-
PDF
- Split View
-
Views
-
Cite
Cite
Mridul Datta, Gary G. Schwartz, Calcium and Vitamin D Supplementation During Androgen Deprivation Therapy for Prostate Cancer: A Critical Review, The Oncologist, Volume 17, Issue 9, September 2012, Pages 1171–1179, https://doi.org/10.1634/theoncologist.2012-0051
Close - Share Icon Share
Abstract
After completing this course, the reader will be able to:
Describe the prevalence of bone loss with androgen deprivation therapy for prostate cancer.
Discuss the possible increased risk of cardiovascular disease and of advanced prostate cancer with high calcium intake.
CME This article is available for continuing medical education credit at CME.TheOncologist.com
Loss of bone mineral density is an unintended consequence of androgen deprivation therapy in men with prostate cancer. Supplementation with calcium and/or vitamin D in these men seems logical and is advocated by many lay and professional groups.
We reviewed guidelines for calcium and vitamin D supplementation and the results of clinical trials of calcium and vitamin D supplementation on bone mineral density in men with prostate cancer undergoing androgen deprivation therapy.
Whether supplementation of men undergoing androgen deprivation therapy with calcium and/or vitamin D results in higher bone mineral density than no supplementation has not been tested. The results of 12 clinical trials show that, at the doses commonly recommended, 500–1,000 mg calcium and 200–500 IU vitamin D per day, men undergoing androgen deprivation lose bone mineral density.
The doses of calcium and vitamin D that have been tested are inadequate to prevent loss of bone mineral density in men undergoing androgen deprivation therapy. In light of evidence that high levels of dietary calcium and calcium supplement use are associated with higher risks for cardiovascular disease and advanced prostate cancer, intervention studies should evaluate the safety as well as the efficacy of calcium and vitamin D supplementation in these men.
Introduction
Although typically considered a disease of postmenopausal women, osteoporosis has become a significant problem among older men, particularly men undergoing androgen deprivation therapy (ADT), the mainstay of treatment for metastatic prostate cancer. Hussain et al. [1] reported prevalences of osteopenia and osteoporosis of 37% and 42%, respectively, in men with prostate cancer, even prior to initiating ADT. Moreover, ADT is used increasingly in men with localized prostate cancer. For example, over a 12-year period (1989–2001), Cooperberg et al. [2] reported a tripling in the use of ADT (from 4.6% to 14.2%) in men with low-risk prostate cancer. A decrease in bone mineral density (BMD) occurs as early as 6 months after initiating ADT [3, 4]. In men undergoing ADT not receiving calcium or vitamin D, Morote et al. [5] reported losses in BMD at 12 months of 3.76% in the total hip and 4.8% in the lumbar spine. The incidence of new vertebral fractures in men receiving ADT is reported to be 1.9% within 12 months [6], increasing to 3.3%–4.9% within 24 months [6, 7]. One man in 10 undergoing ADT develops a new fracture within 24 months [7]. Fractures adversely affect survival and quality of life [8], and significantly increase the cost of medical care [9]. Thus, reducing the burden of osteoporosis in men undergoing ADT is a pressing oncologic need.
Efforts to prevent osteoporosis in women typically involve supplementation with calcium and vitamin D [10, 11]. Similar recommendations have been made for osteoporosis prevention among men undergoing ADT [12–25]. This paper reviews the evidential basis for these recommendations.
Calcium and Vitamin D Intake Among Men: The Recommended and the Real
Dietary recommendations for calcium and vitamin D are set by the Institute of Medicine (IOM) [26]. The 2010 IOM Recommended Dietary Allowances (RDAs) for calcium and vitamin D for men aged 51–70 years are 1,000 mg calcium and 600 IU vitamin D. For men aged >70 years, the recommendation is 1,200 mg calcium and 800 IU vitamin D. The tolerable upper intake limits (ULs) (the level below which a nutrient can be ingested without risking adverse effects) for both age groups are 2,000 mg for calcium and 4,000 IU for vitamin D [26]. Potential adverse outcomes of excess calcium intake include constipation, hypercalciuria, hypercalcemia, vascular and soft tissue calcification, nephrolithiasis, and prostate cancer. Potential adverse outcomes of excess vitamin D intake include higher fall and fracture risk, hypercalciuria, hypercalcemia, and higher risks for prostate cancer and all-cause mortality [27].
