-
PDF
- Split View
-
Views
-
Cite
Cite
Linda T. Vahdat, Rachel Layman, Denise A. Yardley, William Gradishar, Mohamad A. Salkeni, Anil Abraham Joy, Agustin A. Garcia, Patrick Ward, James Khatcheressian, Joseph Sparano, Gladys Rodriguez, Shande Tang, Ling Gao, Rita P. Dalal, John Kauh, Kathy Miller, Randomized Phase II Study of Ramucirumab or Icrucumab in Combination with Capecitabine in Patients with Previously Treated Locally Advanced or Metastatic Breast Cancer, The Oncologist, Volume 22, Issue 3, March 2017, Pages 245–254, https://doi.org/10.1634/theoncologist.2016-0265
Close - Share Icon Share
Abstract
Icrucumab (ICR) and ramucirumab (RAM) bind vascular endothelial growth factor (VEGF) receptors 1 and 2 (VEGFR‐1 and ‐2), respectively. This open‐label, randomized phase II study evaluated their efficacy and safety in combination with capecitabine (CAP) in patients with previously treated unresectable, locally advanced or metastatic breast cancer.
Patients were randomly assigned (1:1:1) to receive CAP (1,000 mg/m2 orally twice daily, days 1–14) alone or in combination with RAM (10 mg/kg intravenously [IV], days 1 and 8) (RAM + CAP) or ICR (12 mg/kg IV, days 1 and 8) (ICR + CAP) every 21 days. The primary endpoint was progression‐free survival (PFS). Secondary endpoints included overall survival (OS), tumor response, safety, and pharmacokinetics.
Of 153 patients randomized, 150 received treatment. Median PFS (95% confidence interval) was 22.1 (12.1–36.1) weeks on RAM + CAP, 7.3 (6.3–13.0) weeks on ICR + CAP, and 19.0 (12.1–24.3) weeks on CAP (hazard ratios [HRs]: 0.691, p = .1315, RAM + CAP versus CAP; 1.480, p = .0851, ICR + CAP versus CAP). Median OS was 67.4 weeks on RAM + CAP, 62.1 weeks on ICR + CAP, and 71.6 weeks on CAP (HRs: 1.833, p = .0283, RAM + CAP versus CAP; 1.468, p = .1550, ICR + CAP versus CAP). There was no statistically significant difference in PFS or OS between either combination arm and CAP. Treatment‐related adverse events more frequent (by ≥10%) on RAM + CAP than on CAP were constipation, decreased appetite, headache, epistaxis, and hypertension. Those more frequent (by ≥10%) on ICR + CAP than CAP were anemia, increased lacrimation, periorbital edema, nausea, vomiting, peripheral edema, facial edema, dehydration, and dyspnea.
Combining RAM or ICR with CAP did not improve PFS in the targeted study population.
Icrucumab and ramucirumab are recombinant human IgG1 monoclonal antibodies that bind vascular endothelial growth factor (VEGF) receptors 1 and 2 (VEGFR‐1 and ‐2), respectively. VEGFR‐1 activation on endothelial and tumor cell surfaces increases tumor vascularization and growth and supports tumor growth via multiple mechanisms, including contributions to angiogenesis and direct promotion of cancer cell proliferation. Strong preclinical and clinical evidence suggests key roles for VEGF and angiogenesis in breast cancer growth, invasion, and metastasis. This randomized phase II study evaluated the efficacy and safety of each antibody in combination with capecitabine in patients with previously treated unresectable, locally advanced or metastatic breast cancer.
Introduction
The tumor microenvironment (vasculature, immune cells, signaling molecules, and extracellular matrix, among other components) is a critical component of tumor progression and a potential therapeutic target . Among the many components critical for tumor progression within the tumor microenvironment, vascular endothelial growth factor (VEGF) and subsequent angiogenesis play key roles in breast cancer growth, invasion, and metastasis . In human breast cancer, intensive neovascularization and tumor angiogenesis correlate with metastases and poor prognosis . Expression of VEGF‐A, the endothelial cell‐specific mitogen most associated with angiogenesis, also correlates with breast cancer risk and outcomes . Inhibiting the VEGF receptor (VEGFR) signaling pathway via anti‐VEGF antibodies, anti‐VEGFR antibodies, and small molecule kinase inhibitors can inhibit or reduce vascularization and tumor growth . Thus, multiple agents that interfere with the VEGF pathway (including bevacizumab, sunitinib, and ramucirumab [RAM]) have been tested in large clinical trials with success.
In addition to the direct antiangiogenic effects of VEGFR blockade, agents targeting the VEGF pathway may inhibit the recruitment of bone marrow‐derived progenitor cells, which have the potential to promote cancer cell growth, invasion, and metastasis [20]. Thus, the tumor microenvironment may be made less favorable for tumor cell survival.
Multiple clinical trials with bevacizumab have shown a benefit for progression‐free survival (PFS) and response rates, but these results have not translated into an improvement in overall survival (OS) . Despite the lack of a survival benefit in the group studies, angiogenesis is still an important and relevant target for tumor progression and an important component of the tumor microenvironment.
RAM (IMC‐1121B) is a recombinant human IgG1 monoclonal antibody that blocks the binding of activating VEGF ligands by specifically binding to VEGFR‐2 with high affinity. Lack of receptor and ligand binding inhibits VEGF‐stimulated proliferation and migration of endothelial cells [27]. DC101, a murine counterpart to RAM that also targets VEGFR‐2, impairs vascular function, increases tumor hypoxia, and inhibits tumor growth in breast cancer xenograft models and may act as a chemosensitizing agent when given in combination with cytotoxic chemotherapies such as paclitaxel, 5‐FU, and docetaxel . Clinical studies of RAM have demonstrated objective antitumor activity and antiangiogenic effects over a wide range of doses [32].
Icrucumab (ICR, IMC‐18F1) is a recombinant human IgG1 monoclonal antibody that blocks the binding of activating VEGF ligands by specifically binding to VEGFR‐1 with high affinity, leading to inhibition of VEGFR‐1‐stimulated proliferation and migration of endothelial cells. ICR in combination with chemotherapy (5‐FU/leucovorin, doxorubicin, or cyclophosphamide) suppresses tumor growth, reduces tumor cell proliferation, and increases tumor cell apoptosis in breast cancer xenograft models [33]. In a phase I trial, ICR demonstrated a tolerable safety profile and a disease control rate (DCR) of 23.1% in patients with advanced solid tumors [34]. Capecitabine (CAP), an orally administered 5‐FU prodrug, is an established therapeutic for advanced breast cancer and other malignancies [35].
This open‐label, multicenter, randomized phase II study evaluated the antitumor activity and safety of CAP in combination with RAM or ICR in patients with unresectable locally advanced or metastatic breast cancer previously treated with anthracycline and taxane therapy.
Methods
Study Design and Patients
Eligible patients were randomly assigned (1:1:1) to one of three study regimens: CAP, RAM + CAP, or ICR + CAP (Fig. 1). Randomization was stratified by triple‐negative receptor status (estrogen receptor‐negative, progesterone receptor‐negative, and human epidermal growth factor receptor‐2 [HER2/neu]‐negative) and by receipt of prior antiangiogenic therapy. Treatment continued until disease progression, development of unacceptable toxicity, noncompliance or withdrawal of consent by the patient, or investigator decision to stop. If a patient was HER2/neu‐positive, then all HER2 therapy was stopped prior to the start of the protocol‐specified therapy.
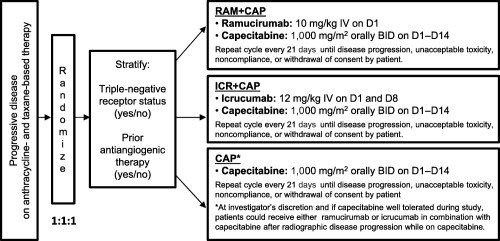
Study design. Abbreviations: BID, twice daily; CAP, capecitabine; D, Day; ICR, icrucumab; IV, intravenously; RAM, ramucirumab.
Eligible patients were ≥18 years old with an Eastern Cooperative Oncology Group (ECOG) performance status of 0‐1, histologically or cytologically confirmed invasive breast cancer that was locally advanced and unresectable or metastatic, disease progression after anthracycline and taxane chemotherapy, and radiographic disease progression within 6 months after ending the most recent systemic treatment. Patients with HER2/neu‐positive disease must have progressed on or after trastuzumab therapy. Patients with hormone receptor‐positive disease must have progressed on or after hormone therapy.
Patients were excluded if they had concurrent active malignancy (other than adequately treated nonmelanomatous skin cancer, curatively treated cervical carcinoma in situ, or other noninvasive carcinoma or in situ neoplasm), known dihydropyrimidine dehydrogenase deficiency, prior CAP treatment for advanced breast cancer, investigational therapy (including hormone therapy) within 2 weeks before randomization, bevacizumab within 4 weeks before randomization, or more than one prior antiangiogenic agent for breast cancer.
This study was conducted in accordance with International Conference on Harmonisation guidelines, good clinical practice, and applicable regulatory requirements. Written informed consent was obtained from all participants.
Study Procedures
Patients in all study arms self‐administered 1,000 mg/m2 of CAP orally twice daily on days 1 to 14 in a 21‐day cycle. On the RAM + CAP arm, patients also received 10 mg/kg of RAM intravenously (IV) over 1 hour on day 1 every 21 days. On the ICR + CAP arm, patients also received 12 mg/kg IV ICR over 1 hour on days 1 and 8 every 21 days. At the investigator’s discretion, patients on the CAP arm could cross over to receive either RAM or ICR in combination with CAP after radiographic disease progression while on CAP.
Baseline evaluations included medical history, physical examination, hematology, chemistry, and echocardiogram. Patients were monitored throughout the study for ECOG performance status, physical examination, vital signs, adverse events, hematology, and chemistry. Tumor assessment was performed every 6 weeks (±3 days) until documented progression for all patients, followed by collection of survival data every 3 months until study termination.
