-
PDF
- Split View
-
Views
-
Cite
Cite
Randall J. Kimple, Janet K. Horton, Chad A. Livasy, Janiel M. Shields, Julia A. Lawrence, WingKeung M. Chiu, Anastasia Ivanova, David W. Ollila, Lisa A. Carey, Jan S. Halle, Carolyn I. Sartor, E. Claire Dees, Phase I Study and Biomarker Analysis of Lapatinib and Concurrent Radiation for Locally Advanced Breast Cancer, The Oncologist, Volume 17, Issue 12, December 2012, Pages 1496–1503, https://doi.org/10.1634/theoncologist.2012-0256
Close - Share Icon Share
Abstract
This phase I study assessed the toxicity and safety of combining daily lapatinib with radiation therapy. Sequential tumor biopsies were obtained to evaluate changes in biomarkers, such as epidermal growth factor receptor (EGFR) and human EGFR-2 (HER2) signaling pathways.
Eligibility for this dose-escalation study included unresectable and locally recurrent or chemotherapy-refractory and locally advanced breast cancer, and adequate organ function. Patients underwent three serial biopsies: at baseline, after 1 week of lapatinib alone, and after 1 week of lapatinib and radiation. Endpoints included determination of toxicity, maximum tolerated dose, and analysis of the effect of lapatinib with or without radiation on EGFR and HER2 signaling pathways by immunohistochemistry.
Doses of lapatinib up to 1,500 mg/day were well tolerated. Toxicity of grade 3 or more was limited to radiation dermatitis and pain. Out of 19 patients treated, in field responses per Response Evaluation Criteria in Solid Tumors criteria were complete in four patients and partial in six patients. Serial biopsies were obtained in 16 patients with no complications. Total Her2 was relatively unchanged while phospho-Her2, phospho-Akt, and phospho-ERK showed variable responses to both lapatinib alone and dual therapy with lapatinib and radiation.
The combination of lapatinib and radiation was well tolerated in this patient cohort. Overall local response rates were comparable to those reported in other studies in this patient population. Biopsies were safely performed at all time points. Inhibition of HER2 and downstream signaling pathways was identified, although no strong correlation with response was seen.
摘要
目的. 本I期研究评估了每日1次拉帕替尼联合放疗方案的毒性反应和安全性。随后通过一系列肿瘤活检来评估生物标志物变化,如表皮生长因子受体(EGFR)和人类EGFR-2(HER2)信号通路。
方法. 本次增量研究的入组标准为不可切除、局部复发或化疗耐药的局部晚期乳腺癌,器官功能尚可。患者连续进行3次活检:基线期、拉帕替尼单药治疗1周后、拉帕替尼联合照射治疗1周后。研究终点包括毒性反应、最大耐受剂量、利用免疫组化分析拉帕替尼联合或不联合照射对EGFR和HER2信号通路的效应。
结果. 拉帕替尼剂量增至1 500 mg/d时可被很好地耐受。3级或3级以上的毒性反应仅为放射性皮炎和疼痛。根据实体肿瘤疗效评价标准,19例接受治疗的患者中,4例患者病灶达完全缓解,6例为部分缓解。16例患者进行反复活检且未出现并发症。总体上HER2相对无变化,而磷酸化HER2、磷酸化Akt、磷酸化ERK对拉帕替尼单药和拉帕替尼与照射联合治疗的应答不同。
结论. 拉帕替尼与照射联合治疗在本研究队列中表现出良好的耐受性。总体局部缓解率与针对相同患者人群的其他研究所报道的结果基本相当。在全部时间点活检均安全进行。研究发现HER2及其下游信号通路受到抑制,但并未观察到其与治疗应答之间的强相关性。
Introduction
Radiation therapy plays a primary role in the treatment of patients with unresectable chest wall recurrences or chemotherapy-refractory, locally advanced breast cancer. Yet, obtaining adequate local control is quite difficult with local failures seen in up to three-quarters of women treated with radiation alone and in about half of women treated with surgical excision followed by radiation [1–4]. Uncontrolled local-regional disease can adversely affect quality of life, making improved local control an important therapeutic goal [5]. Concurrent use of chemotherapy and radiation is a commonly used approach to improve local disease control in many cancers and has been used in patients with breast cancer with unresectable chest wall disease with encouraging results, but often with increased toxicity [6–9].
