-
PDF
- Split View
-
Views
-
Cite
Cite
Manish A. Shah, Jae-Yong Cho, Iain B. Tan, Niall C. Tebbutt, Chia-Jui Yen, Alice Kang, David S. Shames, Lilian Bu, Yoon-Koo Kang, A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction, The Oncologist, Volume 21, Issue 9, September 2016, Pages 1085–1090, https://doi.org/10.1634/theoncologist.2016-0038
Close - Share Icon Share
Abstract
The phase II YO28252 study (01590719) examined first-line onartuzumab plus mFOLFOX6 in patients with metastatic, human epidermal growth factor receptor 2-negative adenocarcinoma of the stomach or gastroesophageal junction. MET immunohistochemistry expression as a biomarker of onartuzumab activity was also examined.
Patients were randomized 1:1 to receive standard mFOLFOX6 plus onartuzumab (10 mg/kg) or placebo in 2-week cycles for 12 cycles, followed by onartuzumab or placebo until disease progression. Coprimary endpoints were progression-free survival (PFS) in intent-to-treat (ITT) and MET-positive populations. The target hazard ratio (HR) was 0.70 for patients in the ITT group and 0.60 in the MET-positive population. Secondary endpoints were overall survival (OS), overall response rate (ORR), and safety.
Overall, 123 patients were enrolled (n = 62 onartuzumab, n = 61 placebo). Median PFS was 6.77 versus 6.97 months for onartuzumab versus placebo, respectively (HR, 1.08; 95% confidence interval [CI], 0.71–1.63; p = .71). In the MET-positive population, median PFS was 5.95 versus 6.80 months, onartuzumab versus placebo (HR, 1.38; 95% CI, 0.60–3.20; p = .45). Median OS was 10.61 months for onartuzumab versus 11.27 months for placebo) (HR, 1.06, 0.64–1.75; p = .83). In the MET-positive population, median OS was 8.51 versus 8.48 months for onartuzumab versus placebo, respectively (HR, 1.12, 95% CI, 0.45–2.78; p = .80). ORR was 60.5% for the onartuzumab group and 57.1% for placebo. Grade 3–5 adverse events (AEs) were seen in 88.3% of patients receiving onartuzumab and in 78.3% of patients receiving placebo, with serious AEs in 55% and 40%, respectively.
The addition of onartuzumab to mFOLFOX6 in gastric cancer did not improve efficacy in an unselected population or in a MET immunohistochemistry-positive population.
Implications for Practice:
The YO28252 study demonstrated that the addition of the anti-MET agent onartuzumab to mFOLFOX6 for treatment of gastric cancer did not improve efficacy in an overall study population or those selected for positive MET status by immunohistochemistry. This highlights the importance of correctly selecting biomarkers for targeted therapies. A multivariate analysis suggested that MET positivity may still be prognostic for worse median overall survival in gastric cancer; therefore, it is important to continue investigation into the optimal approach to inhibit MET signaling in gastric cancer.
Introduction
Adenocarcinomas of the stomach and gastroesophageal junction (GEJ) have a high mortality rate, with approximately 1 million cases per year [1]. The current standards of care for advanced gastric cancer include first-line platinum- and fluoropyrimidine-based regimens such as capecitabine/cisplatin or leucovorin/fluorouracil (5-FU)/oxaliplatin (FOLFOX), taxane-based regimens such as docetaxel/5-FU/cisplatin, and irinotecan-based regimens. For gastric cancer overexpressing human epidermal growth factor-2 (HER2), trastuzumab plus chemotherapy is the current standard treatment [2, 3]. Despite these options, prognosis for advanced gastric cancer is still poor, with median overall survival (OS) of approximately 8–11 months [4]; therefore, there is an urgent need for new therapies.
The MET pathway represents a potential new target in oncology. Signaling through the MET pathway stimulates tissue repair and regeneration in normal tissue but can promote proliferation, survival, and metastasis in malignancies [5]. MET is expressed in a number of cancers, with MET overexpression as assessed by immunohistochemistry (IHC) shown in gastric cancer by Ha et al. [6]. Aberrant upregulation of the MET/hepatocyte growth factor (HGF) pathway is associated with poor prognosis in multiple malignancies, including gastric cancer [7], with MET overexpression by IHC being associated with poor survival in several studies [8]. In a study by Nakajima et al. [9], MET overexpression by IHC in gastric cancer patients was correlated with depth of tumor invasion, lymph node metastases, and poorer survival rates (all p < .001).
