-
PDF
- Split View
-
Views
-
Cite
Cite
J. Randolph Hecht, Al B. Benson, Dmitry Vyushkov, Yingsi Yang, Johanna Bendell, Udit Verma, A Phase II, Randomized, Double‐Blind, Placebo‐Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second‐Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma, The Oncologist, Volume 22, Issue 3, March 2017, Pages 243–e23, https://doi.org/10.1634/theoncologist.2016-0479
Close - Share Icon Share
Abstract
The safety profile in the patient groups who received FOLFIRI and simtuzumab did not differ from that in the FOLFIRI and placebo group.
The addition of simtuzumab to chemotherapy with FOLFIRI does not improve clinical outcomes in patients with metastatic KRAS mutant colorectal carcinoma.
Simtuzumab, a humanized IgG4 monoclonal antibody to lysyl oxidase‐like 2 (LOXL2), blocks desmoplastic reaction in colorectal carcinoma (CRC) cells in vitro.
Patients with metastatic Kirsten rat sarcoma viral oncogene homolog (KRAS) mutant CRC were randomized to receive second‐line 5‐fluorouracil, leucovorin, and irinotecan (FOLFIRI) with either 200 or 700 mg simtuzumab or placebo every 2 weeks in cycles of 28 days. Progression‐free survival (PFS), overall survival (OS), objective response rate (ORR), and safety were assessed.
In total, 249 patients were randomized and treated with FOLFIRI/simtuzumab 700 mg (n = 84), FOLFIRI/simtuzumab 200 mg (n = 85), and FOLFIRI/placebo (n = 80). After a median follow‐up of 5.1, 3.8, and 5.5 months, respectively, median PFS for each of the respective treatment groups was 5.5 months (adjusted HR [95% CI], p value versus placebo; 1.32 [0.92, 1.89]; p = .10), 5.4 months (1.45 [1.01, 2.06]; p = .04), and 5.8 months. Median OS was 11.4 months (1.23 [0.80, 1.91]; p = .25), 10.5 months (1.50 [0.98, 2.30]; p = .06), and 16.3 months, respectively. ORR was 11.9%, 5.9%, and 10%, respectively. Simtuzumab was tolerable in metastatic KRAS mutant CRC patients.
The addition of simtuzumab to FOLFIRI did not improve clinical outcomes in patients with metastatic KRAS mutant CRC.
Discussion
Stroma in the tumor environment is associated with higher numbers of activated fibroblasts, which contribute to the desmoplastic reaction. Tumor‐associated activated fibroblasts secrete oncogenic growth factors, produce extracellular matrix (ECM), and promote epithelial cell transformation. Lysyl oxidase‐like 2 (LOXL2), an extracellular matrix enzyme that remodels ECM, is thought to contribute to the maintenance of the pathologic stromal microenvironment in cancer and fibrotic diseases and to promote tumor angiogenesis and metastatic progression. LOXL2 is expressed in desmoplastic tumors, including CRC, with no expression in the adjacent nonneoplastic areas. Simtuzumab, a humanized IgG4 monoclonal antibody to LOXL2, inhibits its enzymatic activity.
In preclinical studies with antibody precursors to simtuzumab, inhibition of LOXL2 expression reduced the number of activated fibroblasts, decreased ECM deposition, inhibited angiogenesis, and prevented tumor cell invasion and metastases. In a phase I study in patients with advanced solid tumors, simtuzumab reduced the size of several tumors in some patients.
We assessed the additive efficacy of simtuzumab in a multicenter, randomized, double‐blind, placebo‐controlled, phase II study of simtuzumab or placebo in combination with FOLFIRI as a second‐line therapy in patients with metastatic KRAS‐mutant adenocarcinoma of the colon or rectum not amenable to complete surgical resection, who previously failed fluoropyrimidine and oxaliplatin therapy (ClinicalTrials.gov #NCT01479465). Eligible patients had metastatic disease; Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2; adequate bone marrow, hepatic, and renal function; and estimated life expectancy >3 months. Patients were randomized 1:1:1 to receive intravenous FOLFIRI in combination with 700 mg simtuzumab, 200 mg simtuzumab, or placebo every 2 weeks in cycles of 28 days until disease progression or unacceptable toxicity; randomization was stratified by ECOG PS 0 versus >0. From December 2011 to March 2014, 255 patients were enrolled and 249 patients received at least 1 cycle of study drug, with a median number of 6 cycles. Overall, 231 (90.6%) patients discontinued study drug, mainly due to disease progression (185 [72.5%]) and adverse events (12 [4.7%]). The majority of patients were male (128/249, 51.4%) and white (212/249, 85.1%), with a mean (range) age of 60.5 (22.0–85.0) years and a mean 20.3 months from diagnosis. A sequential stepwise hypothesis and a Hochberg testing procedure were used to compare between the two simtuzumab dosing groups versus placebo for PFS, OS, and ORR. The efficacy analyses failed to demonstrate improvement in clinical endpoints upon the addition of simtuzumab to FOLFIRI chemotherapy (Table 1). Results from additional sensitivity analyses were consistent with the primary analyses. The safety profile in the patient groups who received FOLFIRI and simtuzumab did not differ from that in the FOLFIRI and placebo group. Among seven adverse events that led to death, a case of febrile neutropenia was deemed related to treatment with FOLFIRI.