Study End Points
The primary efficacy endpoint was PFS based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Secondary endpoints included objective response rate (ORR), OS, duration of response, safety, and pharmacokinetics (PK). Safety assessments included treatment‐emergent adverse events (TEAEs), serious adverse events (SAEs), physical examinations, vital signs, and laboratory analyses.
Statistical Analysis
A total enrollment of 150 patients was planned to provide the 120 PFS events necessary to yield 73% power for detecting in the final analysis a hazard ratio (HR) of 0.67 (i.e., an increase in median PFS from 4 months [i.e., 16 weeks] in the CAP arm to 6 months [i.e., 24 weeks] in either the RAM + CAP or ICR + CAP arm) by using the log‐rank test at a one‐sided α level of 10%.
Efficacy analyses were based on the modified intent‐to‐treat population (i.e., all randomly assigned patients who received any study drug) as specified in the study protocol. For the primary and secondary analyses, PFS and OS were analyzed using the Kaplan–Meier method with the log‐rank test, stratified by triple‐negative receptor status and prior antiangiogenic treatment. The HR of each combination therapy to CAP alone was estimated using a stratified Cox proportional hazard model. The ORR (complete response [CR] + partial response [PR]) for each combination arm was compared with that for the CAP‐only arm, using a Cochran–Mantel–Haenszel test adjusted for the stratification variables, and 95% confidence intervals [CIs] were estimated.
Safety was assessed in all patients who received at least one dose of study drug and analyzed according to actual treatment received. TEAEs were collected from the time of the first study drug dose to 30 days after the last dose, summarized using the Medical Dictionary for Regulatory Activities, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Adverse events of special interest (AESIs) for RAM and ICR are defined in the supplemental online materials. PK sample collection schedule, PK assay methodology, and PK data analyses are described in the supplemental online materials. Statistical analyses were performed using SAS version 8.2 or later (SAS Institute, Cary, NC) or comparable software.
Results
Study Population
A total of 153 patients were randomized; 150 actually received study treatment (Fig. 2). Treatment arms were balanced (Table 1). Thirty patients on the CAP arm crossed over to combination therapy on either RAM + CAP (29 patients) or ICR + CAP (1 patient) at disease progression.
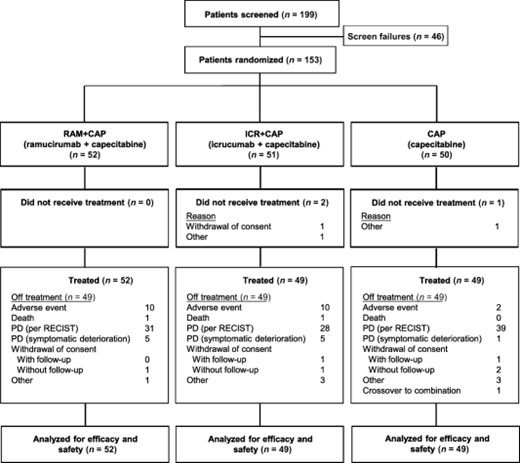
Patient disposition. Abbreviations: CAP, capecitabine; ICR, icrucumab; PD, progressive disease; RAM, ramucirumab; RECIST, Response Evaluation Criteria in Solid Tumors.
| Characteristics . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | All patients (n = 150) . | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 0 | 1 (2.0) | 0 | 1 (0.7) | |
| Female | 52 (100.0) | 48 (98.0) | 49 (100.0) | 149 (99.3) | |
| Age | |||||
| Median (range), years | 55.2 (32–74) | 52.0 (23–70) | 55.0 (34–72) | 54.0 (23–74) | |
| Category, n (%) | |||||
| <65 years | 41 (78.8) | 42 (85.7) | 39 (79.6) | 122 (81.3) | |
| ≥65 years | 11 (21.2) | 7 (14.3) | 10 (20.4) | 28 (18.7) | |
| Race, n (%) | |||||
| American Indian or Alaska native | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| Asian | 2 (3.8) | 1 (2.0) | 1 (2.0) | 4 (2.7) | |
| Black/African American | 13 (25.0) | 8 (16.3) | 4 (8.2) | 25 (16.7) | |
| Native Hawaiian or Pacific Islander | 0 | 0 | 1 (2.0) | 1 (0.7) | |
| White | 36 (69.2) | 37 (75.5) | 40 (81.6) | 113 (75.3) | |
| Other | 1 (1.9) | 2 (4.1) | 2 (4.1) | 5 (3.3) | |
| ECOG PS, n (%) | |||||
| 0 | 23 (44.2) | 26 (53.1) | 32 (65.3) | 81 (54.0) | |
| 1 | 29 (55.8) | 23 (46.9) | 17 (34.7) | 69 (46.0) | |
| HER2 status, n (%) | |||||
| Negative | 21 (40.4) | 27 (55.1) | 26 (53.1) | 74 (49.3) | |
| Triple‐negative receptor status, n (%) | |||||
| Yes | 16 (30.8) | 15 (30.6) | 17 (34.7) | 48 (32.0) | |
| No | 36 (69.2) | 34 (69.4) | 32 (65.3) | 102 (68.0) | |
| Prior antiangiogenic therapy, n (%) | |||||
| Yes | 11 (21.2) | 11 (22.4) | 10 (20.4) | 32 (21.3) | |
| No | 41 (78.8) | 38 (77.6) | 39 (79.6) | 118 (78.7) | |
| Site of metastatic disease, n (%) | |||||
| Bone | 40 (76.9) | 34 (69.4) | 29 (59.2) | 103 (68.7) | |
| Lymph nodes | 27 (51.9) | 28 (57.1) | 28 (57.1) | 83 (55.3) | |
| Liver | 30 (57.7) | 24 (49.0) | 25 (51.0) | 79 (52.7) | |
| Lung | 26 (50.0) | 26 (53.1) | 14 (28.6) | 66 (44.0) | |
| Pleural | 11 (21.2) | 12 (24.5) | 10 (20.4) | 33 (22.0) | |
| Soft tissue | 5 (9.6) | 8 (16.3) | 3 (6.1) | 16 (10.7) | |
| Other | 4 (7.7) | 4 (8.2) | 7 (14.3) | 15 (10.0) | |
| Skin | 2 (3.8) | 1 (2.0) | 4 (8.2) | 7 (4.7) | |
| Peritoneal | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| No. of sites of metastatic disease, n (%) | |||||
| 1 | 6 (11.5) | 7 (14.3) | 11 (22.4) | 24 (16.0) | |
| 2–4 | 42 (80.8) | 39 (79.6) | 36 (73.5) | 117 (78.0) | |
| >4 | 4 (7.7) | 3 (6.1) | 2 (4.1) | 9 (6.0) | |
| Characteristics . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | All patients (n = 150) . | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 0 | 1 (2.0) | 0 | 1 (0.7) | |
| Female | 52 (100.0) | 48 (98.0) | 49 (100.0) | 149 (99.3) | |
| Age | |||||
| Median (range), years | 55.2 (32–74) | 52.0 (23–70) | 55.0 (34–72) | 54.0 (23–74) | |
| Category, n (%) | |||||
| <65 years | 41 (78.8) | 42 (85.7) | 39 (79.6) | 122 (81.3) | |
| ≥65 years | 11 (21.2) | 7 (14.3) | 10 (20.4) | 28 (18.7) | |
| Race, n (%) | |||||
| American Indian or Alaska native | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| Asian | 2 (3.8) | 1 (2.0) | 1 (2.0) | 4 (2.7) | |
| Black/African American | 13 (25.0) | 8 (16.3) | 4 (8.2) | 25 (16.7) | |
| Native Hawaiian or Pacific Islander | 0 | 0 | 1 (2.0) | 1 (0.7) | |
| White | 36 (69.2) | 37 (75.5) | 40 (81.6) | 113 (75.3) | |
| Other | 1 (1.9) | 2 (4.1) | 2 (4.1) | 5 (3.3) | |
| ECOG PS, n (%) | |||||
| 0 | 23 (44.2) | 26 (53.1) | 32 (65.3) | 81 (54.0) | |
| 1 | 29 (55.8) | 23 (46.9) | 17 (34.7) | 69 (46.0) | |
| HER2 status, n (%) | |||||
| Negative | 21 (40.4) | 27 (55.1) | 26 (53.1) | 74 (49.3) | |
| Triple‐negative receptor status, n (%) | |||||
| Yes | 16 (30.8) | 15 (30.6) | 17 (34.7) | 48 (32.0) | |
| No | 36 (69.2) | 34 (69.4) | 32 (65.3) | 102 (68.0) | |
| Prior antiangiogenic therapy, n (%) | |||||
| Yes | 11 (21.2) | 11 (22.4) | 10 (20.4) | 32 (21.3) | |
| No | 41 (78.8) | 38 (77.6) | 39 (79.6) | 118 (78.7) | |
| Site of metastatic disease, n (%) | |||||
| Bone | 40 (76.9) | 34 (69.4) | 29 (59.2) | 103 (68.7) | |
| Lymph nodes | 27 (51.9) | 28 (57.1) | 28 (57.1) | 83 (55.3) | |
| Liver | 30 (57.7) | 24 (49.0) | 25 (51.0) | 79 (52.7) | |
| Lung | 26 (50.0) | 26 (53.1) | 14 (28.6) | 66 (44.0) | |
| Pleural | 11 (21.2) | 12 (24.5) | 10 (20.4) | 33 (22.0) | |
| Soft tissue | 5 (9.6) | 8 (16.3) | 3 (6.1) | 16 (10.7) | |
| Other | 4 (7.7) | 4 (8.2) | 7 (14.3) | 15 (10.0) | |
| Skin | 2 (3.8) | 1 (2.0) | 4 (8.2) | 7 (4.7) | |
| Peritoneal | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| No. of sites of metastatic disease, n (%) | |||||
| 1 | 6 (11.5) | 7 (14.3) | 11 (22.4) | 24 (16.0) | |
| 2–4 | 42 (80.8) | 39 (79.6) | 36 (73.5) | 117 (78.0) | |
| >4 | 4 (7.7) | 3 (6.1) | 2 (4.1) | 9 (6.0) | |
Abbreviations: CAP, capecitabine; ECOG, European Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; ICR, icrucumab; PS, performance status; RAM, ramucirumab.