Epidermal growth factor receptor (EGFR) and its closely related family member human epidermal growth factor receptor 2 (HER2) are members of the ErbB family of growth factor receptors. These transmembrane receptor tyrosine kinases are involved in tumor growth and development. Through activation of a multitude of downstream signaling pathways, including mitogen-activated protein kinases 1/2, extracellular-signal-regulated kinases (ERK) 1/2, phosphatidylinositol 3-kinase, Akt, and nuclear factor-κB, they have been implicated in resistance to both chemotherapy and radiation [10–12].
We have previously shown that inhibition of EGFR and HER2 by lapatinib, a small molecule tyrosine kinase inhibitor with nanomolar specificity for EGFR and its family member HER2, acts synergistically with radiation to improve tumor control both in vitro and in vivo in EGFR- or HER2-expressing cells [13–15]. In addition, trastuzumab, a monoclonal antibody targeting HER2, has shown intriguing promise as a radiosensitizer in a small phase II trial at our institution [7]. Based on preclinical data suggesting good radiosensitization with lapatinib in breast cancer cell lines [13–15], this study was initiated to evaluate the safety of combination therapy with lapatinib and radiation, determine the recommended phase II dose, and evaluate serial biopsies for markers of response to therapy in patients with locally recurrent or chemotherapy-refractory, locally advanced breast cancer. Tumor biopsy samples were obtained at three time points: (a) before treatment to determine EGFR, HER2, and downstream signaling pathway activation at baseline; (b) after 7 days of lapatinib; and (c) after 14 days of lapatinib and 7 days of radiation. The effects on receptor activation, modulation of downstream signaling pathway, and overall tumor proliferation were examined.
Patients and Methods
This open-label, dose-escalation study was conducted in two centers in North Carolina. The primary objective was to determine the recommended phase II dose of lapatinib given concurrently with radiation. Other objectives were to evaluate the impact of an oral EGFR/HER2 inhibitor on receptor and downstream signaling pathways in tumor tissue and to explore correlations between response and inhibition of downstream signaling. The study was approved by each center's institutional review board and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice.
Eligibility Criteria
Patients with unresectable, locally recurrent, or chemotherapy-refractory locally advanced breast cancer were eligible. Disease was required to be evaluable by examination or imaging studies and judged to be amenable to serial biopsies. Patients were at least 18 years old, with an Eastern Cooperative Oncology Group performance status of 0, 1, or 2; adequate hepatic, renal, and bone marrow function; and were able to swallow oral medication. A 3-week interval from the last systemic therapy was required. Patients had to have normal left ventricular ejection fraction (LVEF) as assessed by echocardiogram or multigated acquisition scan. Stable brain metastases were allowed. All patients gave written informed consent.
Study Assessments
Patient demographics and medical history were recorded at baseline. Physical examination, adverse events, and tumor measurements were assessed weekly while on combined therapy, 1 month after completion of combined therapy, and every 6–8 weeks thereafter. Response was assessed per Response Evaluation Criteria in Solid Tumors criteria [16]. Hematology was measured at baseline and then weekly during therapy. Multigated acquisition scan or echocardiogram was performed 8 weeks after initiating therapy and every 8 weeks while on lapatinib.
