory neuropathy | 0% | 40% | 60% | 0% | 0% | 0% | 100% |
| Diarrhea | 0% | 53% | 33% | 13% | 0% | 0% | 99% |
| All Cycles . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Alanine aminotransferase increased | 0% | 65% | 35% | 0% | 0% | 0% | 100% |
| Aspartate aminotransferase increased | 0% | 65% | 35% | 0% | 0% | 0% | 100% |
| Platelet count decreased | 0% | 82% | 6% | 12% | 0% | 0% | 100% |
| Rash maculo‐papular | 0% | 27% | 73% | 0% | 0% | 0% | 100% |
| Hypertension | 0% | 43% | 21% | 36% | 0% | 0% | 100% |
| Fatigue | 0% | 23% | 46% | 31% | 0% | 0% | 100% |
| White blood cell decreased | 0% | 55% | 45% | 0% | 0% | 0% | 100% |
| Electrocardiogram QT corrected interval prolonged | 0% | 28% | 67% | 6% | 0% | 0% | 101% |
| Peripheral sensory neuropathy | 0% | 40% | 60% | 0% | 0% | 0% | 100% |
| Diarrhea | 0% | 53% | 33% | 13% | 0% | 0% | 99% |
Abbreviation: NC/NA, no change from baseline/no adverse event.
Dose‐Limiting Toxicities
| Dose level | Number enrolled | Number evaluable for toxicity | Number with a dose‐limiting toxicity | Dose‐limiting toxicity information |
| 1 | 3 | 3 | ||
| 2 | 9 | 9 | ||
| 3 | 13 | 13 | 1 | Grade 3 thrombocytopenia |
| 4 | 10 | 10 |
| Dose level | Number enrolled | Number evaluable for toxicity | Number with a dose‐limiting toxicity | Dose‐limiting toxicity information |
| 1 | 3 | 3 | ||
| 2 | 9 | 9 | ||
| 3 | 13 | 13 | 1 | Grade 3 thrombocytopenia |
| 4 | 10 | 10 |
Assessment, Analysis, and Discussion
Medullary thyroid cancer (MTC) is a neuroendocrine tumor of the parafollicular or C cells of the thyroid gland that derives from the neural crest and accounts for approximately 4% of thyroid carcinomas [1]. In about 20%–25% of cases, MTC presents as a part of an autosomal dominant inherited disorder, with sporadic tumor accounting for 75% of cases. Activating mutations of the RET (REarranged during Transfection) proto‐oncogene are common, with germline activating RET mutations seen in familial MTC (FMTC) and multiple endocrine neoplasia (MEN) 2a/MEN2b [2–4]. Mutations in RET are also found in sporadic cases, with an estimated 50% of such tumors harboring RET mutations in the absence of germline changes [5–7]. MTC often produces immunoreactive calcitonin and carcinoembryonic antigen (CEA), which can be used as tumor markers [8, 9].
Calcitonin is an excellent tumor marker that correlates with tumor bulk [10, 11]. In patients with detectable serum calcitonin or CEA but without anatomic evidence of disease, careful observation is advised given clinical benefit has not been demonstrated with early therapeutic intervention. Empiric surgical interventions to remove all the lymph nodes of the neck and the mediastinum have been proposed, but results have been disappointing [12, 13]. These procedures often do not find tumor nor result in a biochemical remission. In contrast, patients with rapidly progressive disease by anatomic imaging or in whom a biochemical doubling time of calcitonin levels <2 years is detected should be considered for treatment, ideally in the context of a well‐designed clinical trial [4, 14].