Data on the nutritional status of the U.S. population are collected by the National Health and Nutrition Examination Survey (NHANES) [28]. In NHANES 2003–2006, men aged 51–70 years consumed 204 IU ± 12 IU vitamin D per day (5.1 μg/day ± 0.3 μg/day) and men aged >70 years consumed 224 IU ± 16 IU per day (5.6 μg/day ± 0.4 μg/day) of vitamin D. Forty percent of men aged 51–70 years and 49% of men aged ≥71 years reported using vitamin D supplements daily. Approximately 31% of men in both age groups exceeded the recommended intake of vitamin D [29]. Few foods other than fatty fish and fish liver oil provide meaningful levels of vitamin D, and consequently fortified foods are a major dietary contributor of vitamin D [29]. More than 90% of circulating vitamin D is derived from exposure to sunlight [30].
Data from the NHANES 2003–2006 indicated that dietary calcium intake for men aged 51–70 years was 951 mg ± 19 mg per day and was 871 mg ± 25 mg per day for men aged >70 years. Forty percent of men aged 51–70 years and 43% of men aged ≥71 years reported consuming a multivitamin and mineral supplement in the past month [31]. Fifty-one percent of men aged 51–70 years and 56% of men aged ≥71 years reported using calcium supplements daily [29]. Total calcium intake (diet plus supplements) exceeded the recommended intake in 64% of men aged 31–50 years, in 32% of men aged 51–70 years, and in 31% of men aged ≥71 years [29].
The recent trend of calcium “fortification” of foods has added to the average exposure to dietary calcium. For example, one serving of some breakfast cereals provides 200–1,000 mg calcium and 8 oz of fortified orange juice may contain 500 mg calcium. Other contributors include fortified bread (150–200 mg calcium per slice) and antacids—for example, TUMS® (GlaxoSmithKline, Philadelphia, PA) and Rolaids® (McNeil PPC Inc., Fort Washington, PA), which contain 200–400 and 200–270 mg elemental calcium per tablet, respectively [32, 33]. The consumption of calcium-fortified foods and the use of calcium supplements have made it easy to exceed the UL for calcium unknowingly. This has contributed to the resurgence of the milk-alkali syndrome, recently renamed the “Rolaids-yogurt” or “calcium-alkali” syndrome [34, 35]. The calcium-alkali syndrome is characterized by progressive hypercalcemia, systemic alkalosis, and renal insufficiency and is the third most common cause of hospitalization for hypercalcemia (after hyperparathyroidism and hypercalcemia of malignancy) [34, 35].
Review of Clinical Practice Guidelines
We reviewed clinical practice guidelines for bone health in men with prostate cancer undergoing ADT by searching the Web sites of professional organizations, including the American Urological Association (AUA) [36], the American Society of Clinical Oncology (ASCO) [37], the National Comprehensive Cancer Network (NCCN) [38], the European Association of Urology (EAU) [39], and the National Guideline Clearinghouse [40]. We could not identify guidelines from ASCO, AUA, or the National Guideline Clearinghouse. The EAU guidelines on prostate cancer do not specify recommendations but state that calcium supplementation is protective [39]. The NCCN cites the guidelines of the National Osteoporosis Foundation [41] and recommends 1,200 mg calcium and 800–1,000 IU of vitamin D daily [38].