Study Treatment and Dose Escalation
The following three sequential dose cohorts of lapatinib were investigated in a 3+3 dose escalation design: 500, 1,000, and 1,500 mg orally once daily. Lapatinib alone was administered for 1 week before starting concurrent chemoradiation. Radiotherapy was administered in 1.8–2.0 Gy fractions 5 days a week, to a total dose of 35–70 Gy depending on whether the patient had been previously irradiated. Radiotherapy fields were based on three-dimensional conformal therapy and planned according to standard departmental protocols. Depending upon tumor extent, en face electrons fields, opposed tangential fields, three-dimensional conformal radiotherapy, or mixed photon/electron fields were used at the discretion of the treating radiation oncologist. Cumulative dose to the brachial plexus was limited to 60 Gy. All radiation therapy plans underwent standard quality assurance and peer review per departmental protocol. Lapatinib dose modifications were based on predefined toxicity criteria including skin rash (including dermatitis acneiform), diarrhea leading to dehydration, and decreased ejection fraction.
Initially, patients were stratified based on the presence or absence of previous radiation in the treatment field. However, due to slower than expected patient accrual and the lack of increased toxicity in the prior-radiation arm, the cohorts were combined after completion of the 1,000-mg cohort and are presented as a single group. This study employed a typical 3+3 phase I dose escalation design [17]. Each dose level cohort enrolled three patients, with expansion to six patients if one of the initial three experienced a dose limiting toxicity (DLT). DLT was defined as grade 4 hematologic toxicity; decrease in LVEF >20%; any intolerable grade ≥2 nonhematologic or grade 3 hematologic toxicity requiring dose reduction during combined therapy; any toxicity requiring delay of therapy >2 weeks; and grade ≥3 acute skin toxicity using the Radiation Therapy Oncology Group acute skin toxicity grading system seen at doses up to 50 Gy. Of note, grade 3 skin toxicity experienced at doses above 50 Gy was expected from standard radiotherapy in this clinical population and was therefore not considered a DLT. Toxicity was assessed up to 4 weeks following the completion of drug.
Enrollment in the subsequent cohort began after all patients in the preceding cohort had completed treatment. If more than two of six patients experienced DLT, then the maximum tolerated dose had been exceeded and enrollment continued at the lower dose level until a total of six patients had been enrolled at that dose. No intrapatient dose escalation was allowed. The recommended phase II dose was defined as the dose of lapatinib with radiation, at which no more than one out of six patients experienced a DLT.
Tumor Biopsies
Tumor biopsy, obtained by skin punch or core biopsy, was performed serially: at baseline, after 1 week of lapatinib alone (day 8), and after 1 week of lapatinib and radiation (day 15). Biopsies were saved on saline soaked gauze, fixed in 10% neutral buffered formalin for 48 hours, dehydrated in graded alcohol solutions, and embedded in paraffin.
Immunohistochemistry
Paraffin-embedded tissues were sectioned at 5 μm and immunohistochemistry (IHC) was performed according to the individual antibody manufacturer's instructions in the University of North Carolina Anatomic Pathology Research Core Laboratory. Briefly, tissue sections were deparaffined with xylene; dehydrated with 100%, 95%, and 70% ethanol; and endogenous peroxidase activity was blocked with 3% hydrogen peroxidase with methanol. Samples were steamed for antigen retrieval with 10 mM citrate buffer (pH 6.0) for 30 minutes, blocked with blocking serum, incubated with biotinylated antibody, and incubated with streptavidin conjugated horseradish peroxidase using Vectastain ABC kit (Vector Laboratories, Burlingame, CA) per the manufacturer's protocol. Slides were counterstained with hematoxylin and examined by light microscopy. Positive and negative controls were processed simultaneously to validate appropriate binding. When necessary, IHC procedures were optimized using control tissues. A variety of antibody sources and conditions were used (supplemental online Table 1) and IHC was performed for EGFR, phospho-EGFR, HER2, phospho-HER2, Akt, phospho-Akt, ERK, phospho-ERK, tumor protein 53, and estrogen receptor. Proliferation was assessed by Ki-67 and the percentage of positive cells was determined as the Ki-67 index. When sufficient tissue was available, IHC was performed at all time points.