Metastatic MTC is the most common cause of death in patients with MEN 2a and MEN 2b, and the tumor is unresponsive to conventional doses of radiation therapy. For years, doxorubicin was the only U.S. Food and Drug Administration (FDA)‐approved agent that was used for patients with advanced thyroid cancer; however, response rates in patients with MTC were < 20% often with toxicity [15–17]. In recent years, several tyrosine kinase inhibitors including axitinib, cabozantinib, gefitinib, imatinib, motesanib, sorafenib, sunitinib, and vandetanib have been evaluated in phase I, II, and III clinical trials in patients with advanced MTC [18–29]. Vandetanib, an inhibitor of vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor, and RET [27, 30], was approved by the FDA in April 2011 at a dose of 300 mg per day after a phase III trial demonstrated improvement in median progression‐free survival (PFS) compared with placebo (hazard ratio 0.45; 95% confidence interval [CI] 0.30–0.69; p < .001) and an overall response rate of 45% [31]. Cabozantinib, an inhibitor of hepatocyte growth factor receptor, VEGFR‐2, and RET, also demonstrated clinical activity in patients with medullary thyroid cancer. A phase III trial that demonstrated a median PFS of 11.2 months for cabozantinib compared with 4.0 months for placebo (hazard ratio 0.28; 95% CI 0.19–0.40; p < .001) led to FDA approval in 2012 [32, 33]. The dose of cabozantinib employed in that study and approved by the FDA was 140 mg daily—a dose the FDA approval noted was not tolerated by 79% of patients—and substantially higher than currently administered doses of 40–60 mg daily. Unfortunately for the latter tolerable doses, we lack evidence of efficacy and thus cannot know with certainty the benefit, if any, in MTC.
Bortezomib is a reversible inhibitor of the chymotrypsin‐like activity of the 26S proteasome in mammalian cells. The 26S proteasome is a large protein complex that degrades ubiquitinated proteins and is essential in regulating the intracellular concentration of proteins including multiple signaling cascades within the cell, leading to cell death. Bortezomib is approved for multiple myeloma and mantle cell lymphoma at a dose of 1.3 mg/m2 given on days 1, 4, 8, and 11 of a 21‐day cycle [34]. Moreover, preclinical studies indicate bortezomib reduces both mRNA and protein levels of RET in vitro [35], and published data reported that bortezomib inhibits growth of MTC cell lines and decreases RET expression in vitro [36].
This study was designed to evaluate the safety and tolerability of combined daily oral vandetanib and intravenous (IV) bortezomib on days 1, 4, 8, and 11 of an every‐28‐day cycle to establish a recommended phase II dose of the drug combination in adults with locally advanced or metastatic cancer, including MTC; and to assess the activity of vandetanib plus bortezomib in adults with MTC, using RECIST and tumor biomarkers including CEA and calcitonin as endpoints. Twenty‐two patients were enrolled and received initial doses of bortezomib/vandetanib (mg/m2 B/mg V) of 1/100 (3), 1.3/100 (6), 1.3/200 (6), and 1.3/300 (7). The maximum tolerated dose (MTD) of the combination was vandetanib 300 mg orally daily and bortezomib 1.3 mg/m2 IV on days 1, 4, 8, and 11. Grade 3 toxicities reported were hypertension (24%), fatigue (19%), thrombocytopenia (10%), diarrhea (10%), and arthralgia (10%), with keratoacanthoma, hyperkalemia, pulmonary hemorrhage, edema, and prolonged QT each in one patient (5%). There were no drug‐related grade 4/5 toxicities. There was one dose‐limiting toxicity (DLT) of grade 3 thrombocytopenia with bortezomib and vandetanib doses of 1.3 and 200 in cycle 2. The toxicity resolved and the patient received cycle 3 and subsequent cycles at 1/100. No further DLTs were seen.
Of the 17 patients with MTC, 16 had previously had primary resection, 7 had prior radiation, 6 had prior systemic therapy, and 1 had undergone a craniotomy for metastatic disease. At the time of enrollment, all had metastatic disease. Although the decrease in calcitonin appeared to correlate with RECIST response, the correlation was limited (R2 = 0.54). Importantly, no patient with stable disease duration of <6 months or progressive disease demonstrated a decrease in calcitonin.
In conclusion, the MTD of the combination therapy was vandetanib 300 mg orally daily and bortezomib 1.3 mg/m2 intravenously on days 1, 4, 8, and 11. Although we established a recommended phase II dose for the combination, the activity observed in the 17 patients with MTC enrolled on study was not felt to be sufficient to continue pursuing this as a therapeutic option in MTC. For patients with MTC, many of whom will have an indolent course to their disease and require long‐term therapy, the combination with bortezomib would likely not be sustainable much beyond six cycles. And although RECIST responses were achieved in 4/17 patients, it was felt this might not be better than single‐agent vandetanib with some added toxicity. Thus, a planned phase II study was not pursued.
Disclosures
The authors indicated no financial relationships.
ClinicalTrials.gov Identifier: NCT00923247
Sponsor(s): AstraZeneca/Sanofi Genzyme for vandetanib
Principal Investigator: Ann W. Gramza
IRB Approved: Yes
References
Wells SA Jr,
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