| Characteristics . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | All patients (n = 150) . | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 0 | 1 (2.0) | 0 | 1 (0.7) | |
| Female | 52 (100.0) | 48 (98.0) | 49 (100.0) | 149 (99.3) | |
| Age | |||||
| Median (range), years | 55.2 (32–74) | 52.0 (23–70) | 55.0 (34–72) | 54.0 (23–74) | |
| Category, n (%) | |||||
| <65 years | 41 (78.8) | 42 (85.7) | 39 (79.6) | 122 (81.3) | |
| ≥65 years | 11 (21.2) | 7 (14.3) | 10 (20.4) | 28 (18.7) | |
| Race, n (%) | |||||
| American Indian or Alaska native | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| Asian | 2 (3.8) | 1 (2.0) | 1 (2.0) | 4 (2.7) | |
| Black/African American | 13 (25.0) | 8 (16.3) | 4 (8.2) | 25 (16.7) | |
| Native Hawaiian or Pacific Islander | 0 | 0 | 1 (2.0) | 1 (0.7) | |
| White | 36 (69.2) | 37 (75.5) | 40 (81.6) | 113 (75.3) | |
| Other | 1 (1.9) | 2 (4.1) | 2 (4.1) | 5 (3.3) | |
| ECOG PS, n (%) | |||||
| 0 | 23 (44.2) | 26 (53.1) | 32 (65.3) | 81 (54.0) | |
| 1 | 29 (55.8) | 23 (46.9) | 17 (34.7) | 69 (46.0) | |
| HER2 status, n (%) | |||||
| Negative | 21 (40.4) | 27 (55.1) | 26 (53.1) | 74 (49.3) | |
| Triple‐negative receptor status, n (%) | |||||
| Yes | 16 (30.8) | 15 (30.6) | 17 (34.7) | 48 (32.0) | |
| No | 36 (69.2) | 34 (69.4) | 32 (65.3) | 102 (68.0) | |
| Prior antiangiogenic therapy, n (%) | |||||
| Yes | 11 (21.2) | 11 (22.4) | 10 (20.4) | 32 (21.3) | |
| No | 41 (78.8) | 38 (77.6) | 39 (79.6) | 118 (78.7) | |
| Site of metastatic disease, n (%) | |||||
| Bone | 40 (76.9) | 34 (69.4) | 29 (59.2) | 103 (68.7) | |
| Lymph nodes | 27 (51.9) | 28 (57.1) | 28 (57.1) | 83 (55.3) | |
| Liver | 30 (57.7) | 24 (49.0) | 25 (51.0) | 79 (52.7) | |
| Lung | 26 (50.0) | 26 (53.1) | 14 (28.6) | 66 (44.0) | |
| Pleural | 11 (21.2) | 12 (24.5) | 10 (20.4) | 33 (22.0) | |
| Soft tissue | 5 (9.6) | 8 (16.3) | 3 (6.1) | 16 (10.7) | |
| Other | 4 (7.7) | 4 (8.2) | 7 (14.3) | 15 (10.0) | |
| Skin | 2 (3.8) | 1 (2.0) | 4 (8.2) | 7 (4.7) | |
| Peritoneal | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| No. of sites of metastatic disease, n (%) | |||||
| 1 | 6 (11.5) | 7 (14.3) | 11 (22.4) | 24 (16.0) | |
| 2–4 | 42 (80.8) | 39 (79.6) | 36 (73.5) | 117 (78.0) | |
| >4 | 4 (7.7) | 3 (6.1) | 2 (4.1) | 9 (6.0) | |
| Characteristics . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | All patients (n = 150) . | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 0 | 1 (2.0) | 0 | 1 (0.7) | |
| Female | 52 (100.0) | 48 (98.0) | 49 (100.0) | 149 (99.3) | |
| Age | |||||
| Median (range), years | 55.2 (32–74) | 52.0 (23–70) | 55.0 (34–72) | 54.0 (23–74) | |
| Category, n (%) | |||||
| <65 years | 41 (78.8) | 42 (85.7) | 39 (79.6) | 122 (81.3) | |
| ≥65 years | 11 (21.2) | 7 (14.3) | 10 (20.4) | 28 (18.7) | |
| Race, n (%) | |||||
| American Indian or Alaska native | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| Asian | 2 (3.8) | 1 (2.0) | 1 (2.0) | 4 (2.7) | |
| Black/African American | 13 (25.0) | 8 (16.3) | 4 (8.2) | 25 (16.7) | |
| Native Hawaiian or Pacific Islander | 0 | 0 | 1 (2.0) | 1 (0.7) | |
| White | 36 (69.2) | 37 (75.5) | 40 (81.6) | 113 (75.3) | |
| Other | 1 (1.9) | 2 (4.1) | 2 (4.1) | 5 (3.3) | |
| ECOG PS, n (%) | |||||
| 0 | 23 (44.2) | 26 (53.1) | 32 (65.3) | 81 (54.0) | |
| 1 | 29 (55.8) | 23 (46.9) | 17 (34.7) | 69 (46.0) | |
| HER2 status, n (%) | |||||
| Negative | 21 (40.4) | 27 (55.1) | 26 (53.1) | 74 (49.3) | |
| Triple‐negative receptor status, n (%) | |||||
| Yes | 16 (30.8) | 15 (30.6) | 17 (34.7) | 48 (32.0) | |
| No | 36 (69.2) | 34 (69.4) | 32 (65.3) | 102 (68.0) | |
| Prior antiangiogenic therapy, n (%) | |||||
| Yes | 11 (21.2) | 11 (22.4) | 10 (20.4) | 32 (21.3) | |
| No | 41 (78.8) | 38 (77.6) | 39 (79.6) | 118 (78.7) | |
| Site of metastatic disease, n (%) | |||||
| Bone | 40 (76.9) | 34 (69.4) | 29 (59.2) | 103 (68.7) | |
| Lymph nodes | 27 (51.9) | 28 (57.1) | 28 (57.1) | 83 (55.3) | |
| Liver | 30 (57.7) | 24 (49.0) | 25 (51.0) | 79 (52.7) | |
| Lung | 26 (50.0) | 26 (53.1) | 14 (28.6) | 66 (44.0) | |
| Pleural | 11 (21.2) | 12 (24.5) | 10 (20.4) | 33 (22.0) | |
| Soft tissue | 5 (9.6) | 8 (16.3) | 3 (6.1) | 16 (10.7) | |
| Other | 4 (7.7) | 4 (8.2) | 7 (14.3) | 15 (10.0) | |
| Skin | 2 (3.8) | 1 (2.0) | 4 (8.2) | 7 (4.7) | |
| Peritoneal | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) | |
| No. of sites of metastatic disease, n (%) | |||||
| 1 | 6 (11.5) | 7 (14.3) | 11 (22.4) | 24 (16.0) | |
| 2–4 | 42 (80.8) | 39 (79.6) | 36 (73.5) | 117 (78.0) | |
| >4 | 4 (7.7) | 3 (6.1) | 2 (4.1) | 9 (6.0) | |
Abbreviations: CAP, capecitabine; ECOG, European Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; ICR, icrucumab; PS, performance status; RAM, ramucirumab.
PFS
There were no statistically significant differences in PFS among the arms: RAM + CAP versus CAP, HR = 0.691, p = .1315; ICR + CAP versus CAP, HR = 1.480, p = .0851 (Fig. 3). Median PFS was 22.1 weeks (95% CI, 12.1–36.1) on RAM + CAP, 7.3 weeks (95% CI, 6.3–13.0) on ICR + CAP, and 19.0 weeks (95% CI, 12.1–24.3) on CAP. During the crossover period, median PFS was 12.1 weeks (95% CI, 5.9–26.1) for RAM + CAP crossover patients and unevaluable for the lone ICR + CAP crossover patient.
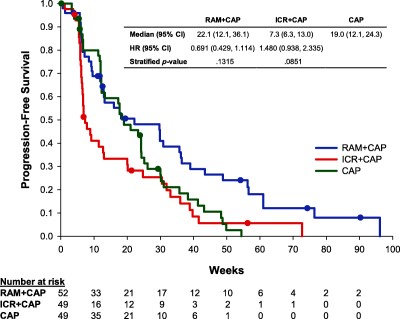
Kaplan–Meier curve for progression‐free survival in modified intent‐to‐treat population. Abbreviations: CAP, capecitabine; CI, confidence interval; HR, hazard ratio; ICR, icrucumab; RAM, ramucirumab.
OS
There were no significant differences in OS among the arms (Table 2). Median OS was 67.4 weeks (95% CI, 41.3–82.6) on RAM + CAP, 62.1 weeks (95% CI, 41.0–84.0) on ICR + CAP, and 71.6 weeks (95% CI, 57.4–89.7) on CAP. Censoring patients for OS analysis at the time of crossover did not change this conclusion.
aEstimated by Kaplan–Meier method.
bHazard ratio for combination therapy/CAP and estimated from Cox model.
cComplete response + partial response + stable disease.
dEstimated using binomial distribution.
eData for patients on the CAP arm who crossed over, at investigator’s discretion, to receive either RAM or ICR in combination with CAP after radiographic disease progression while on CAP.