Trial Information
- Disease
Colorectal cancer
- Stage of disease/treatment
Metastatic/Advanced
- Prior therapy
First‐line oxaliplatin‐ and fluoropyrimidine‐containing regimen
- Type of study ‐ 1
Phase II
- Type of study ‐ 2
Randomized
- ORR
Difference in ORR from placebo, stratified (primary) analysis was 2.0% for the simtuzumab 700 mg arm (p = .69), and −4.1% for the simtuzumab 200 mg arm (p = .33).
- PFS
The median PFS was 5.5 months, (HR 1.32, 0.92, 1.89; p = .10) in the 700 mg simtuzumab arm, 5.4 months (HR 1.45, 1.01, 2.06, p = .04) in the 200 mg simtuzumab arm, and 5.8 month in the control arm
- Primary endpoint
Progression‐free survival
- Secondary endpoint
Overall response rate
- Secondary endpoint
Overall survival
- Secondary endpoint
Safety
- Additional details of endpoints or study design
- Patients had to have measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, defined with all of following criteria: (a) lesions accurately measured in at least one dimension, (b) longest diameter in the plane of measurement was recorded, and (c) minimum size of 10 mm if computed tomography slice thickness ≤5 mm; if thickness was >5 mm, then the minimum size of measurable lesions was twice the slice thickness. Patients were excluded if they had more than one prior chemotherapy regimen for stage IV/metastatic colorectal cancer; underwent an experimental medical treatment within 30 days prior to study entry; received an antitumor therapy (chemotherapy, antibody therapy, molecular targeted therapy, retinoid therapy, hormonal therapy) within 21 days prior to randomization; or had cerebral metastases, uncontrolled hypertension, coronary heart disease, liver disease, or uncontrolled infection. Treatments were administered on day 1 and 15 of each 28‐day cycle. Simtuzumab or placebo was given by IV infusion over 30 minutes. FOLFIRI consisted of 200 mg/m2 leucovorin or 400 mg/m2 dl‐leucovorin administered as a 2‐hour infusion; 180 mg/m2 irinotecan given as a 90‐minute infusion in 500 mL dextrose 5% via a Y‐connector; and 400 mg/m2 fluorouracil bolus followed by a 46‐hour infusion of 2,400 mg/m2 fluorouracil. Efficacy was analyzed in all randomized patients who received at least one dose of study drug (full analysis set [FAS]) based on investigator assessment. Safety analysis set included patients in the FAS population grouped for analyses with treatment assignments designated according to the actual study drug received. Safety assessments included the incidence of adverse events (AEs), infusion site reactions, and clinically relevant changes in laboratory values and vital signs. AEs were coded according to MedDRA version 17.1 and graded per National Cancer Institute Common Toxicity Criteria (CTCAE version 4.03). Overall response rate (ORR) was assessed by the investigator per RECIST v1.1 as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The difference in PFS and OS from placebo was assessed using Kaplan–Meier methods and the stratified log‐rank test, adjusted for the stratification factor ECOG 0 or >0 at randomization. Cochran–Mantel–Haenszel test was used for calculating difference in ORR from placebo. A sequential stepwise hypothesis and a Hochberg testing procedure were used to compare between simtuzumab dosing groups versus placebo for PFS, OS, and ORR, per investigator assessment. A number of sensitivity analyses for PFS, OS, and ORR were also performed to confirm the results of primary analyses. A total of 185 PFS events had to be observed in this study to detect a hazard ratio of 0.6 with approximately 90% power at a two‐sided 0.05 significance level based on Hochberg procedure to claim that at least one of the simtuzumab treatment groups improved PFS significantly compared with placebo. The estimated sample size was 255 patients.
- Investigator’s analysis
Level of activity did not meet planned endpoint.
Drug Information Control Arm
- Generic/Working name
Folinic acid (leucovorin)
- Drug type
Small molecule
- Drug class
Antimetabolite
- Dose
200 mg/m2
- Route
IV over 2 hours
- Schedule of administration
Day 1 and 15 of each 28‐day cycle
- Drug 2
- Generic/working name
5‐FU
- Drug type
Small molecule
- Drug class
Antimetabolite
- Dose
2,400 mg/m2 (bolus 400 mg/m2)
- Route
Continuous intravenous infusion (CIV) over 46 hours
- Schedule of administration
Day 1 and 15 of each 28-day cycle
- Drug 3
- Generic/working name
Irinotecan
- Drug type
Small molecule
- Drug class
Inhibitor of topoisomerase I
- Dose
180 mg/m2
- Route
IV over 1.5 hours
- Schedule of administration
Day 1 and 15 of each 28-day cycle
Drug Information Experimental Arm A: Simtuzumab 200 mg
- Drug 1
- Generic/working name
Simtuzumab (GS‐6624)
- Company name
Gilead Sciences, Inc.