For all samples, IHC was scored for both intensity and percentage of tumor staining by a single breast pathologist (C.A.L.) and by one of the investigators (R.J.K.). Analysis of tumor biopsies was performed by calculating an H-score including percentage and intensity of staining [18]. Comparison between baseline (biopsy 1), postlapatinib (biopsy 2), and postradiation (biopsy 3) biopsies was performed by grouping those with increased, unchanged, and decreased H-scores. Standardized HER2 and estrogen receptor expression from each patient's original biopsy were abstracted from the pathology report [19] and compared to staining on repeat biopsies. All analyses were conducted with SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 21 patients were enrolled; 7, 9, and 3 patients were included in the 500-, 1,000-, and 1,500-mg cohorts, respectively. Two patients did not receive protocol-directed therapy due to withdrawal prior to beginning radiation (symptomatic pulmonary disease and personal choice). Baseline characteristics (Table 1) and the enrollment diagram (Fig. 1) are shown. The 500-mg cohort was expanded to seven patients because one patient had disease that was judged after enrollment to not be amenable to biopsy. This patient was assessable for toxicity endpoints of the trial but not correlative biopsies. One patient discontinued lapatinib and radiation after receiving only 44 Gy because of progressive disease both within and outside the radiation field, but was included in the analysis. All other patients completed the prescribed course of therapy.
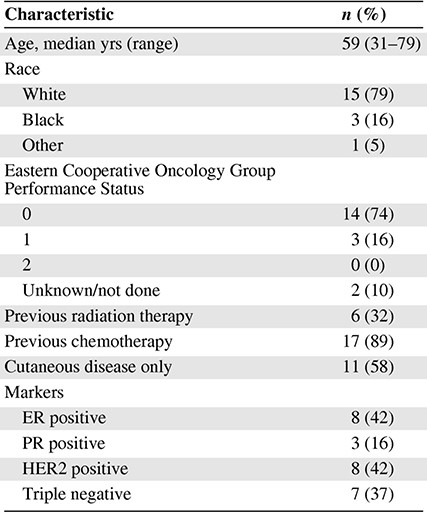
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.

Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
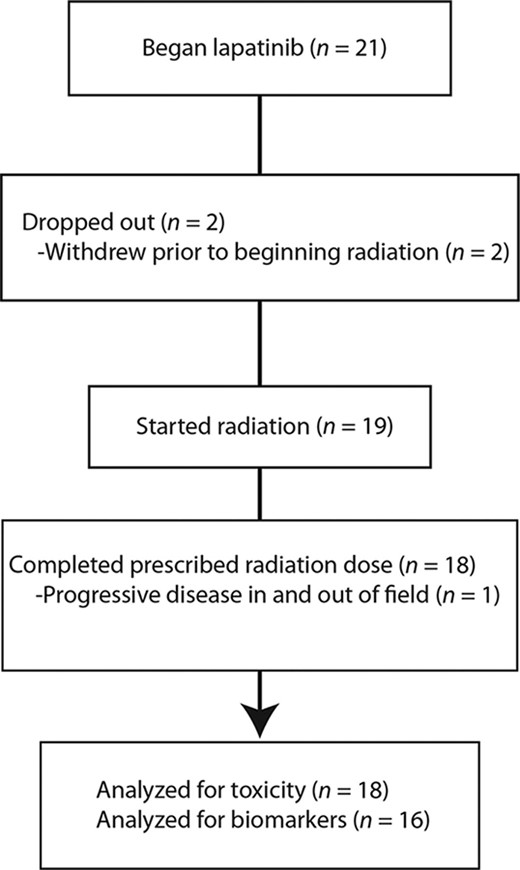
Patients who had not received prior irradiation received a median dose of 60 Gy (range, 44–70 Gy). Previously irradiated patients received a median dose of 50 Gy (range, 45–70.2 Gy). In patients who had received prior irradiation, higher radiation doses were usually given to areas outside the prior treatment fields. No treatment breaks were needed and no dose reductions were made.
