-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer Wu, Jennifer Ng, Paul J. Christos, Alec S. Goldenberg, Joseph Sparano, Max W. Sung, Howard S. Hochster, Franco M. Muggia, Chronic Thalidomide and Chemoembolization for Hepatocellular Carcinoma, The Oncologist, Volume 19, Issue 12, December 2014, Pages 1229–1230, https://doi.org/10.1634/theoncologist.2014-0283
Close - Share Icon Share
Abstract
Transcatheter arterial chemoembolization (TACE) has been used to curtail tumor vasculature and delay tumor progression in hepatocellular carcinoma (HCC). We conducted a phase I trial to evaluate the efficacy and toxicity of thalidomide when combined with TACE in patients with advanced HCC.
Between June 2000 and November 2003, 56 patients with unresectable HCC and amenable to TACE were enrolled. The starting dose of thalidomide was 200 mg/day and was escalated every 2 weeks as tolerated to a maximum dose of 1,000 mg/day. Dose reductions and discontinuation were determined by toxicity. TACE was performed 4 weeks after initiation of thalidomide therapy and repeated as necessary.
Overall, 47 and 55 patients were evaluable for response and toxicity, respectively; the median dose of thalidomide given was 200 mg/day. Three patients (6.38%) patients achieved complete responses, whereas 10 (21.3%) had partial responses, for an overall response rate of 27.7%, and 27 (57.5%) had stable disease. Median progression-free survival was 7 months (95% confidence interval [CI]: 5–10 months), and median OS was 21 months (95% CI: 16–28 months) (Fig. 1). Fatigue and lethargy (49.1%), constipation (47.3%), and nausea (43.6%) were common. Grade 3–4 toxicities consisted mostly of increased aspartate aminotransferase (43.6%) and elevated alanine aminotransferase (38.2%) (Table 1).
Thalidomide and TACE were commonly associated with nonhematologic side effects, with fatigue and constipation being prominent. With a lack of clear therapeutic benefit, this combination is unlikely to be pursued for HCC.
摘要
背景. 经导管肝动脉化疗栓塞术(TACE)在肝细胞癌(HCC)的治疗中用于减少肿瘤血供和延缓肿瘤进展。我们开展了一项 I 期试验,旨在评价沙利度胺联合 TACE 在进展期 HCC 患者中的有效性和毒性。
方法. 2000 年 6 月∼ 2003 年 11 月,共入组 56 例不可手术切除且适于行 TACE 的 HCC 患者。沙利度胺的起始剂量为 200 mg/天,视耐受情况每 2 周上调一次剂量,直至最大剂量 1000 mg/天。根据毒性决定下调给药剂量和停药。TACE 于开始沙利度胺治疗 4 周后实施,并且按需重复实施。
结果. 总体而言,47 例患者可评估治疗反应,55 例可评估毒性;中位沙利度胺剂量为 200 mg/天。3 例患者(6.38%)达到完全缓解,10 例(21.3%)达到部分缓解,总缓解率为 27.7%。27 例患者(57.5%)疾病稳定。中位无进展生存为 7 个月[95% 可信区间(CI):5 ∼ 10 个月],中位总生存(OS)为 21 个月(95%CI:16 ∼ 28 个月)(图 1)。疲乏及嗜睡(49.1%)、便秘(47.3%)和恶心(43.6%)较为常见。3/4 级毒性事件主要包括天门冬氨酸氨基转移酶水平升高(43.6%)和丙氨酸氨基转移酶水平升高(38.2%)(表 1)。
结论. 沙利度胺和 TACE 多与非血液学不良事件相关,主要是疲乏和便秘。由于缺乏明显的治疗获益,这一联合方案不太推荐用于 HCC 治疗。The Oncologist 2014;19:1229–1230
Author Summary
Discussion
The primary endpoint to evaluate the efficacy of the combined regimen of thalidomide and transcatheter arterial chemoembolization (TACE) was overall survival (OS) (Fig. 1). The median OS rate at 1 year with TACE alone was estimated to be 50% (range: 31%–62%) based on randomized controlled studies published prior to 1999 [1–4]. Lengthening of OS by 50% (i.e., from 50% to 75%) due to thalidomide in addition to TACE was sought. In order for the study to have 90% power to detect such a 50% increase in median survival at a one-sided α = 0.1 level, data from 68 patients were needed, and we planned to enroll 75 patients to meet the required number of evaluable subjects at a 10% dropout rate. The survival distribution would be estimated using the Kaplan-Meier method, with corresponding 95% confidence intervals.