Onartuzumab is a fully humanized, monovalent, anti-MET antibody that inhibits HGF binding and receptor activation. Preliminary data from phase I/II studies indicated that MET-targeted agents, including rilotumumab and onartuzumab, are active in gastric cancer [10, 11]. In a phase I study of onartuzumab, one patient with gastric cancer with MET overexpression achieved complete radiographic response after four cycles of monotherapy [10]. The anti-MET agent rilotumumab reported median progression-free survival (PFS) of 5.7 months versus 4.2 months for placebo in a phase II trial [11]. Gastric cancer with MET amplification has also been shown to be responsive to the ALK/ROS/MET inhibitor crizotinib [12]. Together, these data provided evidence that MET is a potentially important target in advanced gastric cancer, and that onartuzumab may be a suitable treatment for tumors with aberrant signaling of the MET pathway. Therefore, the phase II YO28252 study (01590719; www.clinicaltrials.gov) was initiated to examine the efficacy and safety of onartuzumab in combination with mFOLFOX6 as first-line treatment for metastatic, HER2-negative gastric cancer. MET IHC expression as a biomarker of onartuzumab activity was also examined.
Patients and Methods
Study Design
YO28252 was a randomized, phase II, multicenter, double-blind, placebo-controlled study evaluating the safety and efficacy of onartuzumab plus mFOLFOX6 compared with placebo plus mFOLFOX6 in patients with histologically confirmed, metastatic HER2-negative gastric cancer that was not amenable to curative therapy. YO28252 was conducted in more than 30 sites across Australia, Korea, Singapore, Taiwan, Thailand, and the U.S. Eligible patients were randomized 1:1 by an interactive voice response system to receive onartuzumab (10 mg/kg) plus mFOLFOX6 (400 mg/m2 bolus and 2,400 mg/m2 intravenous 5-FU for 46–48 hours, 200 mg/m2 leucovorin, and 85 mg/m2 oxaliplatin for 2 hours) or placebo plus mFOLFOX6 in 2-week cycles for 12 cycles, followed by onartuzumab or placebo until disease progression. Patients were stratified by Lauren histologic subtype and prior gastrectomy.
Patients
Inclusion criteria were as follows: age ≥18 years with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1, with histologically confirmed, inoperable, metastatic adenocarcinoma of the stomach or GEJ. Tissue samples from the primary tumor site or metastatic sites were required for central MET and HER2 status assessment. Exclusion criteria included HER2-positive disease, previous chemotherapy for locally advanced or metastatic disease, or therapy targeting the MET pathway. Further exclusion criteria are detailed in the supplemental online Appendix.
Objectives
The coprimary endpoints were PFS in the intent-to-treat (ITT) population and in the MET-positive population (defined as those with ≥50% of tumor cells with moderate or strong intensity staining) using MET IHC scoring based on a 50% staining algorithm using the Ventana CONFIRM anti-total c-MET (SP44) IHC assay (supplemental online Fig. 1). We also examined an exploratory cutoff for MET positivity of >90% tumor cell staining [13]. Secondary endpoints included OS (in the ITT and MET-positive populations), overall response rate (ORR), and safety. Exploratory biomarker analysis investigated HGF tumor levels as a potential biomarker for efficacy outcomes.
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice and received the appropriate ethics or independent review board approval. Patients provided written informed consent to participate in the study.
Statistical Analysis
With a planned sample size of 120 patients and data cutoff after 84 PFS events observed, the target hazard ratios (HRs) were 0.70 in the ITT population and 0.60 in the MET-positive population. This trial was not planned to have adequate power to detect minimum clinically meaningful differences between the treatment arms. Therefore, formal hypothesis testing was limited because statistically negative outcomes do not necessarily rule out clinically significant treatment effects. P values were two-sided. Kaplan-Meier methodology was used to assess PFS and OS. Response Evaluation Criteria in Solid Tumors version 1.1 was used to evaluate tumor response every 6 weeks; ORR (partial or complete best overall response) was assessed by investigators. Safety was evaluated at every visit using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Results
Patients
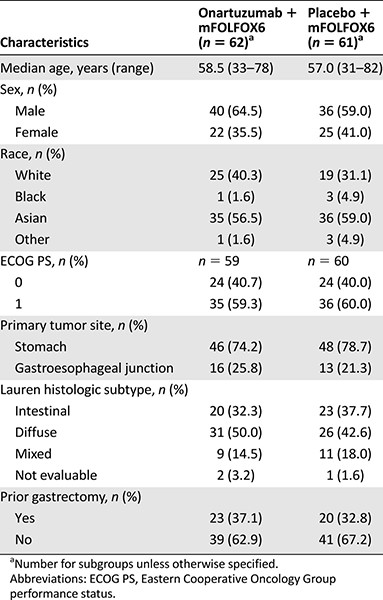
Between July 25, 2012, and May 29, 2013, a total of 123 patients were enrolled and randomly assigned to treatment (n = 62 to the onartuzumab arm; n = 61 to the placebo arm; supplemental online Fig. 2). The ITT population comprised all 123 patients who were randomized to treatment. Only patients who received at least 1 dose of study treatment were included in the safety population (n = 60 in each treatment arm). Baseline characteristics are shown in Table 1; there were no substantial differences between the two arms. MET expression is shown in supplemental online Table 1. Using the 50% cutoff, 28% of patients in the onartuzumab arm and 33% of patients in the placebo arm were included in the MET-positive population (IHC score 2+ or 3+).