Maximally Tolerated Dose
Patients were initially treated with lapatinib at 500 mg/day, with escalations to 1000 mg/day, and 1,500 mg/day. At the 500-mg dose, no patients experienced a DLT. At the 1,000-mg dose, one patient who had not been previously irradiated experienced a DLT (grade 3 moist desquamation developed at 48 Gy). This cohort was expanded by three additional patients. No additional DLTs were observed in this cohort or in the three patients who had been previously irradiated and were treated at the 1,000-mg dose level. As the 1,000-mg dose level was found to be safe regardless of prior radiation, the two arms were combined for the final 1,500-mg cohort. None of the three patients in the final 1,500-mg cohort experienced a DLT. MTD was not reached at the dose approved by the U.S. Food and Drug Administration for the single-agent lapatinib.
Toxicity
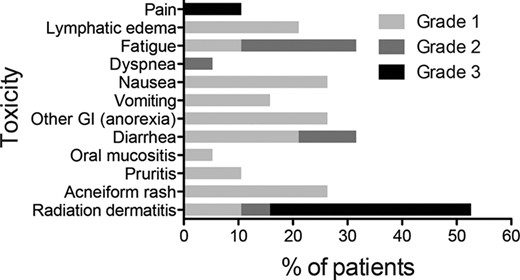
The most common toxicities reported during study treatment, regardless of causality, are listed in Table 2. The majority of toxicities were grade 1 (Fig. 2). Gastrointestinal toxicity was minor, with six patients reporting grade 1 or 2 diarrhea, five with grade 1 or 2 nausea, and three with grade 1 or 2 vomiting. Most grade 3 toxicities were radiation dermatitis, an expected effect of radiation in patients with locally advanced or locally recurrent breast cancer. No cases of grade 3 acneiform rash were reported, although six patients had grade 1 or 2 rash. No grade 4 toxicities were reported and no patient developed symptomatic cardiac dysfunction.
Adverse events of any grade reported in ≥5% of patients during study treatment regardless of causality
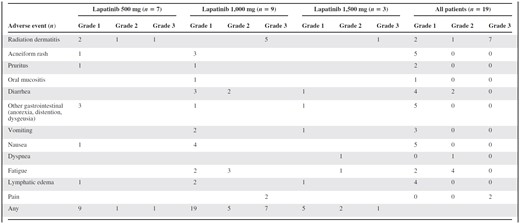
A patient may have had more than one event.
Adverse events of any grade reported in ≥5% of patients during study treatment regardless of causality

A patient may have had more than one event.
Response
Although not the primary objective of this phase I study, some degree of in-field response was seen in 10 of 19 patients. Two patients had progressive disease and seven had stable disease at the conclusion of treatment.
Serial Tumor Biopsies
Successful serial core biopsies were obtained at all three time points for 16 of 21 patients. Two patients had disease that was not able to be biopsied at the initial time point despite being judged amenable to biopsy at the initial screening, so they did not undergo serial biopsies. One additional patient did not undergo the third planned biopsy after being hospitalized for pain control. No complications were observed as a result of the serial biopsies. Three patients had all three biopsies performed but did not have sufficient viable tumor present for analysis at any time point. Samples from one patient were unusable due to difficulties with transport of biopsy material. Seven additional patients had insufficient tissue available for completion of all IHC studies. IHC analyses were performed using a prioritization schema when tissue resources did not permit all analyses to be performed (supplemental online Table 2). Six serial samples were evaluable for the assessment of changes in all signaling pathways at all time points.
Baseline Assessment of Signaling Pathways
Pretreatment biopsies were analyzed for expression of EGFR, phospho-EGFR, HER2, phospho-HER2, phospho-Akt, phospho-ERK, Ki-67, and p65. EGFR expression was seen in 3 of 12 patients. Insufficient samples were available to analyze EGFR activation via phospho-EGFR staining. Four patients were found to be HER2-positive by clinical testing (3+ staining in more than 10% of cells or positive by in situ hybridization). Either HER2 or phospho-HER2 expression was seen in 9 of 11 patients (Table 3), although not always in sufficient intensity to qualify as positive by clinical criteria. No significant relationship between phospho-HER2 at baseline and clinical response was identified. Phospho-Akt was seen in 8 of 10 patients, whereas phospho-ERK was seen in 9 of 10 patients (Table 3). At baseline, 10 patients were evaluable for Ki-67: respectively, seven, one, and two patients had Ki-67 indexes of >25%, 10%–25%, and <10%. p65 staining was seen in seven of nine patients.