Abbreviations: CAP, capecitabine; CI, confidence interval; ICR, icrucumab; mITT, modified intent‐to‐treat; RAM, ramucirumab.
aEstimated by Kaplan–Meier method.
bHazard ratio for combination therapy/CAP and estimated from Cox model.
cComplete response + partial response + stable disease.
dEstimated using binomial distribution.
eData for patients on the CAP arm who crossed over, at investigator’s discretion, to receive either RAM or ICR in combination with CAP after radiographic disease progression while on CAP.
Abbreviations: CAP, capecitabine; CI, confidence interval; ICR, icrucumab; mITT, modified intent‐to‐treat; RAM, ramucirumab.
ORR
The ORR was 28.8% (95% CI, 17.1%–43.1%) on RAM+CAP, 20.4% (95% CI, 10.2%‐34.3%) on ICR + CAP, and 34.7% (95% CI, 21.7%–49.6%) on CAP. These values did not differ significantly between either combination arm and the CAP arm. The DCR (CR + PR + stable disease) was 59.6% (95% CI, 45.1%–73.0%) on RAM + CAP, 34.7% (95% CI, 21.7%–49.6%) on ICR + CAP, and 71.4% (95% CI, 56.7%–83.4%) on CAP. No CRs were observed in any of the 30 patients who crossed over to combination therapy.
Treatment Exposure
The median duration of therapy with RAM and ICR was 14.0 weeks (median, 4.5 infusions per patient) and 6.0 weeks (median, 4.0 infusions per patient), respectively. The median duration of CAP therapy was 16.1, 6.0, and 18.9 weeks on RAM + CAP, ICR + CAP, and CAP, respectively.
The median relative dose intensity of RAM was 98.4%, with 73.1% of patients receiving ≥90% of the 3.3 mg/kg per week protocol‐stipulated dose level of RAM. The median relative dose intensity of ICR was 95.4%, with 55.1% of patients receiving ≥90% of the 8 mg/kg per week protocol‐stipulated dose level of ICR. The median relative dose intensity of CAP on the RAM + CAP, ICR + CAP, and CAP arms was 76.4%, 85.3%, and 80.2%, respectively.
Dose reductions in RAM and ICR were required by 6 patients (11.5%) and 5 patients (10.2%), respectively. Dose reductions in CAP were required by 27 patients (51.9%) on RAM + CAP, 11 (22.4%) on ICR + CAP, and 20 (40.8%) on CAP.
Safety
An overview of adverse events is provided in Table 3. The most common reported TEAEs are listed in Table 4. Over the course of the study, four deaths occurred. Two deaths occurred on the RAM + CAP arm: one due to cardiac failure (related to RAM and/or CAP) and one due to cardiogenic shock (unknown relationship to RAM). One death occurred on the ICR + CAP arm due to respiratory failure (related to ICR). The one death on the CAP arm was due to disease progression (unrelated to study drug).
aAdverse event with missing relationship to study drug (RAM/ICR/CAP) was counted as related.
Abbreviations: —, not applicable; AE, adverse event; CAP, capecitabine; ICR, icrucumab; RAM, ramucirumab; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
aAdverse event with missing relationship to study drug (RAM/ICR/CAP) was counted as related.
Abbreviations: —, not applicable; AE, adverse event; CAP, capecitabine; ICR, icrucumab; RAM, ramucirumab; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Treatment‐emergent adverse events reported in ≥10% of treated patients in any arm
| System organ class/preferred term . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | |
| Blood and lymphatic system disorders | 21 (40.4) | 8 (15.4) | 20 (40.8) | 8 (16.3) | 18 (36.7) | 2 (4.1) |
| Thrombocytopenia | 9 (17.3) | 1 (1.9) | 1 (2.0) | 0 | 9 (18.4) | 0 |
| Neutropenia | 8 (15.4) | 3 (5.8) | 6 (12.2) | 3 (6.1) | 6 (12.2) | 0 |
| Anemia | 7 (13.5) | 1 (1.9) | 15 (30.6) | 5 (10.2) | 12 (24.5) | 2 (4.1) |
| Leukopenia | 4 (7.7) | 0 | 0 | 0 | 7 (14.3) | 0 |
| Cardiac disorders | 6 (11.5) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 4 (8.2) | 0 |
| Ear and labyrinth disorder | 1 (1.9) | 0 | 1 (2.0) | 0 | 2 (4.1) | 0 |
| Eye disorders | 12 (23.1) | 0 | 15 (30.6) | 0 | 5 (10.2) | 0 |
| Lacrimation increased | 4 (7.7) | 0 | 6 (12.2) | 0 | 2 (4.1) | 0 |
| Gastrointestinal disorders | 43 (82.7) | 5 (9.6) | 47 (95.9) | 8 (16.3) | 44 (89.8) | 6 (12.2) |
| Nausea | 27 (51.9) | 0 | 38 (77.6) | 2 (4.1) | 26 (53.1) | 3 (6.1) |
| Diarrhea | 22 (42.3) | 1 (1.9) | 21 (42.9) | 2 (4.1) | 28 (57.1) | 3 (6.1) |
| Constipation | 19 (36.5) | 0 | 14 (28.6) | 0 | 17 (34.7) | 0 |
| Vomiting | 18 (34.6) | 1 (1.9) | 26 (53.1) | 2 (4.1) | 14 (28.6) | 3 (6.1) |
| Stomatitis | 14 (26.9) | 0 | 4 (8.2) | 1 (2.0) | 7 (14.3) | 0 |
| Abdominal pain | 9 (17.3) | 1 (1.9) | 6 (12.2) | 0 | 5 (10.2) | 1 (2.0) |
| Dyspepsia | 6 (11.5) | 0 | 5 (10.2) | 0 | 3 (6.1) | 0 |
| General disorders and administration site conditions | 38 (73.1) | 5 (9.6) | 42 (85.7) | 5 (10.2) | 37 (75.5) | 4 (8.2) |
| Fatigue | 27 (51.9) | 3 (5.8) | 28 (57.1) | 1 (2.0) | 26 (53.1) | 1 (2.0) |
| Pyrexia | 14 (26.9) | 0 | 8 (16.3) | 1 (2.0) | 4 (8.2) | 0 |
| Edema peripheral | 13 (25.0) | 0 | 19 (38.8) | 1 (2.0) | 6 (12.2) | 0 |
| Mucosal inflammation | 9 (17.3) | 2 (3.8) | 4 (8.2) | 0 | 9 (18.4) | 0 |
| Influenza‐like illness | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 1 (2.0) |
| Hepatobiliary disorders | 6 (11.5) | 2 (3.8) | 0 | 0 | 4 (8.2) | 0 |
| Infections and infestations | 22 (42.3) | 0 | 19 (38.8) | 4 (8.2) | 18 (36.7) | 3 (6.1) |
| Sinusitis | 7 (13.5) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Injury, poisoning, and procedural complications | 12 (23.1) | 1 (1.9) | 9 (18.4) | 2 (4.1) | 8 (16.3) | 1 (2.0) |
| Investigations | 24 (46.2) | 3 (5.8) | 14 (28.6) | 0 | 18 (36.7) | 0 |
| Aspartate aminotransferase increased | 9 (17.3) | 0 | 2 (4.1) | 0 | 7 (14.3) | 0 |
| Alanine aminotransferase increased | 5 (9.6) | 0 | 1 (2.0) | 0 | 5 (10.2) | 0 |
| Metabolism and nutrition disorders | 30 (57.7) | 3 (5.8) | 25 (51.0) | 7 (14.3) | 24 (49.0) | 7 (14.3) |
| Decreased appetite | 17 (32.7) | 0 | 11 (22.4) | 2 (4.1) | 10 (20.4) | 1 (2.0) |
| Hypokalemia | 12 (23.1) | 2 (3.8) | 7 (14.3) | 2 (4.1) | 12 (24.5) | 5 (10.2) |
| Hyperglycemia | 9 (17.3) | 0 | 4 (8.2) | 0 | 2 (4.1) | 0 |
| Dehydration | 6 (11.5) | 2 (3.8) | 11 (22.4) | 4 (8.2) | 3 (6.1) | 2 (4.1) |
| Hypocalcemia | 5 (9.6) | 0 | 4 (8.2) | 3 (6.1) | 6 (12.2) | 0 |
| Musculoskeletal and connective tissue disorders | 31 (59.6) | 5 (9.6) | 17 (34.7) | 2 (4.1) | 32 (65.3) | 2 (4.1) |
| Pain in extremity | 15 (28.8) | 2 (3.8) | 3 (6.1) | 0 | 6 (12.2) | 1 (2.0) |
| Arthralgia | 11 (21.2) | 0 | 2 (4.1) | 0 | 8 (16.3) | 0 |
| Back pain | 11 (21.2) | 3 (5.8) | 7 (14.3) | 1 (2.0) | 8 (16.3) | 0 |
| Myalgia | 9 (17.3) | 0 | 4 (8.2) | 0 | 3 (6.1) | 0 |
| Musculoskeletal chest pain | 8 (15.4) | 1 (1.9) | 3 (6.1) | 1 (2.0) | 7 (14.3) | 0 |
