-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Castellano, Emilio Bajetta, Ashok Panneerselvam, Stephen Saletan, Walter Kocha, Thomas O'Dorisio, Lowell B. Anthony, Timothy Hobday, RADIANT‐2 Study Group, Everolimus Plus Octreotide Long‐Acting Repeatable in Patients With Colorectal Neuroendocrine Tumors: A Subgroup Analysis of the Phase III RADIANT‐2 Study, The Oncologist, Volume 18, Issue 1, January 2013, Pages 46–53, https://doi.org/10.1634/theoncologist.2012-0263
Close - Share Icon Share
Abstract
The incidence of colorectal neuroendocrine tumors (NETs) is increasing, and patients with this disease have particularly poor prognoses. Treatment options are limited, and survival times have not improved in the past decade.
A post hoc analysis of the efficacy and tolerability of everolimus plus octreotide long‐acting repeatable (LAR) was conducted in patients with colorectal NETs enrolled in the phase III RAD001 in Advanced Neuroendocrine Tumors, Second Trial (RADIANT‐2) study. The primary endpoint (progression‐free survival [PFS]), secondary endpoints (including objective response rate), and safety were assessed.
Patients with colorectal NETs receiving everolimus plus octreotide LAR had a significantly longer median PFS (29.9 months; n = 19) than did those receiving placebo plus octreotide LAR (6.6 months; n = 20). Everolimus plus octreotide LAR treatment also significantly reduced the risk for disease progression (hazard ratio: 0.34; 95% confidence interval: 0.13–0.89; p = .011). Although no objective responses were observed, tumor shrinkage was more frequently noted in the everolimus plus octreotide LAR arm than in the placebo plus octreotide LAR arm (67% vs. 37%, respectively). The combination of everolimus plus octreotide LAR was generally well tolerated by patients with colorectal NETs; rash and stomatitis were the most commonly reported adverse events.
Everolimus plus octreotide LAR treatment had significant benefits and improved outcomes for patients with advanced colorectal NETs compared with placebo plus octreotide LAR treatment. Results of this exploratory analysis are consistent with those reported from the RADIANT‐2 primary analysis. These findings support additional investigations of everolimus plus octreotide LAR in patients with colorectal NETs.
摘要
引言. 结直肠神经内分泌肿瘤(NET)发病率不断增加,患者预后特别差。该病治疗方法局限,近十年来,其生存时间未见延长。
方法. 在III期RAD001晚期神经内分泌肿瘤第二次试验(RADIANT‐2)入组的结直肠NET患者中,开展一项post hoc分析,评估依维莫司联合长效缓释(LAR)奥曲肽的有效性及耐受性。评估主要终点[无进展生存(PFS)]、次要终点(包括客观缓解率)和安全性。
结果. 接受依维莫司联合奥曲肽LAR的结直肠NET患者,中位PFS(29.9个月, n =19)显著长于安慰剂联合奥曲肽LAR组(6.6个月, n =20)。依维莫司联合奥曲肽LAR同时显著降低了疾病进展的风险(风险比:0.34;95%可信区间:0.13∼0.89; P=0.011)。虽然未见客观缓解,但依维莫司联合奥曲肽LAR组的肿瘤退缩多于安慰剂联合奥曲肽LAR组(分别为67% vs. 37%)。此外,依维莫司联合奥曲肽LAR耐受性良好;皮疹和口腔炎是最常见的不良事件。
结论. 与安慰剂联合奥曲肽LAR组相比,晚期结直肠NET患者接受依维莫司联合奥曲肽LAR后获益显著且转归改善。本项探索性分析结果与RADIANT‐2总体分析结果相一致。这些结果支持在结直肠NET患者中进一步开展依维莫司联合奥曲肽LAR的研究。
Implications for Practice:
The incidence of neuroendocrine tumors (NETs) originating in the colon or rectum is increasing, and patients diagnosed with these tumors have a poor prognosis. The RADIANT‐2 study explored the efficacy of the mammalian target of rapamycin inhibitor everolimus used in combination with the somatostatin analog octreotide long‐acting repeatable (LAR) in patients with NETs and symptoms of carcinoid syndrome. The comparison population received placebo plus octreotide LAR. This article reports the results of subanalyses of a group of patients who had primary colorectal NETs. Patients with colorectal NETs who received everolimus plus octreotide LAR had a significantly longer median survival without disease progression (progression‐free survival) of 29.9 months (n = 19) compared with those who received placebo plus octreotide LAR (6.6 months; n = 20). Although only a small subset of patients enrolled in the RADIANT‐2 study had colorectal NETs, these findings support additional everolimus plus octreotide LAR studies in these patients.