We also informally searched the Internet for recommendations proffered by patient support organizations and by university Web sites. We used the names of known prostate cancer support organizations and the search terms “calcium,” “vitamin D,” “prostate cancer,” and “recommendations.” Representative advice included the following. The Prostate Cancer Foundation advises limiting daily calcium intake to <1,500 mg for preventing prostate cancer but gave no recommendations for bone health during ADT [42]. The Us Too International Prostate Cancer Education & Support Network recommends that men aged >50 years consume ≥1,200 mg calcium daily through their diet and 400–800 IU vitamin D [43]. According to the Prostate Cancer Research Institute, prostate cancer patients should supplement their diet with 1,200–1,500 mg calcium (preferably with calcium citrate) and 2,000 IU Vitamin D [44]. The Bone and Cancer Foundation makes similar recommendations (1,000–1,500 mg calcium and 800–1,200 IU vitamin D daily) [45]. Conversely, the Mayo Clinic advises maintaining calcium intake below the UL (i.e., 2,000 mg/day) [46]. The Harvard School of Public Health advises men not to exceed the RDA for calcium and to limit the intake of calcium supplements to 500 mg if adequate dietary calcium intake cannot be maintained [47]. Reports of a positive association between calcium supplementation and higher cardiovascular risk (see below) prompted the American Society for Bone and Mineral Research to issue a statement of caution regarding “potential cardiovascular risks associated with calcium supplements” [48].
Reviews of the actual practice of calcium supplementation in men undergoing ADT have been published by several groups. The prevalence of physician-recommended calcium supplementation is in the range of 8.7%–50% [49–52]. For example, in a survey of Canadian urologists and radiation oncologists (n = 170), 50% recommended calcium supplements to men with normal BMD starting ADT [50].
Clinical Trial Evidence
Given that calcium and vitamin D supplements are widely consumed by healthy men and are widely recommended to men undergoing ADT, we evaluated the clinical trial evidence for a skeletal benefit of calcium and vitamin D supplementation in men with prostate cancer undergoing ADT. We performed PubMed searches using the medical subject heading terms: “clinical trial,” “prostatic neoplasm,” “calcium,” “calcium, dietary,” “vitamin D,” and “25-hydroxyvitamin D.” We searched the reference lists of the publications that we identified for additional papers and used the Cited Reference Search feature of Web of Science to find related clinical trials. Because several trials that used calcium and/or vitamin D referred to these treatments as “placebo” in the abstract, we retrieved all papers listed as clinical trials and examined the text for details of the comparison groups. We limited our search to papers in English. Trials that did not include calcium or vitamin D (e.g., [7]) or that did not measure BMD were excluded. We also excluded one trial with poor accrual and limited statistical power [53].
We found no trials that addressed the role of calcium and/or vitamin D supplements versus no supplements. However, numerous trials used calcium and vitamin D supplements as a comparison group to compare the effects of other drugs, for example, bisphosphonates, on BMD in men with prostate cancer. The effects observed in the comparison groups in these trials constitute “before–after” data on the effectiveness of calcium and vitamin D supplementation in preserving BMD in men currently undergoing ADT. The results of the 12 evaluable trials are summarized in Table 1.
Change in BMD in men undergoing ADT receiving calcium and vitamin D supplementation alone or with placebo
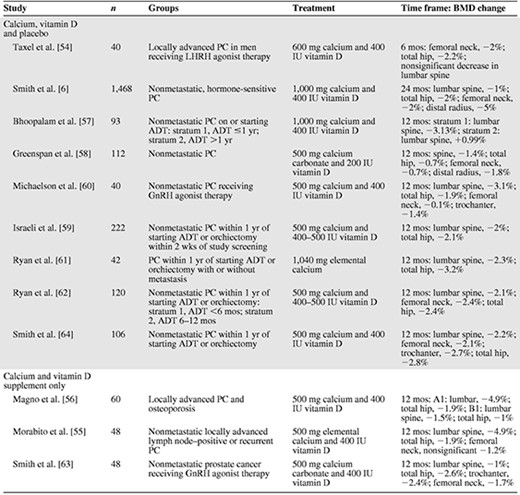
Abbreviations: ADT, androgen deprivation therapy; BMD, bone mineral density; GnRH, gonadotropin-releasing hormone; LHRH, luteinizing hormone releasing hormone; PC, prostate cancer.