Efficacy
Median follow-up was 9.2 months for the onartuzumab arm and 9.1 months for the placebo arm. A total of 30 patients from the onartuzumab arm and 33 patients from the placebo arm had completed 12 cycles of treatment at the data cutoff date of January 29, 2014, with median treatment durations of 5.6 months and 6.5 months, respectively. The median number of cycles completed was 11.0 for onartuzumab-treated patients and 13.5 for placebo-treated patients. Median dose intensity was 91.0% and 94.1%, respectively, for onartuzumab and placebo.
At the data cutoff date of January 29, 2014, the median PFS in the ITT population was 6.77 months in the onartuzumab arm versus 6.97 months with placebo (stratified HR, 1.08; 95% confidence interval [CI], 0.71–1.63; p = .71; Fig. 1A). In the MET-positive population (MET IHC 2+, 3+, 50% staining cut-off), median PFS was 5.95 months versus 6.80 months, respectively, for onartuzumab versus placebo (stratified HR, 1.38; 95% CI, 0.60–3.20; p = .45; Fig. 1B). The stratified HR for PFS in the MET-negative population (MET IHC 0 or 1+, 50% staining cut-off) was 0.99 (95% CI, 0.59–1.68; p = .98; median PFS, 7.03 months for onartuzumab and 7.72 months for placebo). PFS subgroup analyses were consistent with the ITT population (Fig. 1C). Exploratory PFS analyses by different MET-positive definitions (IHC 1+, 2+, 3+ vs. 2+, 3+) and by different MET staining cutoffs (50% vs. 90%) are shown in supplemental online Figure 3. Regardless of the definition of MET positivity or staining cutoff used, median PFS was not significantly different between the treatment arms. Multivariate analysis for PFS showed that ECOG PS (0 vs. 1, p = .004) and tumor location (stomach vs. GEJ, p = .0024) were independent prognostic factors for PFS (supplemental online Table 2).
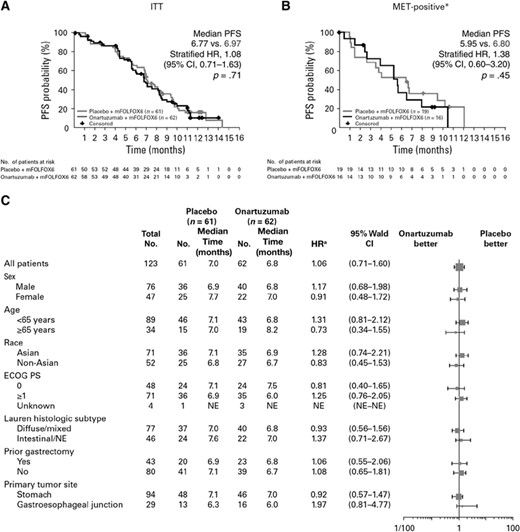
PFS study data. (A): The ITT population. (B): The MET-positive population. (C): Patient subgroups. *, 50% staining cutoff; a, unstratified analysis.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; ITT, intent-to-treat group; NE, not evaluable; PFS, progression-free survival.
At the time of the OS final analysis data cutoff, 34 patients (54.8%) from the onartuzumab arm and 30 patients (49.2%) from the placebo arm had died. Median OS in the ITT population was 10.61 months for onartuzumab versus 11.27 months for placebo (HR, 1.06; 95% CI, 0.64–1.75; p = .83; Fig. 2A). In the MET-positive population, median OS was 8.51 months versus 8.48 months, respectively, for onartuzumab and placebo (HR, 1.12; 95% CI, 0.45–2.78; p = .80; Fig. 2B). The stratified HR for OS in the MET-negative population was 1.09 (95% CI, 0.56–2.12; p = .79; median OS, 10.91 months for onartuzumab and 13.34 months for placebo). Multivariate analysis showed MET positivity was an independent poor prognostic factor for OS, as well as ECOG PS and prior chemotherapy (supplemental online Table 3).