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of tumors that originate from different primary sites, including the gastrointestinal tract, lung, pancreas, and thymus [1]. Approximately 50% of patients have advanced NETs at diagnosis, and 65% die within 5 years [1, 2]. Survival rates vary by primary site and are higher in patients with well‐differentiated tumors than in those with poorly differentiated tumors and with locoregional versus distant disease [1].
Although uncommon among NET subtypes, the incidence of colorectal NETs appears to be increasing despite a decline in the overall incidence of colorectal cancer [3–9]. Colorectal NETs account for a small proportion of these neoplasms and are diagnosed at a rate of 1.06 per 100,000, although the true incidence may be higher because some tumors may be considered benign and are not reported [1, 4].
The apparent increased incidence of colorectal NETs is troubling because this form of NET carries a particularly poor prognosis. Most (62.2%) patients with colorectal NETs present with advanced disease [3]; the median survival duration for patients with metastatic colorectal NETs is 10.4 months [10] compared with 27 and 65 months for patients with NETs of the pancreas and small intestine, respectively [1]. The 3‐year survival rate for patients with advanced colorectal NETs is 13% [10], although this may be overestimated because this study included a large number of patients with poorly differentiated tumors. Regardless, this is lower than for other types of NETs [3, 5] and has not improved in the past decade [1]. Among colorectal NETs, localized NETs of the rectum and colon have similar median survival (290 vs. 261 months, respectively) [1]. Prognosis is increasingly poorer for NETs of the colon compared with rectal NETs because the tumors metastasize regionally (90 vs. 36 months, respectively) and distally (22 vs. 5 months, respectively) [1].
One reason for this poor prognosis is the lack of effective treatment options. Although localized submucosal colorectal NETs may be resectable, there are few effective therapies for advanced disease and a lack of well‐controlled randomized clinical studies to guide evidence‐based practice. Traditional chemotherapeutic and biotherapeutic agents, with their low efficacy and significant toxicity, have limited usefulness for patients with advanced colorectal NETs [4]. Indeed, given the few effective therapies, the North American Neuroendocrine Tumor Society recommends patients with colorectal NETs be enrolled in clinical trials with investigational agents [4].
Recently identified targets for NET treatment include autocrine activation of the mammalian target of rapamycin (mTOR) pathway [11]. mTOR is associated with insulin‐like growth factor (IGF)‐1 mediated regulation of cell growth, proliferation, and angiogenesis and has been implicated in NET pathogenesis [12–14]. The somatostatin analog octreotide downregulates IGF‐1 [15] and improves hormone‐related symptoms associated with NET [16]. In addition, octreotide long‐acting repeatable (LAR) prolongs time to progression in patients with therapy‐naïve metastatic midgut NETs [17–19]. Everolimus, an mTOR inhibitor [14], demonstrated promising antitumor activity for advanced NETs in two phase II studies [20, 21]. In a phase III study in patients with pancreatic NETs (RADIANT‐3), patients receiving everolimus showed a significant increase in progression‐free survival (PFS) compared with placebo (11.0 vs. 4.6 months; hazard ratio [HR]: 0.35; 95% confidence interval [CI]: 0.27–0.45; p < .001) [22]. It appears likely, therefore, that the combination of everolimus plus octreotide LAR may increase antitumor activity by targeting both upstream and downstream components of the mTOR pathway [23].
In the large, double‐blind, placebo‐controlled, phase III, RAD001 in Advanced Neuroendocrine Tumors, Second Trial (RADIANT‐2) trial, the combination of everolimus plus octreotide LAR provided a clinically meaningful 5.1‐month increase in median PFS compared with placebo plus octreotide LAR (16.4 months vs. 11.3 months; HR: 0.77; 95% CI: 0.59–1.00; one‐sided log‐rank test, p = .026) in patients with advanced NETs and a history of secretory symptoms [24]. This exploratory post hoc subgroup analysis was conducted to assess the efficacy and safety of everolimus plus octreotide LAR in patients with colorectal primary NETs.