| Musculoskeletal pain | 6 (11.5) | 1 (1.9) | 1 (2.0) | 0 | 3 (6.1) | 0 |
| Neck pain | 6 (11.5) | 0 | 3 (6.1) | 0 | 0 | 0 |
| Muscle spasms | 5 (9.6) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Neoplasms benign, malignant, and unspecified | 2 (3.8) | 0 | 6 (1.2) | 4 (8.2) | 1 (2.0) | 1 (2.0) |
| Nervous system disorders | 37 (71.2) | 1 (1.9) | 28 (57.1) | 5 (10.2) | 22 (44.9) | 2 (4.1) |
| Headache | 26 (50.0) | 0 | 9 (18.4) | 1 (2.0) | 11 (22.4) | 1 (2.0) |
| Neuropathy peripheral | 10 (19.2) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Dysgeusia | 6 (11.5) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Peripheral sensory neuropathy | 6 (11.5) | 0 | 5 (10.2) | 0 | 2 (4.1) | 0 |
| Dizziness | 5 (9.6) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Psychiatric disorders | 17 (32.7) | 0 | 17 (34.7) | 0 | 17 (34.7) | 1 (2.0) |
| Insomnia | 7 (13.5) | 0 | 9 (18.4) | 0 | 12 (24.5) | 0 |
| Anxiety | 6 (11.5) | 0 | 7 (14.3) | 0 | 5 (10.2) | 0 |
| Depression | 6 (11.5) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Renal and urinary disorders | 6 (11.5) | 0 | 6 (12.2) | 2 (4.1) | 6 (12.2) | 1 (2.0) |
| Respiratory, thoracic, and mediastinal disorders | 38 (73.1) | 7 (13.5) | 34 (69.4) | 11 (22.4) | 20 (40.8) | 0 |
| Cough | 21 (40.4) | 0 | 10 (20.4) | 0 | 9 (18.4) | 0 |
| Dyspnea | 20 (38.5) | 4 (7.7) | 18 (36.7) | 4 (8.2) | 9 (18.4) | 0 |
| Epistaxis | 9 (17.3) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Rhinorrhea | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 0 |
| Oropharyngeal pain | 5 (9.6) | 0 | 3 (6.1) | 0 | 5 (10.2) | 0 |
| Pleural effusion | 5 (9.6) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 43 (82.7) | 9 (17.3) | 24 (49.0) | 3 (6.1) | 42 (85.7) | 7 (14.3) |
| Palmar‐plantar erythrodysesthesia syndrome | 38 (73.1) | 9 (17.3) | 15 (30.6) | 3 (6.1) | 35 (71.4) | 6 (12.2) |
| Skin hyperpigmentation | 7 (13.5) | 0 | 2 (4.1) | 0 | 4 (8.2) | 0 |
| Dry skin | 3 (5.8) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Erythema | 3 (5.8) | 0 | 3 (6.1) | 0 | 7 (14.3) | 0 |
| Vascular disorders | 22 (42.3) | 7 (13.5) | 17 (34.7) | 3 (6.1) | 11 (22.4) | 2 (4.1) |
| Hypertension | 16 (30.8) | 4 (7.7) | 1 (2.0) | 0 | 1 (2.0) | 1 (2.0) |
| System organ class/preferred term . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | |
| Blood and lymphatic system disorders | 21 (40.4) | 8 (15.4) | 20 (40.8) | 8 (16.3) | 18 (36.7) | 2 (4.1) |
| Thrombocytopenia | 9 (17.3) | 1 (1.9) | 1 (2.0) | 0 | 9 (18.4) | 0 |
| Neutropenia | 8 (15.4) | 3 (5.8) | 6 (12.2) | 3 (6.1) | 6 (12.2) | 0 |
| Anemia | 7 (13.5) | 1 (1.9) | 15 (30.6) | 5 (10.2) | 12 (24.5) | 2 (4.1) |
| Leukopenia | 4 (7.7) | 0 | 0 | 0 | 7 (14.3) | 0 |
| Cardiac disorders | 6 (11.5) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 4 (8.2) | 0 |
| Ear and labyrinth disorder | 1 (1.9) | 0 | 1 (2.0) | 0 | 2 (4.1) | 0 |
| Eye disorders | 12 (23.1) | 0 | 15 (30.6) | 0 | 5 (10.2) | 0 |
| Lacrimation increased | 4 (7.7) | 0 | 6 (12.2) | 0 | 2 (4.1) | 0 |
| Gastrointestinal disorders | 43 (82.7) | 5 (9.6) | 47 (95.9) | 8 (16.3) | 44 (89.8) | 6 (12.2) |
| Nausea | 27 (51.9) | 0 | 38 (77.6) | 2 (4.1) | 26 (53.1) | 3 (6.1) |
| Diarrhea | 22 (42.3) | 1 (1.9) | 21 (42.9) | 2 (4.1) | 28 (57.1) | 3 (6.1) |
| Constipation | 19 (36.5) | 0 | 14 (28.6) | 0 | 17 (34.7) | 0 |
| Vomiting | 18 (34.6) | 1 (1.9) | 26 (53.1) | 2 (4.1) | 14 (28.6) | 3 (6.1) |
| Stomatitis | 14 (26.9) | 0 | 4 (8.2) | 1 (2.0) | 7 (14.3) | 0 |
| Abdominal pain | 9 (17.3) | 1 (1.9) | 6 (12.2) | 0 | 5 (10.2) | 1 (2.0) |
| Dyspepsia | 6 (11.5) | 0 | 5 (10.2) | 0 | 3 (6.1) | 0 |
| General disorders and administration site conditions | 38 (73.1) | 5 (9.6) | 42 (85.7) | 5 (10.2) | 37 (75.5) | 4 (8.2) |
| Fatigue | 27 (51.9) | 3 (5.8) | 28 (57.1) | 1 (2.0) | 26 (53.1) | 1 (2.0) |
| Pyrexia | 14 (26.9) | 0 | 8 (16.3) | 1 (2.0) | 4 (8.2) | 0 |
| Edema peripheral | 13 (25.0) | 0 | 19 (38.8) | 1 (2.0) | 6 (12.2) | 0 |
| Mucosal inflammation | 9 (17.3) | 2 (3.8) | 4 (8.2) | 0 | 9 (18.4) | 0 |
| Influenza‐like illness | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 1 (2.0) |
| Hepatobiliary disorders | 6 (11.5) | 2 (3.8) | 0 | 0 | 4 (8.2) | 0 |
| Infections and infestations | 22 (42.3) | 0 | 19 (38.8) | 4 (8.2) | 18 (36.7) | 3 (6.1) |
| Sinusitis | 7 (13.5) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Injury, poisoning, and procedural complications | 12 (23.1) | 1 (1.9) | 9 (18.4) | 2 (4.1) | 8 (16.3) | 1 (2.0) |
| Investigations | 24 (46.2) | 3 (5.8) | 14 (28.6) | 0 | 18 (36.7) | 0 |
| Aspartate aminotransferase increased | 9 (17.3) | 0 | 2 (4.1) | 0 | 7 (14.3) | 0 |
| Alanine aminotransferase increased | 5 (9.6) | 0 | 1 (2.0) | 0 | 5 (10.2) | 0 |
| Metabolism and nutrition disorders | 30 (57.7) | 3 (5.8) | 25 (51.0) | 7 (14.3) | 24 (49.0) | 7 (14.3) |
| Decreased appetite | 17 (32.7) | 0 | 11 (22.4) | 2 (4.1) | 10 (20.4) | 1 (2.0) |
| Hypokalemia | 12 (23.1) | 2 (3.8) | 7 (14.3) | 2 (4.1) | 12 (24.5) | 5 (10.2) |
| Hyperglycemia | 9 (17.3) | 0 | 4 (8.2) | 0 | 2 (4.1) | 0 |
| Dehydration | 6 (11.5) | 2 (3.8) | 11 (22.4) | 4 (8.2) | 3 (6.1) | 2 (4.1) |
| Hypocalcemia | 5 (9.6) | 0 | 4 (8.2) | 3 (6.1) | 6 (12.2) | 0 |
| Musculoskeletal and connective tissue disorders | 31 (59.6) | 5 (9.6) | 17 (34.7) | 2 (4.1) | 32 (65.3) | 2 (4.1) |
| Pain in extremity | 15 (28.8) | 2 (3.8) | 3 (6.1) | 0 | 6 (12.2) | 1 (2.0) |
| Arthralgia | 11 (21.2) | 0 | 2 (4.1) | 0 | 8 (16.3) | 0 |
| Back pain | 11 (21.2) | 3 (5.8) | 7 (14.3) | 1 (2.0) | 8 (16.3) | 0 |
| Myalgia | 9 (17.3) | 0 | 4 (8.2) | 0 | 3 (6.1) | 0 |
| Musculoskeletal chest pain | 8 (15.4) | 1 (1.9) | 3 (6.1) | 1 (2.0) | 7 (14.3) | 0 |
| Musculoskeletal pain | 6 (11.5) | 1 (1.9) | 1 (2.0) | 0 | 3 (6.1) | 0 |
| Neck pain | 6 (11.5) | 0 | 3 (6.1) | 0 | 0 | 0 |
| Muscle spasms | 5 (9.6) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Neoplasms benign, malignant, and unspecified | 2 (3.8) | 0 | 6 (1.2) | 4 (8.2) | 1 (2.0) | 1 (2.0) |
| Nervous system disorders | 37 (71.2) | 1 (1.9) | 28 (57.1) | 5 (10.2) | 22 (44.9) | 2 (4.1) |
| Headache | 26 (50.0) | 0 | 9 (18.4) | 1 (2.0) | 11 (22.4) | 1 (2.0) |
| Neuropathy peripheral | 10 (19.2) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Dysgeusia | 6 (11.5) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Peripheral sensory neuropathy | 6 (11.5) | 0 | 5 (10.2) | 0 | 2 (4.1) | 0 |
| Dizziness | 5 (9.6) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Psychiatric disorders | 17 (32.7) | 0 | 17 (34.7) | 0 | 17 (34.7) | 1 (2.0) |
| Insomnia | 7 (13.5) | 0 | 9 (18.4) | 0 | 12 (24.5) | 0 |
| Anxiety | 6 (11.5) | 0 | 7 (14.3) | 0 | 5 (10.2) | 0 |