Immunohistochemistry score by patient for HER2, phospho-ERK, and phospho-AKT
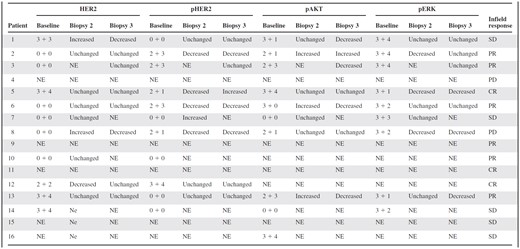
First number represents staining intensity (0 = negative, 1 = borderline, 2 = weak, 3 = moderate) and second number represents the percentage of cells positive (0 = <10%, 1 = 10%–25%, 2 = 26%–50%, 3 = 51%–75%, 4 = >75%). Change in H-score between baseline and biopsy 2 and biopsy 3 indicated.
Abbreviations: CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Immunohistochemistry score by patient for HER2, phospho-ERK, and phospho-AKT

First number represents staining intensity (0 = negative, 1 = borderline, 2 = weak, 3 = moderate) and second number represents the percentage of cells positive (0 = <10%, 1 = 10%–25%, 2 = 26%–50%, 3 = 51%–75%, 4 = >75%). Change in H-score between baseline and biopsy 2 and biopsy 3 indicated.
Abbreviations: CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Post-treatment Assessment of Signaling Pathways
Based on preclinical in vitro work, we expected lapatinib to result in downregulation of HER2 activation [14]. Following 1 week of lapatinib treatment, total HER2 was unchanged in six of nine patients and phospho-HER2 staining was decreased in four of six patients, three of whom had a clinical response to chemoradiation (Table 3). No biopsy pair showed a decrease in phospho-Akt, but three of seven patients (all of whom had a partial response to treatment) demonstrated increased phospho-Akt following lapatinib (Table 3). Phospho-Erk activation was decreased in three of seven patients (Table 3), two of whom had a response to therapy and one who had progressive disease. The Ki-67 index decreased in two of eight patients following lapatinib alone.
Comparison of biopsies taken at baseline with those taken after 1 week of combined lapatinib and radiation showed little change in total EGFR. Decreased total HER2 was seen in two patients who had no response to therapy, whereas no change in HER2 was seen in five patients who had a response to therapy (Table 3). Decreased phospho-HER2 was seen in three of eight patients, with the most striking differences seen in patients with clinical responses (Fig. 3, Table 3). As expected based on preclinical work [13, 14], decreased phospho-ERK was seen in three of seven patients, and decreased phospho-Akt was seen in four of seven patients, respectively (Table 3). The Ki-67 index decreased from baseline in four of eight patients following chemoradiation. Seven of the 16 patients did not have sufficient tissue present in the third biopsy to allow meaningful comparisons with earlier time points due to the absence of viable tumor within the sample tissue.
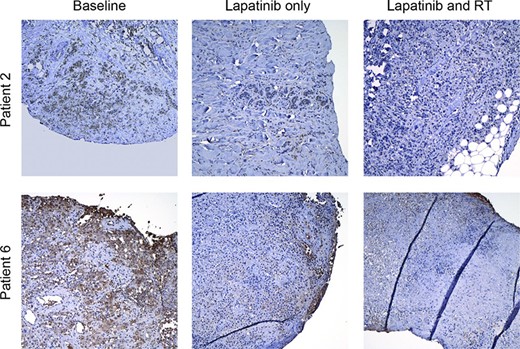
Representative micrographs showing phosphoHER2 staining at baseline (left), after 1 week of lapatinib (middle), and after 1 week of radiation and 2 weeks of lapatinib (right). Abbreviation: RT, radiotherapy.