Methods
The study design has been previously described [24]. In brief, RADIANT‐2 (ClinicalTrials.gov NCT00412061)—a prospective, multicenter, randomized, double‐blind, placebo‐controlled, phase III study—was initiated to compare the efficacy and safety of everolimus plus octreotide LAR and placebo plus octreotide LAR in 429 patients with advanced NETs and a history of secretory symptoms [24]. For this subanalysis, data were evaluated from patients with diagnoses of colorectal primary NETs (Fig. 1).
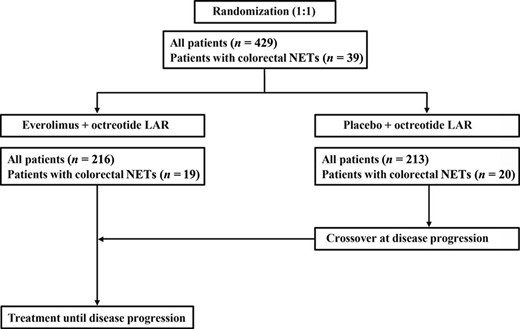
RADIANT‐2 study design for patients with colorectal neuroendocrine tumors.
Abbreviations: LAR, long‐acting repeatable; NET, neuroendocrine tumors.
Protocol approval was sought and obtained from the independent ethics committee or institutional review board at each participating study site. The study was conducted in accordance with Good Clinical Practice guidelines, applicable local regulations, and the Declaration of Helsinki. Written informed consent was obtained from each patient before enrollment.
Patient Eligibility
Briefly, eligible patients were 18 years of age or older, had low‐ or intermediate‐grade advanced (unresectable/metastatic) NETs, radiologic documentation of disease progression within the past 12 months, and history of secretory symptoms (diarrhea, flushing, or both) [24]. For this subanalysis, documentation of the primary tumor site as colonic or rectal was required. Primary tumor sites were determined by the local investigator and reported on the case report form; they were not independently confirmed.
Treatment Administration
Patients were randomly assigned one‐to‐one to receive oral everolimus (10 mg/day) or matching placebo in conjunction with intramuscular octreotide LAR (30 mg) every 28 days. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. Dose adjustments were permitted for patients unable to tolerate the protocol‐specified dosing schedule.
Patients randomly assigned to placebo plus octreotide LAR were permitted to cross over to open‐label everolimus plus octreotide LAR at the time of investigator determination of disease progression according to Response Evaluation Criteria In Solid Tumors (RECIST), version 1.0 [25].
Efficacy and Safety Assessments
The primary endpoint was PFS, defined as the time from randomization to the first documentation of disease progression or death from any cause, determined by an adjudicated central review process according to RECIST version 1.0 [25]. Investigator‐assessed PFS was performed as a key supportive analysis.
Secondary endpoints were confirmed objective response rate, biomarker assessment, overall survival, and safety. Serum chromogranin A (CgA) and 24‐hour urine samples for 5‐hydroxyindoleacetic acid (5‐HIAA) were collected at baseline and analyzed by a central laboratory using Quest Diagnostics enzyme‐linked immunosorbent assay (ELISA) for CgA and high‐performance liquid chromatography (HPLC) for 5‐HIAA. Tumors were measured at baseline and every 12 weeks thereafter. Safety assessments included monitoring of adverse events (AEs) and vital signs, physical examinations every 4 weeks, and regular monitoring of hematologic and clinical biochemistry levels. AEs were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 [26].
Statistical Analysis
Kaplan‐Meier method was used to analyze PFS; treatment groups were compared with log‐rank tests, and HRs were obtained using Cox proportional hazards model.
Results
A total of 429 patients were randomly assigned to receive everolimus plus octreotide LAR (n = 216) or placebo plus octreotide LAR (n = 213; Fig. 2). Overall, 39 patients with colonic/rectal primary tumor sites were enrolled in the study and included in this analysis; of those, 19 received everolimus plus octreotide LAR, and 20 received placebo plus octreotide LAR (Fig. 1).