Change in BMD in men undergoing ADT receiving calcium and vitamin D supplementation alone or with placebo

Abbreviations: ADT, androgen deprivation therapy; BMD, bone mineral density; GnRH, gonadotropin-releasing hormone; LHRH, luteinizing hormone releasing hormone; PC, prostate cancer.
In a 6-month randomized double-blinded, placebo-controlled trial in 40 men undergoing luteinizing hormone-releasing hormone agonist therapy, Taxel et al. [54] evaluated risedronate plus 600 mg calcium and 400 IU vitamin D versus an “identical placebo” plus 600 mg calcium with 400 IU vitamin D. Significant decreases in BMD in the femoral neck (−2%) and total hip (−2.2%) and a nonsignificant decrease in BMD in the lumbar spine were observed in the placebo plus calcium and vitamin D (Ca–D) group. The bone resorption markers N-telopeptide and C-telopeptide increased significantly (21% and 55%, respectively) in this group [54].
Smith et al. [6] compared the human monoclonal antibody denosumab with placebo in men undergoing ADT for nonmetastatic, hormone-sensitive prostate cancer. Participants in both the intervention and placebo groups (n = 1,468) were instructed to consume ≥1,000 mg calcium and ≥400 IU vitamin D daily. At the end of 24 months, BMD in the lumbar spine, total hip, femoral neck, and distal third of the radius decreased by 1%, 2%, 2%, and 5%, respectively, in the placebo plus Ca–D group [6].
Morabito et al. [55] evaluated the bisphosphonate neridronate in men (n = 48) who received bicalutamide. Men in both the intervention and control groups received 500 mg elemental calcium and 400 IU vitamin D3. The experimental group (n = 24) received 25 mg neridronate monthly. At the end of 12 months, participants treated with calcium and vitamin D only (Ca–D) showed significant increases in the bone turnover markers urinary deoxypyridinoline and serum bone alkaline phosphatase. The Ca–D only group showed a significant decrease in BMD in the lumbar spine (−4.9%) and total hip (−1.9%) [55]. Magno et al. [56] evaluated neridronate with two types of ADT, using a similar design and follow-up (12 months). Participants in both the intervention and control groups were supplemented with 500 mg calcium and 400 IU vitamin D. Participants treated with Ca–D only (n = 60) lost BMD in the lumbar spine (group 1, −4.9%; group 2, −1.5%) and total hip (group 1, −1.9%; group 2, −1.0%) and showed an increase in bone turnover markers [56].
Bhoopalam et al. [57] studied the percent change in lumbar spine, total hip, right and left hip, and femoral neck BMD in prostate cancer patients stratified by duration of ADT. Participants in stratum 1 received ADT for ≤1 year and participants in stratum 2 received ADT for >1 year. Participants in both the “placebo” (not further defined by these authors) and intervention (4 mg zoledronic acid) groups received 1,000 mg calcium and 400 IU vitamin D. Men in the placebo plus Ca–D group in stratum 1 showed a 3.1% decrease in BMD in the lumbar spine. Men in the placebo plus Ca–D group in stratum 2 showed an increase in BMD in the lumbar spine of 0.99%. Patients in the placebo plus Ca–D group in stratum 1 also showed a decrease in both the left and right hip and left and right femoral neck BMD. Placebo plus Ca–D group participants in stratum 2 showed a decrease in the left and right hip, but a slight increase in both the left and right femoral neck [57]. Greenspan et al. [58] enrolled 112 men with nonmetastatic prostate cancer to receive either alendronate with 500 mg calcium carbonate and 200 IU vitamin D or matching placebo with 500 mg calcium carbonate and 200 IU vitamin D. Men in the placebo plus Ca–D group lost 1.4% BMD in the posterior–anterior spine, 0.7% in the total hip, 0.7% in the femoral neck, and 1.8% in the one third distal radius [58]. Additionally, increases in urinary (N-telopeptide) and serum (C-telopeptide, N-terminal propeptide, and bone-specific alkaline phosphatase) bone turnover markers and a significant decrease in osteocalcin were observed in this group [58].