- Drug type
Antibody
- Drug class
Inhibitor of lysyl oxidase-like 2 enzyme
- Dose
200 mg per flat dose
- Route
IV
- Schedule of administration
Day 1 and 15 of each 28‐day cycle
- Drug 2
- Generic/working name
Leucovorin
- Drug type
Small molecule
- Drug class
Antimetabolite
- Dose
200 mg/m2
- Route
IV over 2 hours
- Schedule of administration
Day 1 and 15 of each 28‐day cycle
- Drug 3
- Generic/working name
5‐FU
- Drug type
Small molecule
- Drug class
Antimetabolite
- Dose
2,400 mg/m2 (bolus 400 mg/m2)
- Route
Continuous intravenous infusion (CIV) over 46 hours
- Schedule of administration
Day 1 and 15 of each 28-day cycle
- Drug 4
- Generic/working name
Irinotecan
- Drug type
Small molecule
- Drug class
Inhibitor of topoisomerase I
- Dose
180 mg/m2
- Route
IV over 1.5 hours
- Schedule of administration
Day 1 and 15 of each 28-day cycle
Drug Information Experimental Arm B: Simtuzumab 700 mg
- Drug 1
- Generic/working name
Simtuzumab (GS‐6624)
- Company name
Gilead Sciences, Inc.
- Drug type
Antibody
- Drug class
Inhibitor of lysyl oxidase-like 2 enzyme
- Dose
700 mg per flat dose
- Route
IV
- Schedule of administration
Day 1 and 15 of each 28‐day cycle
- Drug 2
- Generic/working name
Leucovorin
- Drug type
Small molecule
- Drug class
Antimetabolite
- Dose
200 mg/m2
- Route
IV over 2 hours
- Schedule of administration
Day 1 and 15 of each 28‐day cycle
- Drug 3
- Generic/working name
5‐FU
- Drug type
Small molecule
- Drug class
Antimetabolite
- Dose
2,400 mg/m2 (bolus 400 mg/m2)
- Route
Continuous intravenous infusion (CIV) over 46 hours
- Schedule of administration
Day 1 and 15 of each 28-day cycle
- Drug 4
- Generic/working name
Irinotecan
- Drug type
Small molecule
- Drug class
Inhibitor of topoisomerase I
- Dose
180 mg/m2
- Route
IV over 1.5 hours
- Schedule of administration
Day 1 and 15 of each 28-day cycle
Patient Characteristics
- Number of patients, male
128
- Number of patients, female
121
- Stage
Advanced, metastatic, stage IV
- Age
Median (range), years: 700 mg simtuzumab, 61 (22–78); 200 mg simtuzumab, 64 (31–83); placebo, 60.5 (32–85)
- Number of prior systemic therapies
1, first‐line oxaliplatin‐ and fluoropyrimidine‐containing regimen
- Performance status: ECOG
0 — 118
1 — 126
2 — 5
Primary Assessment Method
- Control Arm:
- Number of patients enrolled
84
- Number of patients evaluable for toxicity
80
- Number of patients evaluated for efficacy
80
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 8 (10%)
- Response assessment SD
n = 53 (66%)
- Response assessment PD
n = 17 (21%)
- Response assessment OTHER
n = 2 (3%)
- (Median) duration assessments PFS
5.8 months, CI: 4.9–9.0
- (Median) duration assessments OS
16.3 months, CI: 12.0–19.5
- Experimental Arm A: Simtuzumab 200 mg
- Number of patients enrolled
86
- Number of patients evaluable for toxicity
85
- Number of patients evaluated for efficacy
85
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 5 (6%)
- Response assessment SD
n = 50 (59%)
- Response assessment PD
n = 23 (27%)
- Response assessment OTHER
n = 7 (8%)
- (Median) duration assessments PFS
5.4 months, CI: 3.4–5.6
- (Median) duration assessments OS
10.5 months, CI: 9.2–12.6
- Experimental Arm B: Simtuzumab 700 mg
- Number of patients enrolled
85
- Number of patients evaluable for toxicity
84
- Number of patients evaluated for efficacy
84
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 10 (12%)
- Response assessment SD
n = 49 (58%)
- Response assessment PD
n = 23 (27%)
- Response assessment OTHER
n = 2 (2%)
- (Median) duration assessments PFS
5.5 months, CI: 4.0–7.1
- (Median) duration assessments OS
11.4 months, CI: 9.7–15.6
Adverse Events Control Arm
*NC/NA No change from baseline/no adverse event
Adverse events that occurred in ≥10% of patients. n = 80, safety analysis set. Clinical and laboratory adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1 and graded using the National Cancer Institute Common Toxicity Criteria (CTCAE version 4.03).