![RADIANT‐2 CONSORT diagram. Reprinted from Pavel ME, Hainsworth JD, Baudin E et al. Everolimus plus octreotide long‐acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT 2): A randomised, placebo‐controlled, phase 3 study. Lancet 2011;378:2005–2012 [24], with permission from Elsevier.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/oncolo/18/1/10.1634_theoncologist.2012-0263/2/m_oncolo_18_1_46_f2.jpeg?Expires=1747897489&Signature=XTTz9EQMd-5vbtRQAeOZmzubW6luiHF5AfWAwUqKl~fjlF6kLyTOUwAYXAJw65JO0yc7mo5t4yJ37O~llBOOF0Af3l3MDxAUhfrbInTEZxUwM7Ob2UQEO2LioRTz4-GnMjz5GH5-UjcVdAaHTT~dEWYryvTraORdAEIDa8VGFf8rS9lcp7ObBILAUvQdwi3YtlJj4cOiZhDcist9BNcz663YDriiV0NJGZuOUFc-wRElaM7idxrYQ6rWfb5LavcJTYswS0~gz05hu7EGqZgXKBxYA0c44D1Jn5G9Q9GZ28maqQEkhuLdF0OlMv-VK7XPEUdzVGoJeOom8DLVaw91Aw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
RADIANT‐2 CONSORT diagram. Reprinted from Pavel ME, Hainsworth JD, Baudin E et al. Everolimus plus octreotide long‐acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT 2): A randomised, placebo‐controlled, phase 3 study. Lancet 2011;378:2005–2012 [24], with permission from Elsevier.
Patient characteristics are summarized in Table 1. More patients with colorectal primary tumors were younger than 65 years (74%) compared with the overall RADIANT‐2 population (67%). Somatostatin use before enrollment appeared greater in patients with colorectal NETs who received placebo plus octreotide LAR (90%) compared with the overall RADIANT‐2 population (71%). Approximately 90% of patients with colorectal primary NETs had a history of tumor‐related symptoms (35/39). Slightly more than 40% of patients with colorectal tumors had received initial diagnoses >5 years earlier and <10% had received diagnoses within the past 6 months, in line with the overall population.
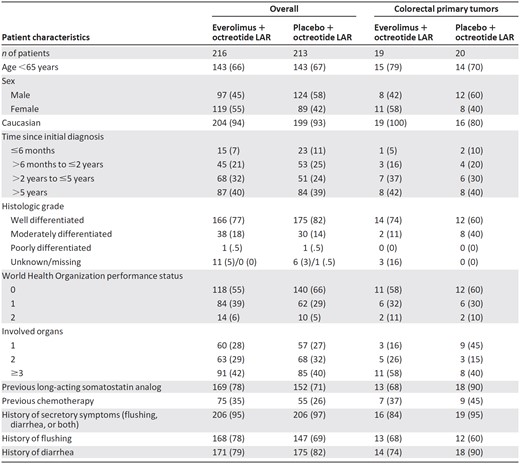
Data are reported as n (%).
Abbreviations: CgA, chromogranin A; HIAA, hydroxyindoleacetic acid; LAR, long‐acting repeatable; ULN, upper limit of normal.

Data are reported as n (%).
Abbreviations: CgA, chromogranin A; HIAA, hydroxyindoleacetic acid; LAR, long‐acting repeatable; ULN, upper limit of normal.
Among patients with colorectal primary tumors, 14 of 19 patients (74%) in the everolimus plus octreotide LAR group and 14 of 20 patients (70%) in the placebo plus octreotide LAR group had colonic NETs; the remaining 5 and 6 patients in these groups had rectal NETs, respectively. A higher proportion of patients receiving everolimus plus octreotide LAR than those receiving placebo plus octreotide LAR were women (58% vs. 40%), had well‐differentiated tumors (74% vs. 60%), and had ≥2 involved organs (84% vs. 65%; Table 1). In addition, within the colorectal NET subgroup, more patients in the placebo plus octreotide LAR arm (40%) than in the everolimus plus octreotide LAR arm (11%) had moderately differentiated NETs; previous somatostatin analog use was also higher in the placebo plus octreotide LAR arm (90%) than in the everolimus plus octreotide LAR arm (68%). However, the numbers were too small to determine whether the differences among treatment arms in the colorectal NET subgroup had an impact on study endpoints.
