-
PDF
- Split View
-
Views
-
Cite
Cite
Dai Chihara, Hidemi Ito, Tomohiro Matsuda, Kota Katanoda, Akiko Shibata, Kumiko Saika, Tomotaka Sobue, Keitaro Matsuo, Decreasing Trend in Mortality of Chronic Myelogenous Leukemia Patients After Introduction of Imatinib in Japan and the U.S., The Oncologist, Volume 17, Issue 12, December 2012, Pages 1547–1550, https://doi.org/10.1634/theoncologist.2012-0197
Close - Share Icon Share
Abstract
Although the impact of imatinib in improving survival outcomes in chronic myelogenous leukemia (CML) patients has been widely reported, its impact on mortality from CML has not been evaluated. A survival benefit demonstrated in clinical trials does not simply translate to a decrease in mortality. To evaluate the impact of imatinib on the public health, we estimated the age-standardized mortality rate of CML patients in Japan and the U.S. using vital statistics data for Japan and data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute for the U.S.
The period covered in this analysis is 1993–2008, during which 64,203 patients in Japan and 26,888 patients in nine registries in the U.S. died as a result of CML. We used joinpoint regression analysis to evaluate the significance of trends in mortality.
Estimated age-standardized mortality rates decreased significantly in both countries after the availability of imatinib. The annual percent changes (95% confidence interval) in the U.S. were −12.3% (−14.8% to −9.7%) for men and −11.6% (−13.1% to −10.1%) for women. In Japan, these were −20.8% (−36.2% to −1.6%) for men and −15.6% (−18.8% to −12.2%) for women. The period of change in the mortality trend seems to correlate with the period in which imatinib appeared in the two countries. The CML mortality rate in 2008 was nearly 30% that of the 1993 level.
This is one example of the advent of a single new drug changing the picture of a single disease, CML. These results may encourage further development of drugs based on the concept of molecular targeting.
摘要
目的. 尽管已广泛报道伊马替尼能够改善慢性髓细胞性白血病(CML)患者生存结果,但该药对CML死亡率的影响尚未得到评估。几项临床试验已证实伊马替尼的生存获益,但这不能简单地转化成死亡率下降。为了评估伊马替尼对公众健康的影响,我们在日本和美国CML患者中估计了年龄标准化死亡率,其中日本患者资料来自人口统计数字,美国患者则采用美国国立癌症研究所监测、流行病学与最终结果(SEER)项目数据。
患者与方法. 本次分析于1993 ~ 2008年开展,64 203例CML患者来自日本,26 888例来自美国9所注册中心。利用joinpoint回归分析来评估死亡率趋势意义。
结果. 使用伊马替尼后两个国家的估计年龄标准化死亡率均显著下降。在美国,年度百分比变化[95%可信区间(CI)]为男性-12.3%(-14.8% ~ -9.7%),女性-11.6%(-13.1% ~ -10.1%)。在日本,年度百分比变化(95%CI)为男性-20.8%(-36.2% ~ -1.6%),女性-15.6%(-18.8% ~ -12.2%)。在两个国家的趋势分析均显示,死亡率变化时间似乎与伊马替尼使用时间相关。2008年CML死亡率水平几乎为1993年的30%。
结论. 本文为一种新药的出现改变了一种疾病(本案例中为CML)治疗现状提供了一个例证。上述结果可能鼓励我们在分子靶向概念基础上深入开展对药物的研发。
Introduction
The efficacy of new oncology drugs is evaluated in clinical trials with regard to adverse effects, the response rate, and the survival rate, and those drugs showing some benefit, mainly in terms of a survival benefit, are approved and introduced into clinical practice. However, a survival benefit demonstrated in clinical trials does not simply translate to a decrease in the mortality rate. The age-standardized mortality rate is a fundamental epidemiological measure that tells us whether or not any lives are saved. Significant decreases in mortality rates have been demonstrated with interventions such as vaccination and drugs like aspirin and antibiotics [1–3]. Similarly, mortality rates also can be evaluated in the field of oncology to confirm the impact of new drugs.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm characterized by the BCR-ABL1 fusion gene located in the Philadelphia chromosome and uncontrolled proliferation of mature and maturing granulocytes with fairly normal differentiation [4]. CML has an annual incidence of one to two cases per 100,000 in white populations, with a slight male predominance [5]. The treatment of patients with CML changed dramatically with the advent of imatinib, the first molecular-targeted drug that inhibits the specific class of tyrosine kinases [6]. In the U.S., a phase I study of imatinib was started in June 1998 and approval by the U.S. Food and Drug Administration was received in May 2001, whereas in Japan, a phase I study was started in June 2000 and approval was received in November 2001. Although the impact of imatinib in improving survival outcomes of CML patients has been widely reported in clinical trials, hospital-based clinical experience, and population-based registry data [7–10], its impact on the mortality rate of CML patients has yet to be evaluated.