Israeli et al. [59] enrolled 222 men with nonmetastatic prostate cancer within 1 year of starting ADT to evaluate the effects of zoledronic acid with 500 mg calcium and a multivitamin containing 400–500 IU vitamin D versus placebo with 500 mg calcium and a multivitamin containing 400–500 IU vitamin D. They reported a 2% decrease in lumbar spine BMD and 2.1% decrease in total hip BMD in patients in the placebo plus Ca–D group. These men also showed a significant increase in both N-telopeptide (42%) and bone-specific alkaline phosphatase (16%) [59]. In 40 men with nonmetastatic prostate cancer receiving a gonadotropin-releasing hormone agonist, Michaelson et al. [60] reported a significant loss in BMD in the placebo plus Ca–D group (500 mg calcium and 400 IU vitamin D). BMD was lost in the lumbar spine (−3.1%), trochanter (−1.4%), total hip (−1.9%), and femoral neck (−0.1%). Placebo plus Ca–D group participants also showed increases in N-telopeptide (10%) and bone-specific alkaline phosphatase (15%) [60].
Ryan et al. [61] evaluated the effectiveness of zoledronic acid plus 1,040 mg elemental calcium versus placebo plus 1,040 mg elemental calcium, administered every 3 months, on BMD and bone turnover markers in men with hormone-sensitive prostate cancer with and without metastasis (n = 42). Participants in the placebo plus calcium group (without scintigraphic evidence of metastatic bone disease in the hip and spine) experienced losses in BMD in the femoral neck (−3.2%) and lumbar spine (−2.2%) [61]. In a second study, Ryan et al. [62] evaluated the effect of zoledronic acid versus placebo (normal saline) on BMD and bone turnover markers in men with nonmetastatic prostate cancer (n = 120) after initiating ADT. Daily supplements of 500 mg calcium and a multivitamin providing 400–500 IU vitamin D were recommended to both groups. BMD decreased significantly in the femoral neck (−2.4%), total hip (−2.4%), and lumbar spine (−2.1%) in the placebo plus Ca–D group. Bone-specific alkaline phosphatase and N-telopeptide levels also increased significantly [62].
Smith et al. [63] examined the effect of raloxifene in men receiving gonadotropin-releasing hormone agonist for prostate cancer (n = 48). Participants in the intervention and control groups received 500 mg calcium carbonate and 400 IU vitamin D from a multivitamin. In the Ca–D only group, BMD decreased in the lumbar spine (−1.0% ± 0.6%), total hip (−2.6% ± 0.7%), trochanter (−2.4% ± 0.8%), and femoral neck (−1.7% ± 0.6%). The bone turnover markers serum amino-terminal propeptide of type I collagen (13.9% ± 11.1%) and urinary deoxypyridinoline (10.3% ± 5.4%) increased [63]. In another study, Smith et al. [64] compared the effectiveness of zoledronic acid plus 500 mg calcium and 400 IU vitamin D with placebo plus 500 mg calcium and 400 IU vitamin D daily in men with nonmetastatic prostate cancer (n = 106) initiating gonadotropin-releasing hormone agonist therapy with or without an antiandrogen. Placebo plus Ca–D group participants showed significant losses in BMD in the lumbar spine (−2.2%), femoral neck (−2.1%), trochanter (−2.7%), and total hip (−2.8%) [64].
In summary, (to our knowledge) no trial has evaluated the effects of calcium or vitamin D versus no supplementation on BMD in men undergoing ADT. Thus, no definitive statement can be made regarding the adequacy and effectiveness of calcium and vitamin D in preventing ADT-related bone loss. However, the results from 12 clinical trials in prostate cancer patients undergoing ADT clearly indicate that calcium supplementation of ∼500–1,000 mg and vitamin D supplementation of 200–500 IU is inadequate to prevent BMD loss. The percent loss in BMD in the lumbar spine from these studies is summarized in Figure 1.