Median CgA levels were 110.3 ng/mL for all patients with colorectal NETs (overall population: 173.9 ng/mL) and 165.0 ng/mL (range: 2.1–2,570.0 ng/mL) and 61.0 ng/mL (range: 4.4–1,760.0 ng/mL) for the everolimus plus octreotide LAR and the placebo plus octreotide LAR arms, respectively. Median 5‐HIAA levels were 69.1 μmol/day for all patients with colorectal NETs (overall population: 16.8 μmol/day) and 69.1 μmol/day in both the everolimus plus octreotide LAR (range: 8.4–733.4 μmol/day) and the placebo plus octreotide LAR arms (range, 4.7–2,679.3 μmol/day).
Treatment
In the colorectal NET subgroup, median treatment duration was 35 weeks for everolimus plus octreotide LAR compared with 28 weeks for placebo plus octreotide LAR. Mean relative dose intensities were 0.90 for everolimus and 1.05 for octreotide LAR (both arms), with at least one dose adjustment or temporary interruption required by 63.2% and 45.0% of patients in the everolimus plus octreotide LAR and the placebo plus octreotide LAR groups, respectively. In the everolimus plus octreotide LAR group, 12 (63.2%) patients had at least one everolimus dose adjustment, and 4 (21.1%) patients had at least one octreotide LAR dose adjustment; of these, eight (42.1%) and two (1.5%) adjustments were due to AEs. In the placebo plus octreotide LAR group, 9 (45.0%) patients had at least one adjustment in placebo dose, eight of which (4.0%) were due to AEs; two (1.0%) patients had at least one adjustment in octreotide LAR dose, both due to AEs.
Efficacy
At the time of data cutoff, only 37% of patients with colorectal NETs in the everolimus plus octreotide LAR arm had disease progression compared with 75% of patients in the placebo plus octreotide LAR arm (Table 2). Median PFS time was increased by 23.3 months in patients with colorectal NETs who received everolimus plus octreotide LAR compared with those treated with placebo plus octreotide LAR (29.9 months vs. 6.6 months, respectively; Fig. 3). In this population, everolimus plus octreotide LAR treatment was associated with a 66% reduction in the estimated risk for disease progression (HR: 0.34; 95% CI: 0.13–0.89, p = .011).
Progression‐free survival by central review in patients with colorectal primary tumors

Hazard ratio (95% CI): 0.34 (0.13–0.89); p = .011.
Abbreviations: CI, confidence interval; LAR, long‐acting repeatable.
Progression‐free survival by central review in patients with colorectal primary tumors

Hazard ratio (95% CI): 0.34 (0.13–0.89); p = .011.
Abbreviations: CI, confidence interval; LAR, long‐acting repeatable.
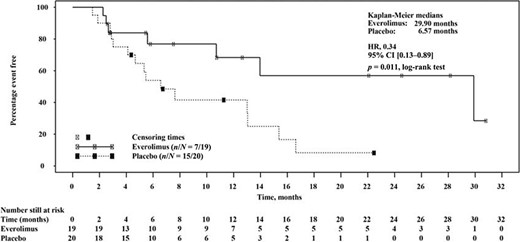
Kaplan‐Meier plot of progression‐free survival times in patients with colorectal neuroendocrine tumors per adjudicated central radiology review. Hazard ratio is obtained from unadjusted Cox model. Both treatments are combined with octreotide long‐acting repeatable. p values were obtained from one‐sided log‐rank tests.
Abbreviations: CI, confidence interval; HR, hazard ratio; LAR, long‐acting repeatable.
When analyzed only for patients with colon as the primary tumor site (n = 14 for both treatment arms), there was a trend similar to that of the overall colorectal population. Median PFS time was 29.9 months in the everolimus plus octreotide LAR arm and 13.0 months in the placebo plus octreotide LAR arm. Treatment with everolimus plus octreotide LAR was associated with a 61% reduction in the risk for disease progression (HR: 0.39; 95% CI: 0.12–1.29, p = .056), although the numbers of patients in each arm were small.