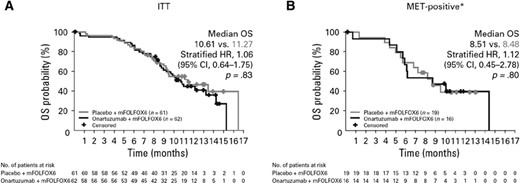
OS study data. (A): The ITT population. (B): The MET-positive population. *, staining cutoff.
Abbreviations: CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat group; OS, overall survival.
The ORR in the ITT population with baseline measurable disease was 60.5% (26 of 43; n = 4 complete responses) for the onartuzumab arm and 57.1% (24 of 42; n = 1 complete response) for placebo. This resulted in a disease control rate (ORR plus stable disease) of 81.4% and 83.3%, respectively.
HGF Biomarker Analysis
Baseline tumor HGF levels assessed by polymerase chain reaction (PCR; 25% upper PCR levels vs. 75% lower PCR levels) did not identify any biomarker activity for PFS or OS in the ITT population (supplemental online Fig. 4A) or in subgroups stratified by MET IHC status, ethnicity (Asian; supplemental online Fig. 4B), or primary tumor location (stomach; supplemental online Fig. 4C). The Subpopulation Treatment Effect Pattern Plots show the moving HR for different levels of HGF expression based on percentile. The graphs suggest that levels of HGF expression were not associated with better outcomes in any of the subgroups analyzed.
Safety
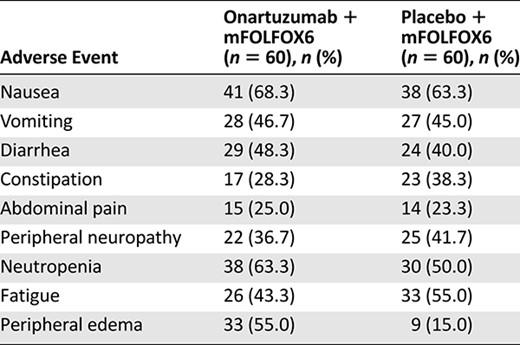
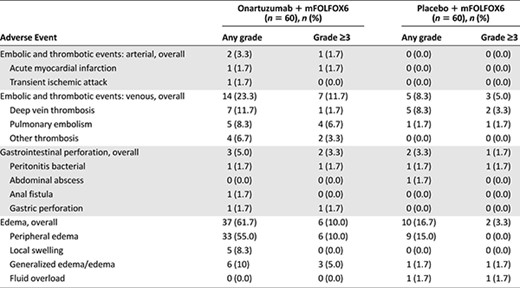
The most frequently reported (≥25%) adverse events (AEs) in both treatment arms (Table 2) included nausea (68.3% vs. 63.3%), vomiting (46.7% vs. 45.0%), and diarrhea (48.3% vs. 40.0%) for onartuzumab versus placebo, respectively. Related AEs were reported in 100% of patients who received onartuzumab and 93.3% of patients who received placebo. Grade 3–5 AEs were seen in 88.3% and 78.3% of patients in the onartuzumab and placebo arms, respectively, with serious AEs in 55% and 40% of patients, respectively. Grade 3–5 AEs observed more frequently with onartuzumab than with placebo (≥5% difference) included neutropenia (58% vs. 45%), thrombocytopenia (10% vs. 3%), peripheral edema (10% vs. 0%), and pulmonary embolism (7% vs. 2%). AEs of special interest, including edema and thrombotic events, are shown in Table 3. Four grade 5 AEs occurred in the onartuzumab arm (gastric perforation, septic shock, sepsis, and hypotension); 2 occurred in the placebo arm (congestive heart failure and cerebral hemorrhage).


Discussion
Aberrant upregulation of the MET pathway is associated with poor prognosis in gastric cancer [9]. Onartuzumab inhibits MET signaling by blocking ligand-dependent receptor activation. In this multicenter, randomized study, the addition of onartuzumab to first-line mFOLFOX6 for metastatic gastric cancer did not improve PFS in the ITT population (HR, 1.08) or in the MET-positive subgroup (IHC 2+, 3+; HR, 1.38). Secondary endpoints of improved OS and ORR with the addition of onartuzumab were also not achieved. These data suggest that inhibition of the HGF/MET pathway may be ineffective for the treatment of gastric cancer. The RILOMET-1 phase III study of HGF inhibitor rilotumumab for gastric cancer also reported negative results and closed prematurely because of lack of activity [14]. Circulating HGF levels have been explored in other tumor types as a potential biomarker for MET inhibitor efficacy [15], and high levels of circulating HGF were found in the patient who had a complete response in the phase I study [10]; however, HGF levels determined by PCR had no relationship with efficacy in this study. This suggests that alternative biomarkers are needed to define the optimal patient population for onartuzumab. The lack of further biomarker analysis in YO28252 may be considered a limitation and the small total numbers, particularly in the subgroup analyses, must be taken into account when reviewing the data presented.