| Depression | 6 (11.5) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Renal and urinary disorders | 6 (11.5) | 0 | 6 (12.2) | 2 (4.1) | 6 (12.2) | 1 (2.0) |
| Respiratory, thoracic, and mediastinal disorders | 38 (73.1) | 7 (13.5) | 34 (69.4) | 11 (22.4) | 20 (40.8) | 0 |
| Cough | 21 (40.4) | 0 | 10 (20.4) | 0 | 9 (18.4) | 0 |
| Dyspnea | 20 (38.5) | 4 (7.7) | 18 (36.7) | 4 (8.2) | 9 (18.4) | 0 |
| Epistaxis | 9 (17.3) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Rhinorrhea | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 0 |
| Oropharyngeal pain | 5 (9.6) | 0 | 3 (6.1) | 0 | 5 (10.2) | 0 |
| Pleural effusion | 5 (9.6) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 43 (82.7) | 9 (17.3) | 24 (49.0) | 3 (6.1) | 42 (85.7) | 7 (14.3) |
| Palmar‐plantar erythrodysesthesia syndrome | 38 (73.1) | 9 (17.3) | 15 (30.6) | 3 (6.1) | 35 (71.4) | 6 (12.2) |
| Skin hyperpigmentation | 7 (13.5) | 0 | 2 (4.1) | 0 | 4 (8.2) | 0 |
| Dry skin | 3 (5.8) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Erythema | 3 (5.8) | 0 | 3 (6.1) | 0 | 7 (14.3) | 0 |
| Vascular disorders | 22 (42.3) | 7 (13.5) | 17 (34.7) | 3 (6.1) | 11 (22.4) | 2 (4.1) |
| Hypertension | 16 (30.8) | 4 (7.7) | 1 (2.0) | 0 | 1 (2.0) | 1 (2.0) |
Treatment‐emergent adverse events reported in ≥10% of treated patients in any arm
| System organ class/preferred term . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | |
| Blood and lymphatic system disorders | 21 (40.4) | 8 (15.4) | 20 (40.8) | 8 (16.3) | 18 (36.7) | 2 (4.1) |
| Thrombocytopenia | 9 (17.3) | 1 (1.9) | 1 (2.0) | 0 | 9 (18.4) | 0 |
| Neutropenia | 8 (15.4) | 3 (5.8) | 6 (12.2) | 3 (6.1) | 6 (12.2) | 0 |
| Anemia | 7 (13.5) | 1 (1.9) | 15 (30.6) | 5 (10.2) | 12 (24.5) | 2 (4.1) |
| Leukopenia | 4 (7.7) | 0 | 0 | 0 | 7 (14.3) | 0 |
| Cardiac disorders | 6 (11.5) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 4 (8.2) | 0 |
| Ear and labyrinth disorder | 1 (1.9) | 0 | 1 (2.0) | 0 | 2 (4.1) | 0 |
| Eye disorders | 12 (23.1) | 0 | 15 (30.6) | 0 | 5 (10.2) | 0 |
| Lacrimation increased | 4 (7.7) | 0 | 6 (12.2) | 0 | 2 (4.1) | 0 |
| Gastrointestinal disorders | 43 (82.7) | 5 (9.6) | 47 (95.9) | 8 (16.3) | 44 (89.8) | 6 (12.2) |
| Nausea | 27 (51.9) | 0 | 38 (77.6) | 2 (4.1) | 26 (53.1) | 3 (6.1) |
| Diarrhea | 22 (42.3) | 1 (1.9) | 21 (42.9) | 2 (4.1) | 28 (57.1) | 3 (6.1) |
| Constipation | 19 (36.5) | 0 | 14 (28.6) | 0 | 17 (34.7) | 0 |
| Vomiting | 18 (34.6) | 1 (1.9) | 26 (53.1) | 2 (4.1) | 14 (28.6) | 3 (6.1) |
| Stomatitis | 14 (26.9) | 0 | 4 (8.2) | 1 (2.0) | 7 (14.3) | 0 |
| Abdominal pain | 9 (17.3) | 1 (1.9) | 6 (12.2) | 0 | 5 (10.2) | 1 (2.0) |
| Dyspepsia | 6 (11.5) | 0 | 5 (10.2) | 0 | 3 (6.1) | 0 |
| General disorders and administration site conditions | 38 (73.1) | 5 (9.6) | 42 (85.7) | 5 (10.2) | 37 (75.5) | 4 (8.2) |
| Fatigue | 27 (51.9) | 3 (5.8) | 28 (57.1) | 1 (2.0) | 26 (53.1) | 1 (2.0) |
| Pyrexia | 14 (26.9) | 0 | 8 (16.3) | 1 (2.0) | 4 (8.2) | 0 |
| Edema peripheral | 13 (25.0) | 0 | 19 (38.8) | 1 (2.0) | 6 (12.2) | 0 |
| Mucosal inflammation | 9 (17.3) | 2 (3.8) | 4 (8.2) | 0 | 9 (18.4) | 0 |
| Influenza‐like illness | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 1 (2.0) |
| Hepatobiliary disorders | 6 (11.5) | 2 (3.8) | 0 | 0 | 4 (8.2) | 0 |
| Infections and infestations | 22 (42.3) | 0 | 19 (38.8) | 4 (8.2) | 18 (36.7) | 3 (6.1) |
| Sinusitis | 7 (13.5) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Injury, poisoning, and procedural complications | 12 (23.1) | 1 (1.9) | 9 (18.4) | 2 (4.1) | 8 (16.3) | 1 (2.0) |
| Investigations | 24 (46.2) | 3 (5.8) | 14 (28.6) | 0 | 18 (36.7) | 0 |
| Aspartate aminotransferase increased | 9 (17.3) | 0 | 2 (4.1) | 0 | 7 (14.3) | 0 |
| Alanine aminotransferase increased | 5 (9.6) | 0 | 1 (2.0) | 0 | 5 (10.2) | 0 |
| Metabolism and nutrition disorders | 30 (57.7) | 3 (5.8) | 25 (51.0) | 7 (14.3) | 24 (49.0) | 7 (14.3) |
| Decreased appetite | 17 (32.7) | 0 | 11 (22.4) | 2 (4.1) | 10 (20.4) | 1 (2.0) |
| Hypokalemia | 12 (23.1) | 2 (3.8) | 7 (14.3) | 2 (4.1) | 12 (24.5) | 5 (10.2) |
| Hyperglycemia | 9 (17.3) | 0 | 4 (8.2) | 0 | 2 (4.1) | 0 |
| Dehydration | 6 (11.5) | 2 (3.8) | 11 (22.4) | 4 (8.2) | 3 (6.1) | 2 (4.1) |
| Hypocalcemia | 5 (9.6) | 0 | 4 (8.2) | 3 (6.1) | 6 (12.2) | 0 |
| Musculoskeletal and connective tissue disorders | 31 (59.6) | 5 (9.6) | 17 (34.7) | 2 (4.1) | 32 (65.3) | 2 (4.1) |
| Pain in extremity | 15 (28.8) | 2 (3.8) | 3 (6.1) | 0 | 6 (12.2) | 1 (2.0) |
| Arthralgia | 11 (21.2) | 0 | 2 (4.1) | 0 | 8 (16.3) | 0 |
| Back pain | 11 (21.2) | 3 (5.8) | 7 (14.3) | 1 (2.0) | 8 (16.3) | 0 |
| Myalgia | 9 (17.3) | 0 | 4 (8.2) | 0 | 3 (6.1) | 0 |
| Musculoskeletal chest pain | 8 (15.4) | 1 (1.9) | 3 (6.1) | 1 (2.0) | 7 (14.3) | 0 |
| Musculoskeletal pain | 6 (11.5) | 1 (1.9) | 1 (2.0) | 0 | 3 (6.1) | 0 |
| Neck pain | 6 (11.5) | 0 | 3 (6.1) | 0 | 0 | 0 |
| Muscle spasms | 5 (9.6) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Neoplasms benign, malignant, and unspecified | 2 (3.8) | 0 | 6 (1.2) | 4 (8.2) | 1 (2.0) | 1 (2.0) |
| Nervous system disorders | 37 (71.2) | 1 (1.9) | 28 (57.1) | 5 (10.2) | 22 (44.9) | 2 (4.1) |
| Headache | 26 (50.0) | 0 | 9 (18.4) | 1 (2.0) | 11 (22.4) | 1 (2.0) |
| Neuropathy peripheral | 10 (19.2) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Dysgeusia | 6 (11.5) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Peripheral sensory neuropathy | 6 (11.5) | 0 | 5 (10.2) | 0 | 2 (4.1) | 0 |
| Dizziness | 5 (9.6) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Psychiatric disorders | 17 (32.7) | 0 | 17 (34.7) | 0 | 17 (34.7) | 1 (2.0) |
| Insomnia | 7 (13.5) | 0 | 9 (18.4) | 0 | 12 (24.5) | 0 |
| Anxiety | 6 (11.5) | 0 | 7 (14.3) | 0 | 5 (10.2) | 0 |
| Depression | 6 (11.5) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Renal and urinary disorders | 6 (11.5) | 0 | 6 (12.2) | 2 (4.1) | 6 (12.2) | 1 (2.0) |
| Respiratory, thoracic, and mediastinal disorders | 38 (73.1) | 7 (13.5) | 34 (69.4) | 11 (22.4) | 20 (40.8) | 0 |
| Cough | 21 (40.4) | 0 | 10 (20.4) | 0 | 9 (18.4) | 0 |
| Dyspnea | 20 (38.5) | 4 (7.7) | 18 (36.7) | 4 (8.2) | 9 (18.4) | 0 |
| Epistaxis | 9 (17.3) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Rhinorrhea | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 0 |
| Oropharyngeal pain | 5 (9.6) | 0 | 3 (6.1) | 0 | 5 (10.2) | 0 |
| Pleural effusion | 5 (9.6) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 43 (82.7) | 9 (17.3) | 24 (49.0) | 3 (6.1) | 42 (85.7) | 7 (14.3) |
| Palmar‐plantar erythrodysesthesia syndrome | 38 (73.1) | 9 (17.3) | 15 (30.6) | 3 (6.1) | 35 (71.4) | 6 (12.2) |