In the overall RADIANT‐2 population, no complete responses were observed according to RECIST version 1.0 criteria. No patients with colorectal primary tumors experienced partial response (≥30% decrease in sum of diameters). However, some extent of tumor shrinkage was reported for 67% of patients with colorectal NETs who received everolimus plus octreotide LAR therapy compared with 37% of those who received placebo plus octreotide LAR therapy (Fig. 4).
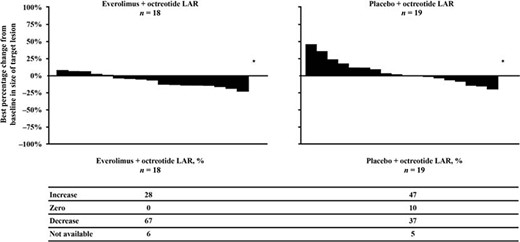
Best percentage change from baseline in target lesion size of colorectal tumors.
Abbreviation: LAR, long‐acting repeatable.
Safety
Among patients with colorectal NETs, most reported AEs were mild (grade 1/2) and were consistent with the safety profile of everolimus plus octreotide LAR in the overall RADIANT‐2 study population (Table 3). Overall, 30 of 39 patients reported drug‐related AEs, irrespective of relationship to study drug (100% in the everolimus plus octreotide LAR arm and 55.5% in the placebo plus octreotide LAR).
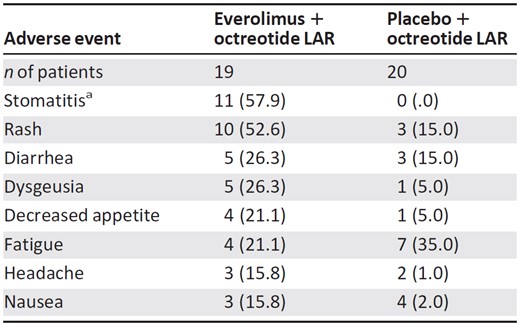
Data are reported as n (%).
aIncludes stomatitis, mouth ulceration, and lip ulceration.
Abbreviation: LAR, long‐acting repeatable.

Data are reported as n (%).
aIncludes stomatitis, mouth ulceration, and lip ulceration.
Abbreviation: LAR, long‐acting repeatable.
The most frequent (>5 patients) all‐grade, treatment‐related AEs reported in patients receiving everolimus plus octreotide LAR were rash (n = 10), stomatitis (n = 9), diarrhea (n = 5), and dysgeusia (n = 5; Table 3). These results are similar to those reported for the overall population [24]. Grade 3/4 AEs were reported by six (31.6%) patients in the everolimus plus octreotide LAR arm and five (25.0%) patients receiving placebo plus octreotide LAR. The most common grade 3/4 AE (reported in ≥3 patients) was diarrhea (n = 2). These AEs were reversible with dose reduction or discontinuation.
In the colorectal NET population, 16 deaths occurred throughout the duration of the study and follow‐up (four during the double‐blind phase). One death occurred in the everolimus plus octreotide LAR arm, and three deaths occurred in the placebo plus octreotide LAR arm. The patient in the everolimus plus octreotide LAR arm died of disease progression 22 days after the last dose of everolimus. Death was not believed to have resulted from study medication. Patients in the placebo plus octreotide LAR arm died of liver failure due to disease progression (143 days after the start of medication), cardiorespiratory arrest (grade 4; 468 days after the start of medication), and disease progression (9 days after discontinuation from the study because of disease progression). The other 12 patients died >28 days after discontinuation from the study and were not considered to have been on treatment.
Discussion
Surveillance, Epidemiology, and End Results data show that many patients with NETs present with advanced disease; patients with colorectal NETs have particularly poor prognoses [1]. Because of the aggressive nature of colorectal NETs, patient survival may be measured in months rather than years; this discouraging outlook has not noticeably improved in the past decade [1, 3, 5]. To date, treatment options for these patients have been limited; low efficacy and significant toxicity have been reported with the use of chemotherapeutic and biotherapeutic agents [4]. The need is urgent for new treatment options that reduce the risk for disease progression and improve survival rates without compromising patient well‐being [27, 28].