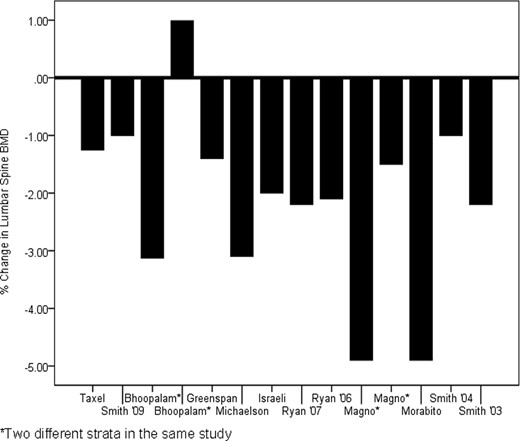
Percent change in lumbar spine bone mineral density (BMD) within the comparison groups (supplemented with 500–1,000 mg calcium and 200–500 IU vitamin D) reported in different clinical trials.
Vitamin D and Calcium Supplementation and Non-Skeletal Disease Risk
Vitamin D and Prostate Cancer
Interest in the role of vitamin D in prostate cancer was stimulated in 1992 by the demonstration that mortality rates for prostate cancer in the U.S. were inversely related to the availability of sunlight and by the subsequent demonstration of vitamin D receptors in human prostate cells [65, 66]. Normal human prostate cells synthesize the vitamin D hormone that exerts prodifferentiating and antiproliferative effects on prostate cells [67]. The results of many epidemiologic studies have shown that exposure of individuals to ultraviolet light, the major source of vitamin D, appears to protect against prostate cancer [68]. However, the results of prospective serological studies have been mixed, with some studies paradoxically reporting a higher prostate cancer risk associated with high levels of serum 25-hydroxyvitamin D [68, 69]. Despite unambiguous evidence that vitamin D reduces prostate cancer growth in the laboratory setting (e.g., [70]), concern about risk enhancement with high levels of vitamin D led the IOM to include prostate cancer among the potential adverse effects of high vitamin D intake. The apparent disparate effects of high doses of vitamin D in intact men, in contrast to the laboratory setting, may be related to the physiologic effects of vitamin D, for example, in stimulating intestinal calcium absorption.
Calcium and Prostate Cancer
Calcium supplements are recommended because they are presumed to be beneficial, or at least are presumed to be harmless. The latter presumption may be incorrect. The subject of calcium intake and prostate cancer risk has generated a large literature [71–76] whose origins lie in ecologic (group level) studies showing a correlation between the prostate cancer mortality rate and per capita consumption of dairy products [77]. These ecologic studies were followed by epidemiologic studies of calcium and dairy intake in individuals, which are the subject of several recent meta-analyses [75, 78, 79]. For example, the World Cancer Research Fund/American Institute for Cancer Research concluded that foods containing calcium are a probable cause of prostate cancer [78]. Similarly, the Agency for Health Care Research and Quality noted that three of the four cohort studies rated “A” for methodological quality show a significantly greater risk for prostate cancer with diets high in calcium [79]. For example, in the Health Professionals Study, Giovannucci et al. [80] reported significantly higher risks for advanced and fatal cancer at calcium intakes of 1,500–1,999 mg/day. The risk was greatest at intakes >2,000 mg/day. Dietary calcium and calcium supplements were each associated with greater risk.
Although many epidemiologic studies have identified calcium as the probable dietary factor associated with this higher risk, it was rarely possible to separate the effects of calcium from those of other components of dairy foods (e.g., insulin-like binding proteins and estrogen) that have been implicated in conferring a greater prostate cancer risk [71]. However, compelling evidence implicating calcium per se comes from a study of Singapore Chinese. The Singapore Chinese diet is extremely low in dairy but contains calcium in foods such as soy and green vegetables. Butler et al. [81] observed a greater risk for prostate cancer among Singapore Chinese men in the highest quartile of calcium consumption. (Note that the calcium intake was quite low by western standards—median intake, 659 mg/day.)