| Skin hyperpigmentation | 7 (13.5) | 0 | 2 (4.1) | 0 | 4 (8.2) | 0 |
| Dry skin | 3 (5.8) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Erythema | 3 (5.8) | 0 | 3 (6.1) | 0 | 7 (14.3) | 0 |
| Vascular disorders | 22 (42.3) | 7 (13.5) | 17 (34.7) | 3 (6.1) | 11 (22.4) | 2 (4.1) |
| Hypertension | 16 (30.8) | 4 (7.7) | 1 (2.0) | 0 | 1 (2.0) | 1 (2.0) |
| System organ class/preferred term . | RAM + CAP (n = 52) . | ICR + CAP (n = 49) . | CAP (n = 49) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | |
| Blood and lymphatic system disorders | 21 (40.4) | 8 (15.4) | 20 (40.8) | 8 (16.3) | 18 (36.7) | 2 (4.1) |
| Thrombocytopenia | 9 (17.3) | 1 (1.9) | 1 (2.0) | 0 | 9 (18.4) | 0 |
| Neutropenia | 8 (15.4) | 3 (5.8) | 6 (12.2) | 3 (6.1) | 6 (12.2) | 0 |
| Anemia | 7 (13.5) | 1 (1.9) | 15 (30.6) | 5 (10.2) | 12 (24.5) | 2 (4.1) |
| Leukopenia | 4 (7.7) | 0 | 0 | 0 | 7 (14.3) | 0 |
| Cardiac disorders | 6 (11.5) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 4 (8.2) | 0 |
| Ear and labyrinth disorder | 1 (1.9) | 0 | 1 (2.0) | 0 | 2 (4.1) | 0 |
| Eye disorders | 12 (23.1) | 0 | 15 (30.6) | 0 | 5 (10.2) | 0 |
| Lacrimation increased | 4 (7.7) | 0 | 6 (12.2) | 0 | 2 (4.1) | 0 |
| Gastrointestinal disorders | 43 (82.7) | 5 (9.6) | 47 (95.9) | 8 (16.3) | 44 (89.8) | 6 (12.2) |
| Nausea | 27 (51.9) | 0 | 38 (77.6) | 2 (4.1) | 26 (53.1) | 3 (6.1) |
| Diarrhea | 22 (42.3) | 1 (1.9) | 21 (42.9) | 2 (4.1) | 28 (57.1) | 3 (6.1) |
| Constipation | 19 (36.5) | 0 | 14 (28.6) | 0 | 17 (34.7) | 0 |
| Vomiting | 18 (34.6) | 1 (1.9) | 26 (53.1) | 2 (4.1) | 14 (28.6) | 3 (6.1) |
| Stomatitis | 14 (26.9) | 0 | 4 (8.2) | 1 (2.0) | 7 (14.3) | 0 |
| Abdominal pain | 9 (17.3) | 1 (1.9) | 6 (12.2) | 0 | 5 (10.2) | 1 (2.0) |
| Dyspepsia | 6 (11.5) | 0 | 5 (10.2) | 0 | 3 (6.1) | 0 |
| General disorders and administration site conditions | 38 (73.1) | 5 (9.6) | 42 (85.7) | 5 (10.2) | 37 (75.5) | 4 (8.2) |
| Fatigue | 27 (51.9) | 3 (5.8) | 28 (57.1) | 1 (2.0) | 26 (53.1) | 1 (2.0) |
| Pyrexia | 14 (26.9) | 0 | 8 (16.3) | 1 (2.0) | 4 (8.2) | 0 |
| Edema peripheral | 13 (25.0) | 0 | 19 (38.8) | 1 (2.0) | 6 (12.2) | 0 |
| Mucosal inflammation | 9 (17.3) | 2 (3.8) | 4 (8.2) | 0 | 9 (18.4) | 0 |
| Influenza‐like illness | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 1 (2.0) |
| Hepatobiliary disorders | 6 (11.5) | 2 (3.8) | 0 | 0 | 4 (8.2) | 0 |
| Infections and infestations | 22 (42.3) | 0 | 19 (38.8) | 4 (8.2) | 18 (36.7) | 3 (6.1) |
| Sinusitis | 7 (13.5) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Injury, poisoning, and procedural complications | 12 (23.1) | 1 (1.9) | 9 (18.4) | 2 (4.1) | 8 (16.3) | 1 (2.0) |
| Investigations | 24 (46.2) | 3 (5.8) | 14 (28.6) | 0 | 18 (36.7) | 0 |
| Aspartate aminotransferase increased | 9 (17.3) | 0 | 2 (4.1) | 0 | 7 (14.3) | 0 |
| Alanine aminotransferase increased | 5 (9.6) | 0 | 1 (2.0) | 0 | 5 (10.2) | 0 |
| Metabolism and nutrition disorders | 30 (57.7) | 3 (5.8) | 25 (51.0) | 7 (14.3) | 24 (49.0) | 7 (14.3) |
| Decreased appetite | 17 (32.7) | 0 | 11 (22.4) | 2 (4.1) | 10 (20.4) | 1 (2.0) |
| Hypokalemia | 12 (23.1) | 2 (3.8) | 7 (14.3) | 2 (4.1) | 12 (24.5) | 5 (10.2) |
| Hyperglycemia | 9 (17.3) | 0 | 4 (8.2) | 0 | 2 (4.1) | 0 |
| Dehydration | 6 (11.5) | 2 (3.8) | 11 (22.4) | 4 (8.2) | 3 (6.1) | 2 (4.1) |
| Hypocalcemia | 5 (9.6) | 0 | 4 (8.2) | 3 (6.1) | 6 (12.2) | 0 |
| Musculoskeletal and connective tissue disorders | 31 (59.6) | 5 (9.6) | 17 (34.7) | 2 (4.1) | 32 (65.3) | 2 (4.1) |
| Pain in extremity | 15 (28.8) | 2 (3.8) | 3 (6.1) | 0 | 6 (12.2) | 1 (2.0) |
| Arthralgia | 11 (21.2) | 0 | 2 (4.1) | 0 | 8 (16.3) | 0 |
| Back pain | 11 (21.2) | 3 (5.8) | 7 (14.3) | 1 (2.0) | 8 (16.3) | 0 |
| Myalgia | 9 (17.3) | 0 | 4 (8.2) | 0 | 3 (6.1) | 0 |
| Musculoskeletal chest pain | 8 (15.4) | 1 (1.9) | 3 (6.1) | 1 (2.0) | 7 (14.3) | 0 |
| Musculoskeletal pain | 6 (11.5) | 1 (1.9) | 1 (2.0) | 0 | 3 (6.1) | 0 |
| Neck pain | 6 (11.5) | 0 | 3 (6.1) | 0 | 0 | 0 |
| Muscle spasms | 5 (9.6) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Neoplasms benign, malignant, and unspecified | 2 (3.8) | 0 | 6 (1.2) | 4 (8.2) | 1 (2.0) | 1 (2.0) |
| Nervous system disorders | 37 (71.2) | 1 (1.9) | 28 (57.1) | 5 (10.2) | 22 (44.9) | 2 (4.1) |
| Headache | 26 (50.0) | 0 | 9 (18.4) | 1 (2.0) | 11 (22.4) | 1 (2.0) |
| Neuropathy peripheral | 10 (19.2) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Dysgeusia | 6 (11.5) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Peripheral sensory neuropathy | 6 (11.5) | 0 | 5 (10.2) | 0 | 2 (4.1) | 0 |
| Dizziness | 5 (9.6) | 0 | 6 (12.2) | 0 | 3 (6.1) | 0 |
| Psychiatric disorders | 17 (32.7) | 0 | 17 (34.7) | 0 | 17 (34.7) | 1 (2.0) |
| Insomnia | 7 (13.5) | 0 | 9 (18.4) | 0 | 12 (24.5) | 0 |
| Anxiety | 6 (11.5) | 0 | 7 (14.3) | 0 | 5 (10.2) | 0 |
| Depression | 6 (11.5) | 0 | 6 (12.2) | 0 | 4 (8.2) | 0 |
| Renal and urinary disorders | 6 (11.5) | 0 | 6 (12.2) | 2 (4.1) | 6 (12.2) | 1 (2.0) |
| Respiratory, thoracic, and mediastinal disorders | 38 (73.1) | 7 (13.5) | 34 (69.4) | 11 (22.4) | 20 (40.8) | 0 |
| Cough | 21 (40.4) | 0 | 10 (20.4) | 0 | 9 (18.4) | 0 |
| Dyspnea | 20 (38.5) | 4 (7.7) | 18 (36.7) | 4 (8.2) | 9 (18.4) | 0 |
| Epistaxis | 9 (17.3) | 0 | 0 | 0 | 3 (6.1) | 0 |
| Rhinorrhea | 6 (11.5) | 0 | 2 (4.1) | 0 | 1 (2.0) | 0 |
| Oropharyngeal pain | 5 (9.6) | 0 | 3 (6.1) | 0 | 5 (10.2) | 0 |
| Pleural effusion | 5 (9.6) | 2 (3.8) | 16 (32.7) | 6 (12.2) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 43 (82.7) | 9 (17.3) | 24 (49.0) | 3 (6.1) | 42 (85.7) | 7 (14.3) |
| Palmar‐plantar erythrodysesthesia syndrome | 38 (73.1) | 9 (17.3) | 15 (30.6) | 3 (6.1) | 35 (71.4) | 6 (12.2) |
| Skin hyperpigmentation | 7 (13.5) | 0 | 2 (4.1) | 0 | 4 (8.2) | 0 |
| Dry skin | 3 (5.8) | 0 | 0 | 0 | 5 (10.2) | 0 |
| Erythema | 3 (5.8) | 0 | 3 (6.1) | 0 | 7 (14.3) | 0 |
| Vascular disorders | 22 (42.3) | 7 (13.5) | 17 (34.7) | 3 (6.1) | 11 (22.4) | 2 (4.1) |
| Hypertension | 16 (30.8) | 4 (7.7) | 1 (2.0) | 0 | 1 (2.0) | 1 (2.0) |
Serious AEs were more frequent on the RAM + CAP (38.5%) and ICR + CAP (51.0%) arms than on the CAP arm (12.2%). Grade ≥3 TEAEs were more frequent on the RAM + CAP (67.3%) and ICR + CAP (69.4%) arms than on the CAP arm (40.8%). Discontinuation of any study drug due to a TEAE was more frequent on the combination arms than on the CAP arm: 15 patients (28.8%) on RAM + CAP, 17 patients (34.7%) on ICR + CAP, and 1 patient (2.0%) on CAP. Instances of grade ≥3 AESIs were more frequent on the RAM + CAP (36.5%) and ICR + CAP (36.7%) arms than on the CAP arm (14.3%).