Here, to evaluate the clinical impact of imatinib on the public health, we estimated the mortality rates of CML patients in Japan and the U.S. using vital statistics data for Japan and data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute for the U.S. [11, 12].
Materials and Methods
Japanese data were collected from all 47 prefectures, whereas data for the U.S. were sourced from the SEER 9 database, which covers only nine cities and states (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah) [11, 12]. The period covered in this analysis is 1993–2008. Rates of sex-specific mortality were age standardized by the world standard population [13]. Mortality rates were calculated as cases of death resulting from CML per 100,000 person-years. We used joinpoint regression analysis to evaluate the significance of the trend, as described in detail elsewhere [14]. For the analysis, uncorrelated error models were used. The minimum number of joinpoints was set as zero and the maximum number was set as three. The standard error of the age-standardized rate was estimated for each year. All computations were performed using STATA, version 11 (STATA Corp., College Station, TX), except for the joinpoint regression analysis, for which we used the Joinpoint Regression Program, version 3.3 (U.S. National Cancer Institute, Bethesda, MD). For the joinpoint regression analysis, two-sided p-values <.05 were considered statistically significant.
Results
Totals of 64,203 patients from Japan and 26,888 patients from nine cities and states of the U.S. died as a result of CML during this period. The estimated age-standardized mortality rates of CML patients in Japan and the U.S. are shown as circles in Figure 1. The line shows the age-standardized modeled mortality rate estimated by joinpoint regression analysis. Age-standardized mortality rates with 95% confidence intervals (CIs) in both countries are shown in supplemental online Table 1. The annual percent change (APC) and the trend in the CML morality rate estimated by joinpoint regression analyses are summarized in Table 1.
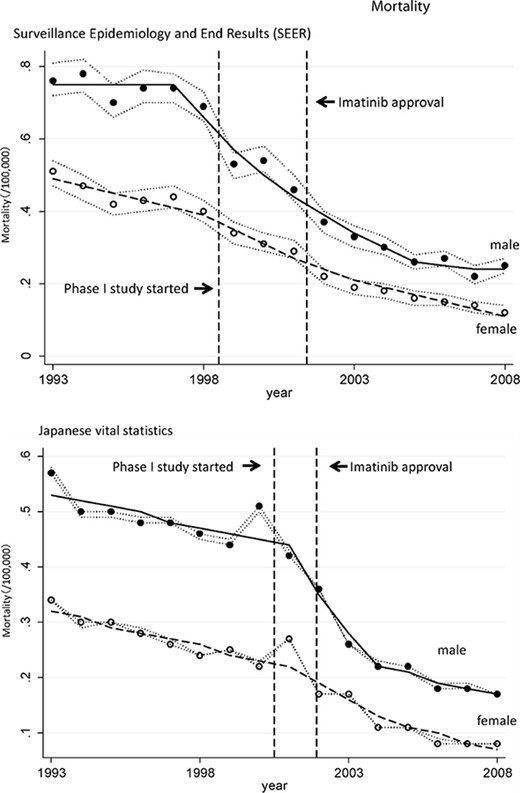
Mortality rate of patients with chronic myeloid leukemia (CML) in Japan and the U.S. Shown is the age-standardized mortality rate of CML patients in the U.S. and Japan. Segi's world standard population was applied. Circles indicate the observed age-adjusted mortality rates and lines indicate age-adjusted modeled mortality rates estimated by joinpoint regression analyses. The dotted line around the circles indicates the 95% confidential interval of the mortality rate.
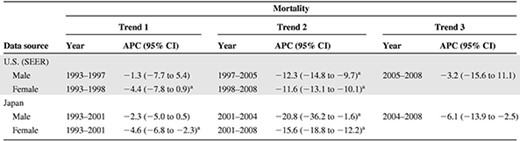
aAPC is statistically significantly different from zero (two-sided p < .05, calculated using a t-test).
Abbreviations: APC, annual percent change; CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.

aAPC is statistically significantly different from zero (two-sided p < .05, calculated using a t-test).