| Serious Adverse Events Control Arm . | ||
|---|---|---|
| Name . | Grade . | Attribution . |
| Febrile neutropenia | 4 | Probable |
| Dehydration | 2 | Probable |
| Dehydration | 3 | Probable |
| Vomiting | 2 | Probable |
| Nausea | 2 | Probable |
| Nausea | 3 | Probable |
| Diarrhea | 3 | Probable |
| Abdominal pain | 3 | Probable |
| Enteritis | 3 | Probable |
| Gastroenteritis | 3 | Probable |
| Serious Adverse Events Control Arm . | ||
|---|---|---|
| Name . | Grade . | Attribution . |
| Febrile neutropenia | 4 | Probable |
| Dehydration | 2 | Probable |
| Dehydration | 3 | Probable |
| Vomiting | 2 | Probable |
| Nausea | 2 | Probable |
| Nausea | 3 | Probable |
| Diarrhea | 3 | Probable |
| Abdominal pain | 3 | Probable |
| Enteritis | 3 | Probable |
| Gastroenteritis | 3 | Probable |
SAEs deemed related to simtuzumab/placebo. As this was a double‐blind study, the SAEs considered related to simtuzumab were recorded for both treatment arms. SAEs related to FOLFIRI were not collected. n = 80, safety analysis set.
Adverse Events Both Experimental Arms
*NC/NA No change from baseline/no adverse event
Adverse events that occurred in ≥10% of patients. n = 169, safety analysis set. Clinical and laboratory adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1 and graded using the National Cancer Institute Common Toxicity Criteria (CTCAE version 4.03).
| Serious Adverse Events Experimental Arm . | ||
|---|---|---|
| Name . | Grade . | Attribution . |
| Sepsis | 4 | Probable |
| Urosepsis | 4 | Probable |
| Febrile neutropenia | 3 | Probable |
| Febrile neutropenia | 4 | Probable |
| Thrombocytopenia | 4 | Probable |
| Diverticulitis | 3 | Probable |
| Lung infection | 2 | Probable |
| Lower respiratory tract inflammation | 4 | Probable |
| Pneumothorax | 4 | Probable |
| Diarrhea | 3 | Probable |
| Delirium | 4 | Probable |
| Confusional state | 4 | Probable |
| Serious Adverse Events Experimental Arm . | ||
|---|---|---|
| Name . | Grade . | Attribution . |
| Sepsis | 4 | Probable |
| Urosepsis | 4 | Probable |
| Febrile neutropenia | 3 | Probable |
| Febrile neutropenia | 4 | Probable |
| Thrombocytopenia | 4 | Probable |
| Diverticulitis | 3 | Probable |
| Lung infection | 2 | Probable |
| Lower respiratory tract inflammation | 4 | Probable |
| Pneumothorax | 4 | Probable |
| Diarrhea | 3 | Probable |
| Delirium | 4 | Probable |
| Confusional state | 4 | Probable |
SAEs deemed related to simtuzumab. SAEs related to FOLFIRI were not collected. n = 169, safety analysis set.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Pharmacokinetics/Pharmacodynamics
Not Collected
- Investigator’s Assessment
Level of activity did not meet planned endpoint
Colorectal cancer (CRC) accounts for 9.7% of all incident cancers worldwide and is the fourth leading cause of cancer mortality [1]. One of the frequent genetic mutations in CRC is in the KRAS gene in the chromosomal instability pathway [2]. KRAS mutation‐positive patients do not respond to anti‐epidermal growth factor receptor antibody therapy [3] and have poorer survival than patients with the KRAS wild‐type gene [4]. The currently available therapies for advanced KRAS‐mutated CRC include chemotherapy alone or in combination with antiangiogenic antibodies such as bevacizumab, aflibercept, or ramucirumab [5]. New therapies are urgently needed for this patient population.
Activated stroma in the tumor environment is associated with higher numbers of activated fibroblasts, which contribute to the desmoplastic reaction . Tumor‐associated activated fibroblasts secrete oncogenic growth factors, produce extracellular matrix (ECM), and promote epithelial cell transformation . Lysyl oxidase‐like 2 (LOXL2), an enzyme that remodels the ECM, contributes to the maintenance of the pathologic stromal microenvironment in cancer and fibrotic diseases [9] and promotes tumor angiogenesis [10] and metastatic progression [11]. LOXL2 is expressed in desmoplastic tumors, including CRC, with no expression in the adjacent nonneoplastic areas .