PK
RAM PK parameters are summarized in supplemental online Table S1. RAM exhibited low clearance (~0.01 L/hour), a small volume of distribution (~3 L), and a long half‐life (~7–9 days). A small amount of accumulation was observed after three doses of RAM at 10 mg/kg once every 3 weeks. The accumulation ratio based on maximal concentration was 1.11. The steady state appeared to be achieved by the fourth infusion (supplemental online Table S2).
For ICR, because of sample processing errors, PK data were very limited (i.e., from two patients in cycle 1, two patients in cycle 2, and one patient in cycle 3) and allowed no reliable estimation of PK parameters.
Discussion
This study evaluated the antitumor activity and safety of CAP in combination with RAM or ICR as compared with CAP alone in patients with unresectable, locally advanced or metastatic breast cancer previously treated with anthracycline and taxane therapy. Neither combination therapy resulted in a statistically significant improvement in PFS (RAM + CAP versus CAP, HR = 0.691, p = .1315; ICR + CAP versus CAP, HR = 1.480, p = .0851) or OS over CAP alone. Crossing over from CAP to RAM + CAP did not appear to confer any dramatic survival benefit, as shown by the median PFS of 12.1 weeks for the 29 patients who did cross over.
The adverse event profile of RAM + CAP and ICR + CAP combinations in this study appeared to be worse than that of CAP given alone. The incidence of SAEs was more frequent on the RAM + CAP (38.5%) and ICR + CAP (51.0%) arms than on the CAP arm (12.2%). So too was the incidence of discontinuation of any study drug due to a TEAE (28.8% on RAM + CAP and 34.7% on ICR + CAP versus 2.0% on CAP). CAP dose reductions were required more often on the RAM + CAP (51.9%) arm than on either the ICR + CAP (22.4%) or CAP (40.8%) arm. The median cumulative CAP dose (RAM + CAP, 89,508 mg/m2; ICR + CAP, 53,364 mg/m2; CAP, 132,197 mg/m2) and median duration of CAP therapy (RAM + CAP, 16.1 weeks; ICR + CAP, 6.0 weeks; CAP, 18.9 weeks) were both greater on the CAP arm than on either combination arm. However, the median CAP dose intensity (RAM + CAP 7,128 mg/m2 per week, ICR + CAP 7,963 mg/m2 per week, CAP 7,489 mg/m2 per week) was greatest in the ICR + CAP arm, most likely an artifact related to the shorter median duration of treatment on the ICR + CAP arm. Aside from the lesser tolerability of the RAM + CAP and ICR + CAP combinations, no new safety signals arose from this trial, and the safety profile is consistent with the RAM and ICR safety profiles to date.
The RAM PK parameters derived from noncompartmental analysis in this study are consistent with those observed in other RAM trials . Because of the limited number of samples, however, no definitive conclusions about the ICR PK profile could be made.
Several trials have investigated the combination of antiangiogenic agents and cytotoxic chemotherapy. Bevacizumab in combination with paclitaxel for the treatment of patients who have not received chemotherapy for metastatic HER2‐negative breast cancer received accelerated approval by the U.S. Food and Drug Administration in 2008 on the basis of results from the ECOG trial E2100, which reported a 5.5‐month improvement in PFS with bevacizumab over paclitaxel alone [21]. In the subsequent confirmatory trials AVADO (comparing bevacizumab + docetaxel versus docetaxel alone) [22] and RIBBON‐1 (comparing bevacizumab + taxane/anthracycline versus taxane/anthracycline alone or bevacizumab + CAP versus CAP alone) [23], PFS statistically improved with the addition of bevacizumab but less so than in the E2100 trial. It is important to note, however, that none of these large trials demonstrated an improvement in OS. Based upon the risk–benefit ratio, the bevacizumab indication for the treatment of metastatic breast cancer was withdrawn.
Small molecule kinase inhibitors of VEGFR have fared no better. A randomized phase III trial comparing sunitinib + paclitaxel versus bevacizumab + paclitaxel was halted prematurely due to inferior clinical outcomes on the sunitinib‐containing arm [40]. In addition, the phase III RESILIENCE trial, which compared the combination of sorafenib + CAP to placebo + CAP in patients with locally advanced or metastatic breast cancer, failed to demonstrate improvement in either median PFS (5.5 months with sorafenib + CAP versus 5.4 months with placebo + CAP, HR = 0.973, p = .406) or median OS (18.9 months with sorafenib + CAP and 20.3 months with placebo + CAP, HR = 1.195, p = .93) [41].
The efficacy results for the RAM combination in this study are similar to those from two recent trials in which RAM was combined with other agents in patients with metastatic breast cancer. The ROSE/TRIO‐12 trial was a multicenter, randomized, double‐blind study of RAM + docetaxel versus placebo + docetaxel in patients with previously untreated HER2‐negative, unresectable, locally recurrent or metastatic breast cancer [38]. The predefined PFS endpoint in that trial was not met. The I4T‐IE‐JVCD study was a multicenter, randomized, open‐label, phase II trial of RAM + eribulin versus eribulin alone in women with locally recurrent or metastatic breast cancer who had received two to four prior chemotherapy regimens in the relapsed/metastatic setting [39]. RAM added to eribulin did not significantly improve PFS, OS, or ORR as a third‐ to fifth‐line therapy in patients with metastatic breast cancer.
The aggregate clinical data suggest the existence of parallel and escape pathways that mitigate or nullify the effects of VEGF‐directed therapy in breast cancer. This in turn raises the fundamental question of whether VEGF is a valid target for the treatment of breast cancer or, at the very least, whether other pathways need to be inhibited in conjunction with VEGF in order to have a clinically meaningful impact. Further insight is therefore needed to better understand the chemosensitization effects at the cellular and molecular levels and the likelihood of improving efficacy with combinations of targeted agents.
Conclusion
This study did not meet its primary end point of improved PFS in patients with previously treated locally advanced or metastatic breast cancer by adding RAM or ICR to CAP.
Acknowledgments
The authors wish to acknowledge Jude Richard (INC Research, Austin, TX) for medical writing assistance. A.A.G. is currently affiliated with the Section of Hematology/Oncology, LSU Health New Orleans, New Orleans, LA; R.P.D. is a former employee of Eli Lilly and Company.
Author Contributions
Conception/Design: Linda T. Vahdat, Joseph Sparano, Shande Tang
Provision of study material or patients: Linda T. Vahdat, Rachel Layman, Denise A. Yardley, William Gradishar, Anil Abraham Joy, Agustin A. Garcia, Patrick Ward, James Khatcheressian, Joseph Sparano, Gladys Rodriguez, Kathy Miller
Collection and/or assembly of data: Linda T. Vahdat, Rachel Layman, Denise A. Yardley, William Gradishar, Mohamad A. Salkeni, Anil Abraham Joy, Agustin A. Garcia, Patrick Ward, James Khatcheressian, Joseph Sparano, Gladys Rodriguez, Shande Tang, Rita P. Dalal, Kathy Miller
Data analysis and interpretation: Linda T. Vahdat, Rachel Layman, Anil Abraham Joy, Agustin A. Garcia, James Khatcheressian, Joseph Sparano, Gladys Rodriguez, Shande Tang, Ling Gao, Rita P. Dalal, John Kauh, Kathy Miller
Manuscript writing: Linda T. Vahdat, Rachel Layman, Denise A. Yardley, William Gradishar, Mohamad A. Salkeni, Anil Abraham Joy, Agustin A. Garcia, Patrick Ward, James Khatcheressian, Joseph Sparano, Gladys Rodriguez, Shande Tang, Ling Gao, Rita P. Dalal, John Kauh
Final approval of manuscript: Linda T. Vahdat, Rachel Layman, Denise A. Yardley, William Gradishar, Mohamad A. Salkeni, Anil Abraham Joy, Agustin A. Garcia, Patrick Ward, James Khatcheressian, Joseph Sparano, Gladys Rodriguez, Shande Tang, Ling Gao, Rita P. Dalal, John Kauh, Kathy Miller
Disclosures
Linda T. Vahdat: Eisai (H), Celldex, Novartis, Genentech, Polyphor, Immunomedics, Pfizer (RF); Anil Abraham Joy: Eli Lilly and Company (C/A); Shande Tang: Eli Lilly and Company (E); Ling Gao: Eli Lilly and Company (E, L, OI); Joseph Sparano: Eli Lilly and Company (C/A); Rita P. Dalal: Eli Lilly and Company (E, L, OI); John Kauh: Eli Lilly and Company (E, L). The other authors indicated no financial relationships.
(C/A), Consultant/advisory relationship; (E), Employment; (H), Honoraria received; (IP), Intellectual property rights/inventor/patent holder; (L), Leadership position; (OI), Ownership interest; (RF), Research funding
References
Author notes
Clinicaltrials.gov Identifier: NCT01234402
Disclosures of potential conflicts of interest may be found at the end of this article.