In this exploratory subgroup analysis of the RADIANT‐2 study, patients with colorectal NETs treated with the combination of everolimus plus octreotide LAR had significantly longer median PFS times than those patients treated with placebo plus octreotide LAR (29.9 vs. 6.6 months). Treatment with everolimus plus octreotide LAR was also associated with significant (66%) reduction in the estimated risk for disease progression or death (HR: 0.34; 95% CI: 0.13–0.89; p = .011). Data reported here indicate that PFS times were similar between the patients with colonic primary tumor site and the combined colorectal NET population (29.9 months).
Although no objective responses were observed per RECIST criteria, tumor shrinkage was more frequently observed in patients with colorectal NETs who received everolimus plus octreotide LAR than in those patients who received placebo plus octreotide LAR (67% vs. 37%, respectively). The combination of everolimus plus octreotide LAR was well tolerated by patients with colorectal NETs. Most reported AEs were low grade, and, as expected, the most commonly reported AEs were rash and stomatitis.
As previously reported [24], in the primary RADIANT‐2 study, everolimus plus octreotide LAR delayed disease progression by 5.1 months in patients with advanced NETs compared with placebo plus octreotide LAR (as assessed by central review). In the primary study, everolimus plus octreotide LAR was associated with a 23% reduction in the estimated risk for progression (HR: 0.77; 95% CI: 0.59–1.00; p = .026). The PFS benefit of everolimus plus octreotide LAR treatment was further supported by local investigator assessment (p = .018). The primary study results also indicated that everolimus plus octreotide LAR was generally well tolerated, with treatment‐related AEs being primarily grade 1/2 in severity. AEs and hematologic changes were consistent with the known individual safety profiles of everolimus and octreotide LAR in cancer.
Data from the current subgroup analysis indicate that, compared with the overall RADIANT‐2 population, patients with colorectal primary tumors treated with everolimus plus octreotide LAR had longer median PFS times (29.9 months vs. 16.4 months), whereas patients treated with placebo plus octreotide LAR had shorter median PFS times (6.6 months vs. 11.3 months) and poorer prognoses. This exploratory analysis showed treatment with everolimus plus octreotide LAR resulted in a greater reduction in risk for disease progression among the colorectal subgroup compared with the overall population (61% vs. 23%, respectively), although the number of patients with colorectal NETs was small. In both the overall and the colorectal NET populations, tumor shrinkage (non‐RECIST) occurred more frequently in the everolimus plus octreotide LAR arm than in the placebo plus octreotide LAR arm (75% and 67% vs. 45% and 37%, respectively). The safety and tolerability of everolimus plus octreotide LAR therapy among patients with colorectal NETs are consistent with the safety observations from the overall RADIANT‐2 study population.
Somatostatin analogs such as octreotide and lanreotide are efficacious in the symptomatic relief of gastrointestinal and pancreatic NET in locoregional and metastatic disease [16, 29]. Octreotide LAR has also demonstrated efficacy in the first‐line treatment of metastatic midgut NETs by prolonging time to progression and has become the standard of care for these patients [17, 18]. However, the use of everolimus plus octreotide LAR therapy to target both upstream and downstream components of the mTOR pathway may have greater efficacy than a single agent [11]. In support of this, studies have demonstrated that the addition of everolimus to octreotide LAR improves efficacy in the management of advanced NET [21, 24].
Some study limitations should be noted. This subgroup analysis was based on a small patient population (n = 39); as such, minor imbalances between treatment groups in patient characteristics and prognostic factors (e.g., sex, histologic grading, number of involved organs, previous somatostatin use, and history of secretory symptoms) might have had a disproportionate impact on study outcomes, including PFS assessment. The method of selecting patients for this subanalysis might also have affected the results because identifying the primary tumor site as colorectal was based on information provided at the enrolling site, without independent central confirmation. As such, further prospective studies are needed to confirm the data reported.
In conclusion, in this exploratory analysis of the RADIANT‐2 colorectal population, the combination of everolimus plus octreotide LAR demonstrated a significant 23.3‐month prolongation of median PFS time and a greater frequency of tumor shrinkage compared with placebo plus octreotide LAR. These findings suggest that such combination therapy in patients with advanced colorectal NETs—a subgroup of patients with a particularly poor prognosis—may be efficacious and that everolimus may be beneficial in treating these patients in the clinic. Additional research exploring mTOR pathway inhibition as a therapeutic strategy in this specific histologic subtype of NET is warranted.