![Overall survival. The sample includes 56 patients, with 40 deaths. Median overall survival (OS) was 21 months (95% confidence interval [CI]:16–28 months). The 1-year OS rate was 70.8% (95% CI: 54.7%–82.0%). The 3-year OS rate was 27.2% (95% CI: 14.3%–41.9%).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/oncolo/19/12/10.1634_theoncologist.2014-0283/2/m_oncolo_19_12_1229_f1.jpeg?Expires=1750454326&Signature=XWN6htCtM3ikpwKiWJ5lLEIc84ga4cuEeyz3OAUso005zwex3fiH8odNae9otL~Z4P-yPmpjnIOWzMAAnGWDQc7oCkKQ~oNQTYEJhzGnXmLWOKTVFotDBLfcHFctcDxnNjg0RKThBNPbyr4LfE-sT7isoxmHxfw6b8K6WlJi7Hju7fBiX6ZcWBNbpEZciZpgvjhYujMuu2dJ9jRV8gZGTB5r5riOcjTNI-iN1YshVF~XQK4AatqpJ3QbzvBTxWp1pEsV4ChK~5V3QLLo~SUM1MFIKRDVJEaLU2gtA5PklUiQiJjrFSxbDUjX441URSSqbGnmqWETvpyNhKoTDpiWug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Overall survival. The sample includes 56 patients, with 40 deaths. Median overall survival (OS) was 21 months (95% confidence interval [CI]:16–28 months). The 1-year OS rate was 70.8% (95% CI: 54.7%–82.0%). The 3-year OS rate was 27.2% (95% CI: 14.3%–41.9%).
Adverse events due to thalidomide only or a combination of thalidomide and transcatheter arterial chemoembolization (all grades >10%)
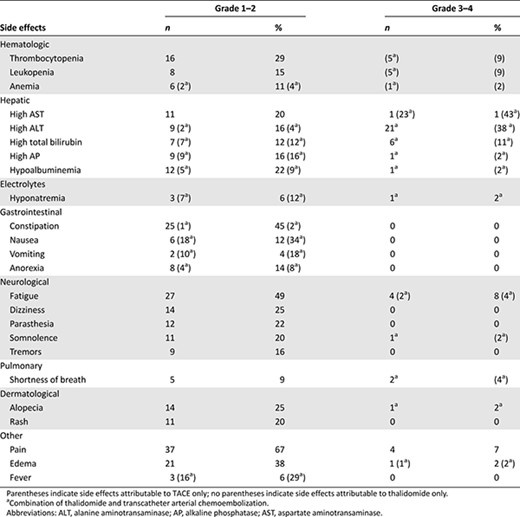
Adverse events due to thalidomide only or a combination of thalidomide and transcatheter arterial chemoembolization (all grades >10%)

In our study using the combination of thalidomide and TACE, the OS rate at 1 year was only 71%, short of the expected 75%, and therefore did not reach our target. In addition, the secondary objective of progression-free survival (PFS) was only 25%, a disappointing number that is lower than 42% for historical TACE alone [1]. Moreover, our study yielded a relatively low response rate (RR) of 27.7%, much lower than the historical RR of TACE alone, which can be up to 35% [2]. Consequently, this study was terminated. The final median OS of 21 months in this study was slight longer than that shown in the meta-analysis performed by Marelli et al., in which the median OS was 18 months [2].
Acknowledgments
We thank Dr. Leonard Liebes and Dr. Matthew Volm for their contribution to this study.
Author disclosures and references available online.
Access the full results at: Wu-14-283.theoncologist.com
ClinicalTrials.gov Identifier: NCT00006198 Sponsor(s): National Cancer for Research Resources
Principal Investigator: Alec S. Goldenberg IRB Approved: Yes