The mechanism(s) linking a high dietary intake of calcium to a higher risk for prostate cancer is unclear. However, a potential mechanism involves the effects of dietary calcium increasing the level of ionized calcium in blood [82, 83]. Prostate cancer cells express both the calcium-sensing receptor [84] and calcium-dependent voltage-gated channels [85]. Stimulation of these receptors by extracellular calcium increases prostate cancer cell growth in vitro and in vivo. It is noteworthy in this regard that, in a large case–control study of African-American men, significantly greater risks for advanced prostate cancer were found among men with diets high in calcium and among men with genotypes associated with high intestinal calcium absorption [86]. These data are consistent with the findings from two (but not all [87]) prospective studies showing that higher prediagnostic levels of total serum calcium and of ionized calcium, within the normal reference ranges, predict death from prostate cancer [88, 89].
Calcium Supplementation and Cardiovascular Disease
Calcium supplements also have attracted concern as a possible contributor to the risk for cardiovascular disease [90–95] (see recent reviews [90, 92, 96, 97]). For example, in a meta-analysis of randomized, placebo-controlled trials of calcium supplements, Bolland et al. [91] reported a 30% greater risk for myocardial infarction (MI) in individuals who consumed ≥500 mg/day of calcium supplements (pooled relative risk, 1.27; 95% confidence interval [CI], 1.01–1.59). In a reanalysis of data from the Women's Health Initiative [98], 1,000-mg calcium supplements, with or without 400 IU vitamin D, led to a ∼25% higher risk for MI [99]. Sambrook et al. [100] reported significantly higher all-cause mortality and a trend toward more deaths from MIs in a group of elderly men and women (n = 602) who received 600 mg calcium supplementation daily and spent about 30–40 minutes in the sunshine, versus a control group and those who spent only 30–40 minutes in the sunshine but received no supplemental calcium. Analyzing data from a large prospective study, the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition, Li et al. [101] reported that participants consuming calcium supplements had double the risk for an MI (hazard ratio, 2.39; 95% CI, 1.12–5.12).
The results of studies of dietary calcium and cardiovascular risk are consistent with the results of studies of serum calcium. For example, Fraser et al. [95] combined data from three NHANES surveys and reported that a higher serum calcium level was significantly associated with cardiovascular risk factors involving lipid and glucose metabolism. Impaired glucose tolerance is an independent risk factor for cardiovascular disease and is associated with a higher serum calcium level independently of age and other factors [102]. Jorde et al. [93] evaluated total serum calcium levels in Norwegian men (n = 12,895) and women (n = 14,293) and reported a significant positive associations between the total serum calcium level and systolic and diastolic blood pressure and serum total and high-density lipoprotein cholesterol levels. The total serum calcium level was a significant predictor of MI in men, with an odds ratio (OR) of 1.21 per 0.1 mmol/L greater total serum calcium level. Similar results linking an elevated serum calcium level with a higher risk for cardiovascular disease [91, 95, 99] and mortality [94, 103] have been reported by others. Although the mechanism(s) by which calcium increases the risk for an MI is incompletely understood, it is likely that these involve elevated levels of ionized serum calcium. Even a modestly higher total serum calcium level (0.02 mmol/L) within the normal reference range has been reported to be an independent risk factor for an MI in middle-aged men (OR, 2.33; 95% CI, 1.21–4.51) [94].
Discussion
Despite considerable published opinion advocating calcium and vitamin D supplementation in men undergoing ADT, we could find no clinical trial evidence evaluating calcium and vitamin D versus no calcium and/or no vitamin D on inhibiting BMD loss in men undergoing ADT. However, results from 12 clinical trials of other agents that employed calcium and vitamin D supplementation as the comparison group indicate that daily calcium supplementation of 500–1,000 mg and vitamin D intake of 200–500 IU above routine dietary intake is ineffective in preventing ADT-related BMD loss (Fig. 1).
A methodological problem affecting many of these trials (e.g., [57, 59, 64]) is that it is unclear how much elemental calcium (the biologically active fraction of calcium) was provided or consumed. The concentration of elemental calcium varies in different forms of calcium supplements; for example, it is 40% in calcium carbonate but only 21% in calcium citrate [104]. Furthermore, the contribution of calcium from the diet (apart from supplements) was rarely specified. Assuming a daily average intake of ∼900 mg/day (based on NHANES), participants likely consumed ∼1,500–2,000 mg calcium daily. Thus, the BMD loss suggests that the amount of calcium needed to prevent BMD loss in men during ADT is higher than the current UL for calcium, which was established for a healthy population. However, if calcium supplements are beneficial for preventing BMD loss in men undergoing ADT, which has yet to be demonstrated, their benefits must be weighed against their potential risks.