Acknowledgments
This work was supported by Novartis Pharmaceuticals. The study was designed by the academic investigators and representatives of the sponsor. Data were collected with the use of the sponsor's data management systems and analyzed by the sponsor's statistical team. All authors contributed to the interpretation of data and subsequent writing, reviewing and amending of the manuscript; the first draft was prepared by the first author and a medical writer funded by Novartis. All authors vouch for the accuracy and completeness of the reported data and attest that the study conformed to the protocol and statistical analysis plan.
We thank the participating patients and their families, as well as the global network of research nurses, trial coordinators, and operations staffs, for their contributions, and we thank the investigators who participated in this trial, including the following: Australia: P. Mainwaring, D. Morris, T. Price, D. Wyld; Belgium: I. Borbath, M. Peeters, E. Van Cutsem, J.‐L. Van Laethem; Canada: W. Kocha, R. Letourneau, J. Maroun, M. Moore, L. Sideris, L. Siu; Czech Republic: O. Louthan, J. Novotny, P. Vitek; Finland: M. Välimäki; France: E. Baudin, G. Cadiot, J.A. Chayvialle, S. Dominguez, B. Goichot, R. Guimbaud, C. Lepage, P. Rougier, P. Ruszniewski, J.F. Seitz, M. Ychou; Germany: C. Auernhammer, M. Bläker, D. Hörsch, M.E. Pavel, J. Schmoll, B. Wiedenmann; Greece: G. Kaltsas, G. Nikou; Israel: D. Gross, I. Shimon; Italy: E. Bajetta, N. Fazio, G. Luppi, S. Ricci, F. Santeusanio, S. Siena, P. Tomassetti; Netherlands: W.W. De Herder, E. De Vries; Slovakia: S. Kinova; Spain: J. Sastre; Sweden: B. Eriksson, D. Granberg, K. Öberg; Turkey: N. Aykan, S. Yalcin; United Kingdom: A. Grossman; United States: B. Baltz, C. Becerra, J. Beck, J. Brell, P. Byeff, T. Cartwright, E. Chiorean, A. Cohn, P. Conkling, S. DelPrete, L. DeMarco, T. Dragovich, J.R. Eckardt, G. Eckhardt, W. Edenfield, P. Engstrom, J.D. Hainsworth, J. Hamm, J. Hecht, B. Hellerstedt, K. Holen, L. Kvols, T. Lairmore, N. LoConte, D. Loesch, A. Maniam, A. Montero, M. Morse, N. Neubauer, J. Picus, M. Pipas, R. Raju, D. Richards, R. Ruxer, T. Ryan, D. Slater, D. Smith, L. White Jr, S. Williamson, E.M. Wolin, J.C. Yao.
Writing assistance (in the form of copyediting, editorial assistance, and table and figure development) was provided by Sally‐Anne Mitchell, Ph.D., and Jennifer M. Kulak, Ph.D., of ApotheCom. Financial support for writing assistance was provided by Novartis Pharmaceuticals.
Author Contributions
Conception and design: Daniel Castellano, Stephen Saletan, Lowell B. Anthony
Provision of study materials or patients: Daniel Castellano, Walter Kocha, Lowell B. Anthony, Timothy Hobday
Collection and/or assembly of data: Daniel Castellano, Emilio Bajetta, Walter Kocha, Lowell B. Anthony, Timothy Hobday
Data analysis and interpretation: Daniel Castellano, Ashok Panneerselvam, Stephen Saletan, Thomas O'Dorisio, Lowell B. Anthony
Manuscript writing: Daniel Castellano, Ashok Panneerselvam, Stephen Saletan, Walter Kocha, Timothy Hobday
Final approval of manuscript: Daniel Castellano, Emilio Bajetta, Ashok Panneerselvam, Stephen Saletan, Walter Kocha, Thomas O'Dorisio, Lowell B. Anthony, Timothy Hobday
Disclosures
Ashok Panneerselvam: Novartis (E); Stephen Saletan: Novartis (E); Walter Kocha: Novartis, Pfizer (C/A); Novartis (RF); Lowell B. Anthony: Novartis, Helsinn, Pfizer (C/A); Pfizer, Novartis, Amgen (H); Imcline, Novartis (RF); Timothy Hobday: Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