Discussion
This study showed that lapatinib given at 1,500 mg/day with chest wall radiation was well tolerated in patients with unresectable chest wall recurrences or chemotherapy-refractory, locally advanced breast cancer. There were no DLTs in the 1,500 mg dose escalation cohort, albeit with a small number of patients treated. Further expansion was precluded due to poor accrual and insufficient funds. The entire planned course of radiation was delivered in all but one patient who developed progressive disease within the radiation field leading to the decision to cease therapy on trial. The recommended phase II dose (1,500 mg/day) was the same as the MTD determined by Harrington et al. in their phase I study in patients with locally advanced squamous cell carcinoma of the head and neck treated with lapatinib and concurrent radiation [20].
Patients with local-regional recurrence after mastectomy have a poor prognosis; 5-year survival rates are routinely less than 50% [21, 22]. Uncontrolled local-regional disease is highly morbid [3] and has been associated with worse survival (35% vs. 64% at 5 years) compared to those patients whose local-regional disease is controlled [23]. Local control rates with radiation alone, the current standard of care, have been in the range of 30%–60% [1, 4, 21, 23–24]. Not surprisingly, several of these series suggested that tumors treated to higher radiation doses had improved local control [1, 4, 21, 23]. However, improved local control comes at the risk of increased toxicity and wound healing complications [25], and it is often limited in the setting of prior irradiation.
The best-studied radiation sensitizer for chest wall recurrence is hyperthermia, which has been the subject of five randomized trials. Although studies have been plagued by poor accrual, in a combined analysis, hyperthermia has been shown to improve local control at the expense of increased toxicity [26]. Because of the resources involved in delivering hyperthermia (cost, equipment, time, and personnel), this is not an option at the majority of radiation oncology centers. In many other disease sites, concurrent chemotherapy and radiation are used as standard therapy to improve local control and survival. However, data supporting chemoradiation in breast cancer are limited to several phase I and small phase II trials. Formenti et al. used twice-weekly paclitaxel and concurrent radiation in women with operable stage IIB–III breast cancer with minimal grade 3 skin desquamation (7%) and a 34% pathologic complete remission rate [27]. Chakravarthy et al. found a 34% pathologic complete response rate and a low rate of grade 3 or 4 toxicity in a small cohort of patients with operable breast cancer treated with neoadjuvant paclitaxel and radiation [28]. Suh et al. at the University of Michigan reported on a phase I trial investigating the use of gemcitabine with radiation for patients with unresectable chest wall recurrences; they reported grade 3 neutropenia in two of three patients treated at the initial dose level and grade 3 or 4 acute skin toxicity in all patients [6]. Our study was designed based on promising preclinical data regarding the efficacy of lapatinib as a breast cancer radiosensitizer [13, 15] and with the hope that the use of a dual EGFR/HER2 targeted inhibitor would be more tolerable than standard systemic therapy.
The toxicity profile in this study was consistent with what we have previously seen in a phase II trial investigating concurrent trastuzumab and radiation in a similar cohort of patients [7]. Acneiform rash was seen in approximately 30% of patients in this study. Although definitive conclusions are limited by sample size, the rate did not increase with lapatinib dose escalation. This is similar to what was reported by Harrington et al. [20] and was in the range of the 43% rate reported in a recent analysis of nine clinical trials using lapatinib monotherapy [29].
Although the small size of our trial limited our ability to investigate the association of rash with response, there is a growing body of evidence suggesting that rash is not predictive of response in all EGFR inhibitors [20, 30]. Consistent with what has been reported in other investigations of lapatinib, diarrhea was seen in about 30% of patients, was mild in all cases with no grade 3 or higher toxicity seen, and was successfully managed with conservative therapy according to standard guidelines [31]. Cardiac events have been seen less frequently with lapatinib than with the HER2 monoclonal antibody trastuzumab, perhaps suggesting different pathologic mechanisms. We did not see any cases of left ventricular dysfunction in the patients treated on this study despite the frequency of prior treatment with cardiotoxic drugs (i.e., trastuzumab and adriamycin) in a number of patients.