The consensus of observational epidemiologic studies is that dietary calcium increases the risk for prostate cancer, especially the risk for advanced and/or fatal disease [75, 78]. It may be questioned whether these epidemiologic studies, which often involve cancer incidence, are relevant to a population of men with prevalent cancer. However, the observation that calcium promotes prostate cancer invasion and metastasis in vivo [84, 85] supports the hypothesis that calcium supplementation increases prostate cancer aggressiveness in men with prevalent disease. It is noteworthy that recent data from the Health Professionals Follow Up Study indicate that men with prostate cancer with the highest intake of whole milk (a major source of calcium) experienced a significantly higher risk for prostate cancer progression [105].
To our knowledge, there is only one trial that evaluated the role of dietary calcium and prostate cancer incidence. Baron et al. [19] conducted a randomized trial of calcium supplementation and the risk for colorectal cancer. These data were examined to evaluate the association of calcium supplementation with prostate cancer risk. Six hundred seventy-two men were assigned to receive either 3 g calcium carbonate daily (1,200 mg elemental calcium) or placebo for 4 years and were followed for up to 12 years. The mean dietary intakes of calcium were comparable in the two groups. A (nonsignificant) lower incidence of prostate cancer was observed within 2 years of calcium supplementation. The risks for prostate-specific antigen (PSA) conversion (an increase in PSA from baseline to year 4, with 4 ng/mL and 6 ng/mL PSA used as cutoffs) were similar in the placebo and calcium-supplemented groups [19]. These data provide some reassurance that calcium supplements do not cause prostate cancer in the short term. However, these data are not strictly relevant to the role of calcium as a tumor promoter, the concern in men with extant prostate cancer.
The fact that men undergoing ADT are known to have a higher risk for cardiovascular disease [106], combined with the possibility that this risk is augmented by calcium supplementation, raises an additional concern about the safety of calcium supplementation. Because the major causes of mortality in men undergoing ADT are prostate cancer and cardiovascular disease [107], unless these diseases are the specific objects of study, it is conceivable that an increase in morbidity or mortality from these causes resulting from the use of calcium supplements could go unrecognized.
Conclusions
Calcium and vitamin D supplements are widely prescribed to men with prostate cancer undergoing ADT. Whether supplementation of men undergoing ADT with calcium and/or vitamin D results in a higher BMD than in those with no supplementation has not been tested. Available clinical trial data regarding supplemental calcium at 500–1,000 mg/day and vitamin D at 200–500 IU/day indicate that these regimens are inadequate to prevent BMD loss. Calcium supplements have been implicated in greater risks for cardiovascular disease and advanced prostate cancer. Thus, clinical trials to determine the risk–benefit ratio of calcium and vitamin D supplementation in men undergoing ADT for prostate cancer are urgently needed. Key safety endpoints in such trials should include markers of prostate cancer growth, for example, PSA and PSA velocity, as well as surrogate markers of cardiovascular disease.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Mridul Datta is supported by the Comprehensive Cancer Center of Wake Forest University Cancer Control Traineeship, NCI/NIH grant #R25CA122061.
Author Contributions
Conception/Design: Gary G. Schwartz, Mridul Datta
Manuscript writing: Gary G. Schwartz, Mridul Datta
Final approval of manuscript: Gary G. Schwartz, Mridul Datta
References
Institute of Medicine. In
Author notes
Disclosures: The authors indicated no financial relationships.
Section Editors: Chris Parker: Amgen, Astellas, Bayer, BNIT, Janssen, Takeda (H); M. Dror Michaelson: None
Reviewer “A”: Novartis (C/A, H, RF)
Reviewer “B”: None