Simtuzumab, a humanized IgG4 monoclonal antibody against LOXL2, inhibits its enzymatic activity [13]. In preclinical studies with antibody precursors to simtuzumab, inhibition of LOXL2 expression reduced numbers of activated fibroblasts, decreased ECM deposition [9], inhibited angiogenesis [14], and prevented tumor cell invasion and metastases [15]. Of note, in preclinical models, simtuzumab had better efficacy in KRAS mutant cell lines (Gilead Sciences, Inc., data on file). In a phase I study in patients with advanced solid tumors, single agent simtuzumab reduced the size of several tumors in select patients [16].
In a multicenter, randomized, double‐blind, placebo‐controlled phase II study, we evaluated the efficacy of simtuzumab or placebo in combination with FOLFIRI as a second‐line therapy in patients with metastatic KRAS‐mutant CRC who progressed following oxaliplatin‐ and fluoropyrimidine‐containing first‐line therapy (ClinicalTrials.gov #NCT01479465).
The key endpoints included progression‐free survival (PFS), overall survival (OS), objective response rate (ORR) per RECIST v1.1, and safety. Enrolled patients had stage IV disease; ECOG PS ≤2; adequate bone marrow, hepatic, and renal function; and estimated life expectancy >3 months. Patients were randomized 1:1:1 to receive intravenous FOLFIRI in combination with simtuzumab or placebo until disease progression or unacceptable toxicity; randomization was stratified by Eastern Cooperative Oncology Group performance status (ECOG PS) 0 vs >0. Patients were scheduled for 2 visits per cycle and a computed tomography (CT) or magnetic resonance imaging (MRI) scan every 8 weeks.
From December 2011 to March 2014, 255 patients were randomized, 249 patients received at least one 28‐day cycle of study drug (full analysis set [FAS]), and 233 (94%) patients received ≥2 cycles, with a median number of 6 cycles. The majority of enrolled patients were male (128/249, 51.4%) and white (212/249, 85.1%), with a mean (range) age of 60.5 (22.0–85.0) years (Table 2). The mean (SD) time since first diagnosis of colorectal cancer was 20.3 (20.31) months. In total, 42% of patients had 3 or more target lesions at baseline. The most common target lesion sites included liver (73%), lung (45%), and lymph nodes (16%) (Table 2).
Overall, 231 (90.6%) patients had discontinued study, mainly due to disease progression (185 [72.5%]) and adverse events (12 [4.7%]). A sequential stepwise hypothesis and a Hochberg testing procedure were used to compare between the two simtuzumab dosing groups versus placebo for PFS, OS, and ORR. A number of sensitivity analyses were also conducted for PFS, OS, and ORR to confirm the results of the primary analyses. The study failed to reach any of the prespecified efficacy endpoints. Median PFS for FOLFIRI/simtuzumab 700 mg, FOLFIRI/simtuzumab 200 mg, and FOLFIRI/placebo was 5.5 months (adjusted hazard ratios [HRs] with 95% confidence interval [CIs] for the stratified primary analysis, p value versus placebo; 1.32 [0.92, 1.89]; p = .10), 5.4 months (1.45 [1.01, 2.06] p = .04), and 5.8 months, respectively (Fig. 1), after a median follow‐up of 5.1, 3.8, and 5.5 months, respectively. Median OS for the FOLFIRI/simtuzumab 700 mg, FOLFIRI/simtuzumab 200 mg, and FOLFIRI/placebo was 11.4 months (adjusted HR, [95% CIs] for the stratified OS primary analysis, p value versus placebo; 1.23 [0.80, 1.91]; p = .25), 10.5 months (1.50 [0.98, 2.30]; p = .06), and 16.3 months, respectively (Fig. 2). The adjusted HRs for PFS and OS for the simtuzumab arms compared with placebo were all greater than 1. The ORRs in FOLFIRI/simtuzumab 700 mg, FOLFIRI/simtuzumab 200 mg, and FOLFIRI/placebo were 11.9%, 5.9%, and 10.0%, respectively. The difference (95% CI) in ORR for patients treated with 700 mg FOLFIRI plus simtuzumab versus patients treated with FOLFIRI plus placebo was 2.0% (−8.1, 11.9; p = .69) and −4.1% (−13.3, 4.6; p = .33) for patients treated with 200 mg FOLFIRI plus simtuzumab compared with FOLFIRI plus placebo. Results from sensitivity analyses were consistent with the primary analyses.
The safety profile in the patient groups who received FOLFIRI combined with simtuzumab was not different from patients who received FOLFIRI with placebo. The most common adverse events (AEs) deemed by the investigator to be related to study treatment were fatigue, diarrhea, nausea and neutropenia. AEs greater than or equal to grade 3 were reported in 59.5% in the FOLFIRI/simtuzumab 700 mg group, 67.1% in the FOLFIRI/simtuzumab 200 mg group, and 61.3% in the FOLFIRI/placebo group. Seven AEs leading to death occurred during this study; one case of febrile neutropenia was deemed related to FOLFIRI treatment. None of the deaths on study were considered related to simtuzumab.