The safety profile of onartuzumab was as expected, with edema, venous thromboembolism, and AEs leading to treatment discontinuation being more frequent in the onartuzumab arm than in the placebo arm. No new safety signals were observed.
Although onartuzumab is a potent, selective inhibitor of the MET signaling pathway, its addition to standard chemotherapy for gastric cancer did not improve efficacy in the ITT or MET-positive populations at increasingly stringent thresholds of MET-positive criteria. One consideration could be that the presence of chemotherapy could cause a negative interaction with onartuzumab, thereby counteracting any benefit from the addition of onartuzumab. However, based on the data available and the lack of efficacy of onartuzumab in other nonchemotherapy regimens, this appears unlikely. The YO28252 data suggest that either inhibition of MET signaling by a ligand-blocking antibody is not an appropriate strategy in gastric cancer, perhaps due to redundant mechanisms of pathway activation, or that MET IHC does not select appropriately for MET-driven tumors. The mode of action of onartuzumab may be worth considering, as both onartuzumab and rilotumumab are designed to inhibit ligand-dependent activation of MET signaling. These drugs, therefore, may be ineffective at inhibiting signaling from receptors with activating mutations. Adenosine triphosphate-competitive small molecules would be expected to inhibit ligand-independent MET receptor activity driven by mutations, as seen in promising response data from crizotinib in MET-amplified patients [13]. However, this hypothesis is linked with the identification of the corresponding predictive biomarker because receptor activation and amplification may point to different biology and response to different anti-MET therapies.
Alternative biomarkers to guide patient selection may be required to see the best results for onartuzumab in gastric cancer. One such option is MET amplification, which may be a more appropriate biomarker to select for tumors that will respond to MET inhibitors. Preliminary data for the MET inhibitor AMG337 showed that, in 10 patients with MET-amplified gastric cancer, 1 patient achieved a complete response and 4 had partial responses; however, further investigations have been terminated [16].
There are a number of reasons why the promising preclinical and phase I findings of onartuzumab perhaps did not translate to promising phase II data in gastric cancer. These include the increased patient population in phase II trials, alternative oncogenic signaling pathways that may not be blocked by onartuzumab, and the use of IHC as the chosen MET biomarker. The multivariate analysis suggested that MET positivity may still be prognostic for worse median OS in gastric cancer; therefore, it is important to continue investigation into the optimal approach to inhibit MET signaling in gastric cancer.
Conclusion
The YO28252 study demonstrated that the addition of onartuzumab to mFOLFOX6 in metastatic gastric cancer did not provide a clinical benefit in either the ITT population or in patients with MET-positive tumors as defined by MET IHC.
Acknowledgments
This study was supported by F. Hoffmann-La Roche Ltd. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
This study was previously presented at the American Society of Clinical Oncology Gastrointestinal Cancers meeting in 2015.
Author Contributions
Provision of Study Material or Patients: Manish A. Shah, Jae-Yong Cho, Iain B. Tan, Niall C. Tebbutt, Chia-Jui Yen, Yoon-Koo Kang
Data Analysis and Interpretation: Manish A. Shah, Jae-Yong Cho, Iain B. Tan, Niall C. Tebbutt, Chia-Jui Yen, Alice Kang, David S. Shames, Lilian Bu, Yoon-Koo Kang
Manuscript Writing: Manish A. Shah, Jae-Yong Cho, Iain B. Tan, Niall C. Tebbutt, Chia-Jui Yen, Alice Kang, David S. Shames, Lilian Bu, Yoon-Koo Kang
Final Approval of Manuscript: Manish A. Shah, Jae-Yong Cho, Iain B. Tan, Niall C. Tebbutt, Chia-Jui Yen, Alice Kang, David S. Shames, Lilian Bu, Yoon-Koo Kang
Disclosures
Manish A. Shah: Lilly (C/A), Lilly, Merck, Sanofi-Aventis, Berg Pharma, Genentech (RF); Iain B. Tan: Advisory board (C/A); Niall C. Tebbutt: Roche (C/A); Alice Kang: Roche China Holding (E); David S. Shames: Genentech (E), UT Southwestern (IP), Roche Holdings (OI); Yoon-Koo Kang: Roche, Novartis, Bayer, Sanofi-Aventis, Taiho, Ono (C/A), Bayer, Sanofi-Aventis, Roche, Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board