Abbreviations: APC, annual percent change; CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
As depicted in Figure 1, the age-standardized mortality rate of patients with CML decreased significantly in Japan and the U.S. The change in trend in the U.S. occurred in 1997 in males (95% CI, 1995–1999) and in 1998 in females (95% CI, 1996–2000), and it occurred in 2001 in Japan in both males and females (95% CI, 1999–2002). The periods of change in the mortality trend seem to correlate with the period in which imatinib appeared in the two countries.
Discussion
We showed a significant decrease in the mortality rate of CML patients in the imatinib era, which is reasonable considering the very strong clinical impact of imatinib. By 2008, the mortality rate decreased to ∼20%–30% of the 1993 level in both countries. Studies have shown decreases in mortality rates associated with screening methods for certain cancers [15, 16]. To date, however, no single drug has been proven to decrease the mortality rate. This is the first study to report that a single cancer drug has the ability to change the mortality rate of patients with a certain disease in the oncology field. The appearance of a new and outstanding treatment or prevention modality sometimes changes the whole picture of a disease, and our results show that the molecular-targeted drug imatinib could be one such drug. These findings may encourage further development of drugs based on the concept of molecular targeting.
The possibility exists that the observed decrease in the CML mortality rate might have resulted from a decrease in incidence. To evaluate this, we estimated the age-adjusted incidence of CML from the Japanese cancer registry data and SEER 9 data [11, 12, 17]. Although the results showed a constant decrease in the two countries (supplemental online figure), the APC in the mortality rate after imatinib was far greater than the change in incidence, supporting the idea that the observed decrease in the mortality rate is not only a result of the decrease in incidence but also a result of improvement in treatment. Previous studies using population- and hospital-based registries have shown an improvement in survival outcomes after the introduction of imatinib [7, 8].
Although the reason for the decrease in incidence is unclear, one possibility is that the precise diagnostic criteria introduced in the last decade that stipulate detection of the BCR-ABL1 fusion gene might have decreased the diagnosis rate of CML without this fusion gene [18]. The U.S. and Japan use the International Classification of Diseases – Oncology (ICD-O) for their cancer registries. ICD-O-3 version coding classifies myeloproliferative neoplasm (MPN) as a malignant disease; however, ICD-O-2 (used in the U.S. starting in 2001 and in Japan starting in 2002) did not include MPN as a malignant disease. This could lead to the coding of MPN as CML, which would result in overestimation of the CML incidence. The trend in incidence of MPN cannot be estimated properly because these diseases were not coded before ICD-O-3, which makes it difficult to confirm this hypothesis. Little is known about the features that predispose patients to a CML etiology. Although some exposures, such as obesity, radiation, smoking, and hair dye, are reported as the increasing the risk for CML leukemogenesis [19, 20], these factors are still controversial. Also, there are no data in cancer registries that can assess whether or not these exposures decreased within our study period.
To conclude that only imatinib decreased the mortality rate remains controversial because the decreasing trend in the mortality rate began to occur before imatinib approval in both countries, especially in the U.S. As we described above, this may be partially related to the decrease in incidence. Also, improvements in treatment, such as stem cell transplantation and infection control, may have contributed to this decreasing trend. However, the decreasing trend in the mortality rate after imatinib approval is more remarkable than the trend before imatinib approval, as we show with Trend 2 in Table 1. We can conclude that something happened in the treatment of CML during this period that may be attributed to imatinib.
Summary
In conclusion, we found a significant decrease in the population-based mortality rate of patients with CML in both Japan and the U.S. that may be related to the introduction of imatinib in the two countries. An increasingly large number of molecular-targeted drugs is now available or under development. Evaluation of the impact of these drugs may require assessment of both survival and mortality outcomes from the time the drugs appear in practice.
Acknowledgments
We thank all of the SEER and Japanese vital statistics staff.
Author Contributions
Conception/Design: Keitaro Matsuo, Tomotaka Sobue
Provision of study material or patients: Tomohiro Matsuda, Kota Katanoda, Akiko Shibata, Kumiko Saika, Tomotaka Sobue
Collection and/or assembly of data: Tomohiro Matsuda, Kota Katanoda, Akiko Shibata, Kumiko Saika, Tomotaka Sobue
Data analysis and interpretation: Keitaro Matsuo, Dai Chihara, Hidemi Ito
Manuscript writing: Keitaro Matsuo, Dai Chihara, Hidemi Ito
Final approval of manuscript: Keitaro Matsuo, Dai Chihara, Hidemi Ito, Tomohiro Matsuda, Kota Katanoda, Akiko Shibata, Kumiko Saika, Tomotaka Sobue
References
Author notes
Disclosures: The authors indicated no financial relationships.