In conclusion, the addition of simtuzumab to FOLFIRI did not improve PFS, OS, or ORR in patients with metastatic KRAS mutant CRC.
Acknowledgments
Medical writing and editorial support was provided by Ewa Wandzioch Ph.D., of AlphaBioCom, LLC, King of Prussia, Pennsylvania, and was funded by Gilead Sciences, Inc.
Disclosures
J. Randolph Hecht: Amgen, Genentech, Boston Biomedical (C/A), Amgen, Symphogen,Merrimack Pharmaceuticals (RF); Al B. Benson III: Genentech/Roche, Sanofi, Bristol‐Myers Squibb, Merck Serono, Merck/Schering Plough, Spectrum Pharmaceuticals, Lilly/ImClone, Celgene, Genomic Health, Vicus Therapeutics, Pharmacyclics, Precision Therapeutics, Taiho Pharmaceutical, Bayer, Alchemia, Infinity Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, EMD Serono (C/A), Genentech/Roche, Sanofi, Bristol‐Myers Squibb, Merck Serono, Merck/Schering Plough, Spectrum Pharmaceuticals, Lilly/ImClone, Celgene, Genomic Health, Vicus Therapeutics, Pharmacyclics, Precision Therapeutics, Taiho Pharmaceutical, Bayer, Alchemia, Infinity Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, EMD Serono, AVEO, Gilead Sciences (H), Genentech, Gilead Sciences, Amgen, Astellas Pharma, Bayer/Onyx, Novartis, Alchemia, AVEO, Infinity Pharmaceuticals, Merck Serono, EMD Serono (RF); Yingsi Yang: Gilead Sciences, Inc. (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
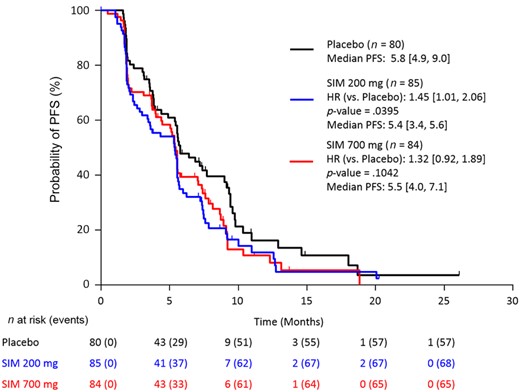
Stratified Kaplan–Meier plot of progression‐free survival by investigator assessment (FAS population).
Abbreviations: CI, confidence interval; FAS, full analysis set (all patients randomized and treated with ≥1 dose of study drug); HR, hazard ratio (numbers in brackets are 95% CIs); PFS, progression‐free survival; SIM, simtuzumab.
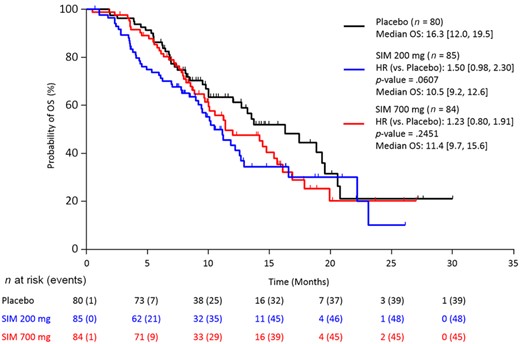
Stratified Kaplan–Meier plot of overall survival (FAS population).
Abbreviations: CI, confidence interval; FAS, full analysis set (all patients randomized and treated with ≥1 dose of study drug); HR, hazard ratio (numbers in brackets are 95% CIs); OS, overall survival; SIM, simtuzumab.
aStratified primary analysis per investigator assessment. The results per Independent Review Committee were similar to those obtained by investigator assessment.
bp values based on stratified two‐sided log‐rank test.
cp values based on Cochran–Mantel–Haenszel test adjusted for stratification factors.
Abbreviations: CI, confidence interval; CR, complete response; FAS, full analysis set (all patients randomized and treated with ≥1 dose of study drug); HR, hazard ratio; KM, Kaplan–Meier; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; PR, partial response.
aStratified primary analysis per investigator assessment. The results per Independent Review Committee were similar to those obtained by investigator assessment.
bp values based on stratified two‐sided log‐rank test.
cp values based on Cochran–Mantel–Haenszel test adjusted for stratification factors.
Abbreviations: CI, confidence interval; CR, complete response; FAS, full analysis set (all patients randomized and treated with ≥1 dose of study drug); HR, hazard ratio; KM, Kaplan–Meier; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; PR, partial response.
| Characteristic . | Simtuzumab 700 mg/FOLFIRI n = 84 . | Simtuzumab 200 mg/FOLFIRI n = 85 . | Placebo/FOLFIRI n = 80 . |
|---|---|---|---|
| Mean age in years (range) | 60.1 (22.0–78.0) | 62.6 (31.0–83.0) | 58.8 (32.0–85.0) |
| ECOG performance status, n (%) | |||
| 0 | 35 (41.7) | 40 (47.1) | 43 (53.8) |
| 1 | 48 (57.1) | 42 (49.4) | 36 (45.0) |
| 2 | 1 (1.2) | 3 (3.5) | 1 (1.3) |
| Mean time since first diagnosis of colorectal cancer in months (SD) | 20.7 (24.72) | 20.5 (17.53) | 19.6 (17.87) |
| Patients with prior chemoimmunotherapy, n (%) | 84 (100) | 85 (100) | 80 (100) |
| Patients with prior radiotherapy related to current disease, n (%) | 13 (15.5) | 17 (20.0) | 16 (20.0) |
| No. of target lesions at baseline, n (%) | |||
| 1 | 12 (14.3) | 11 (12.9) | 14 (17.5) |
| 2 | 31 (36.9) | 35 (41.2) | 39 (48.8) |
| 3 | 20 (23.8) | 18 (21.2) | 16 (20.0) |
| 4 | 12 (14.3) | 15 (17.6) | 9 (11.3) |
| 5 | 8 (9.5) | 5 (5.9) | 2 (2.5) |
| Lesion site, n (%) | |||
| Liver | 59 (70.2) | 64 (75.3) | 58 (72.5) |
| Lung | 42 (50.0) | 37 (43.5) | 34 (42.5) |
| Lymph nodes (shortest diameter) | 14 (16.7) | 15 (17.6) | 11 (13.8) |
| Mean tumor sum of diameter at baseline per investigator in millimeters (SD) | 90.3 (57.38) | 87.9 (58.04) | 77.2 (52.54) |
| Mean tumor sum of diameter at baseline per IRR in millimeters (SD) | 89.7 (50.93) | 91.1 (50.67) | 82.8 (61.45) |
| Mean tumor marker CEA in μg/L (SD) | 264.7 (861.88) | 462.8 (1325.24) | 320.3 (970.59) |
| Characteristic . | Simtuzumab 700 mg/FOLFIRI n = 84 . | Simtuzumab 200 mg/FOLFIRI n = 85 . | Placebo/FOLFIRI n = 80 . |
|---|---|---|---|
| Mean age in years (range) | 60.1 (22.0–78.0) | 62.6 (31.0–83.0) | 58.8 (32.0–85.0) |
| ECOG performance status, n (%) | |||
| 0 | 35 (41.7) | 40 (47.1) | 43 (53.8) |
| 1 | 48 (57.1) | 42 (49.4) | 36 (45.0) |
| 2 | 1 (1.2) | 3 (3.5) | 1 (1.3) |
| Mean time since first diagnosis of colorectal cancer in months (SD) | 20.7 (24.72) | 20.5 (17.53) | 19.6 (17.87) |
| Patients with prior chemoimmunotherapy, n (%) | 84 (100) | 85 (100) | 80 (100) |
| Patients with prior radiotherapy related to current disease, n (%) | 13 (15.5) | 17 (20.0) | 16 (20.0) |
| No. of target lesions at baseline, n (%) | |||
| 1 | 12 (14.3) | 11 (12.9) | 14 (17.5) |
| 2 | 31 (36.9) | 35 (41.2) | 39 (48.8) |
| 3 | 20 (23.8) | 18 (21.2) | 16 (20.0) |
| 4 | 12 (14.3) | 15 (17.6) | 9 (11.3) |
| 5 | 8 (9.5) | 5 (5.9) | 2 (2.5) |
| Lesion site, n (%) | |||
| Liver | 59 (70.2) | 64 (75.3) | 58 (72.5) |
| Lung | 42 (50.0) | 37 (43.5) | 34 (42.5) |
| Lymph nodes (shortest diameter) | 14 (16.7) | 15 (17.6) | 11 (13.8) |
| Mean tumor sum of diameter at baseline per investigator in millimeters (SD) | 90.3 (57.38) | 87.9 (58.04) | 77.2 (52.54) |
| Mean tumor sum of diameter at baseline per IRR in millimeters (SD) | 89.7 (50.93) | 91.1 (50.67) | 82.8 (61.45) |
| Mean tumor marker CEA in μg/L (SD) | 264.7 (861.88) | 462.8 (1325.24) | 320.3 (970.59) |
Abbreviations: CEA, carcinoembryonic antigen; FAS, full analysis set (all patients randomized and treated with ≥1 dose of study drug); IRR, Independent Radiology Review; SD, standard deviation.
| Characteristic . | Simtuzumab 700 mg/FOLFIRI n = 84 . | Simtuzumab 200 mg/FOLFIRI n = 85 . | Placebo/FOLFIRI n = 80 . |
|---|---|---|---|
| Mean age in years (range) | 60.1 (22.0–78.0) | 62.6 (31.0–83.0) | 58.8 (32.0–85.0) |
| ECOG performance status, n (%) | |||
| 0 | 35 (41.7) | 40 (47.1) | 43 (53.8) |
| 1 | 48 (57.1) | 42 (49.4) | 36 (45.0) |
| 2 | 1 (1.2) | 3 (3.5) | 1 (1.3) |
| Mean time since first diagnosis of colorectal cancer in months (SD) | 20.7 (24.72) | 20.5 (17.53) | 19.6 (17.87) |
| Patients with prior chemoimmunotherapy, n (%) | 84 (100) | 85 (100) | 80 (100) |
| Patients with prior radiotherapy related to current disease, n (%) | 13 (15.5) | 17 (20.0) | 16 (20.0) |
| No. of target lesions at baseline, n (%) | |||
| 1 | 12 (14.3) | 11 (12.9) | 14 (17.5) |
| 2 | 31 (36.9) | 35 (41.2) | 39 (48.8) |
| 3 | 20 (23.8) | 18 (21.2) | 16 (20.0) |
| 4 | 12 (14.3) | 15 (17.6) | 9 (11.3) |
| 5 | 8 (9.5) | 5 (5.9) | 2 (2.5) |
| Lesion site, n (%) | |||
| Liver | 59 (70.2) | 64 (75.3) | 58 (72.5) |
| Lung | 42 (50.0) | 37 (43.5) | 34 (42.5) |
| Lymph nodes (shortest diameter) | 14 (16.7) | 15 (17.6) | 11 (13.8) |
| Mean tumor sum of diameter at baseline per investigator in millimeters (SD) | 90.3 (57.38) | 87.9 (58.04) | 77.2 (52.54) |
| Mean tumor sum of diameter at baseline per IRR in millimeters (SD) | 89.7 (50.93) | 91.1 (50.67) | 82.8 (61.45) |
| Mean tumor marker CEA in μg/L (SD) | 264.7 (861.88) | 462.8 (1325.24) | 320.3 (970.59) |
| Characteristic . | Simtuzumab 700 mg/FOLFIRI n = 84 . | Simtuzumab 200 mg/FOLFIRI n = 85 . | Placebo/FOLFIRI n = 80 . |
|---|---|---|---|
| Mean age in years (range) | 60.1 (22.0–78.0) | 62.6 (31.0–83.0) | 58.8 (32.0–85.0) |
| ECOG performance status, n (%) | |||
| 0 | 35 (41.7) | 40 (47.1) | 43 (53.8) |
| 1 | 48 (57.1) | 42 (49.4) | 36 (45.0) |
| 2 | 1 (1.2) | 3 (3.5) | 1 (1.3) |
| Mean time since first diagnosis of colorectal cancer in months (SD) | 20.7 (24.72) | 20.5 (17.53) | 19.6 (17.87) |
| Patients with prior chemoimmunotherapy, n (%) | 84 (100) | 85 (100) | 80 (100) |
| Patients with prior radiotherapy related to current disease, n (%) | 13 (15.5) | 17 (20.0) | 16 (20.0) |
| No. of target lesions at baseline, n (%) | |||
| 1 | 12 (14.3) | 11 (12.9) | 14 (17.5) |
| 2 | 31 (36.9) | 35 (41.2) | 39 (48.8) |
| 3 | 20 (23.8) | 18 (21.2) | 16 (20.0) |
| 4 | 12 (14.3) | 15 (17.6) | 9 (11.3) |
| 5 | 8 (9.5) | 5 (5.9) | 2 (2.5) |
| Lesion site, n (%) | |||
| Liver | 59 (70.2) | 64 (75.3) | 58 (72.5) |
| Lung | 42 (50.0) | 37 (43.5) | 34 (42.5) |
| Lymph nodes (shortest diameter) | 14 (16.7) | 15 (17.6) | 11 (13.8) |
| Mean tumor sum of diameter at baseline per investigator in millimeters (SD) | 90.3 (57.38) | 87.9 (58.04) | 77.2 (52.54) |
| Mean tumor sum of diameter at baseline per IRR in millimeters (SD) | 89.7 (50.93) | 91.1 (50.67) | 82.8 (61.45) |
| Mean tumor marker CEA in μg/L (SD) | 264.7 (861.88) | 462.8 (1325.24) | 320.3 (970.59) |
Abbreviations: CEA, carcinoembryonic antigen; FAS, full analysis set (all patients randomized and treated with ≥1 dose of study drug); IRR, Independent Radiology Review; SD, standard deviation.
ClinicalTrials.gov Identifier: NCT01479465
Sponsor(s): Gilead Sciences, Inc., Foster City, California, USA
Principal Investigator: J. Randolph Hecht
IRB Approved: Yes
References
NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. 2016. Available at https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed May 12,