Performing serial tumor biopsies in irradiated tissue can be safely accomplished and may provide valuable information confirming in vitro-generated hypotheses, identifying prognostic or predictive markers, and identifying new avenues of investigation [32]. However, this study also highlights the inherent difficulty in obtaining serial biopsies in this manner: a significant number of biopsies contained scant or no tumor tissue, which not only limits the accuracy of scoring, but also may lead to underappreciation of intratumoral heterogeneity.
Although we were able to detect changes in downstream pathways, the semiquantitative nature of IHC made it difficult to identify predictors of response in this small dose-escalation study. We did see results that appeared similar to those described by Spector et al. from a small clinical study: relatively unchanged total HER2 and variable changes in phospho-HER2, phospho-Akt, and phospho-ERK [33]. Interestingly, we did not identify the patterns of change predicted by our in vitro work [13–15]. These differences between in vitro analysis and patient biopsies may have several explanations, including the small sample size of our study, changes in molecular responses that occur between 1 day (in vitro studies) and 1 week (clinical trial) after therapy, heterogeneity in patient tumors, or even fundamental differences between drug responses in patient tumors and cell lines maintained in vitro. It may be that serial biopsy analysis of this type may be best used in larger phase II trials or within the confines of an expanded dose cohort in a phase I trial.
Conclusion
Lapatinib (1,500 mg/day), when given concurrently with radiation to patients with unresectable chest wall recurrences or chemotherapy-refractory, locally advanced breast cancer, was well tolerated. This dose was associated with an acceptable tolerability profile, similar to that observed with radiation alone for treatment of this disease; it resulted in a nearly 50% response rate. Further studies investigating rational combinations of targeted therapy and radiation for women with locally advanced breast cancer are warranted.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank the generous patients who participated in this trial; Nana Feinberg and Mervi Eeva in the University of North Carolina Anatomic Pathology Translational Core Laboratory (UNC APTCL) for expert technical assistance; and Laura Esler, Kim Keller, Rachel Phipps, and the other staff members in the Lineberger Comprehensive Cancer Center Clinical Protocol Office who helped with the patient care and data collection in this study.
This work was supported by the National Cancer Institute grants R21 CA119590 (E.C.D.), CA115888 (C.I.S. and J.M.S.), P50-CA58223; General Clinical Research Center grant RR00046, and GlaxoSmithKline. R.J.K. has been designated a B. Leonard Holman Pathway Fellow by the American Board of Radiology and was supported by a Phillips Medical Systems/Radiological Society of North America Research Resident Grant and by a Resident/Fellows in Radiation Oncology Seed Grant from the American Society for Radiation Oncology. The UNC APTCL is supported, in part, by grants from the University of North Carolina University Cancer Research Fund.
Author Contributions
Conception/Design: Jan S. Halle, Carolyn I. Sartor, E. Claire Dees
Provision of study materials or patients: Randall J. Kimple, Janet K. Horton, Julia A. Lawrence, David W. Ollila, Lisa A. Carey, Jan S. Halle, Carolyn I. Sartor, E. Claire Dees
Collection and/or assembly of data: Randall J. Kimple, Janet K. Horton, Carolyn I. Sartor, E. Claire Dees
Data analysis and interpretation: Randall J. Kimple, Janet K. Horton, Chad A. Livasy, Janiel M. Shields, WingKeung M. Chiu, Anastasia Ivanova, E. Claire Dees
Manuscript writing: Randall J. Kimple, Janet K. Horton
Final approval of manuscript: Randall J. Kimple, Janet K. Horton, Chad A. Livasy, Janiel M. Shields, Julia A. Lawrence, WingKeung M. Chiu, Anastasia Ivanova, David W. Ollila, Lisa A. Carey, Jan S. Halle, Carolyn I. Sartor, E. Claire Dees
References
Author notes
Disclosures: Chad A. Livasy: Genentech (H); Lisa A. Carey: Blue Cross/Blue Shield of North Carolina Board of Trustees (E); Sanofi, Aventis, Genentech/Roche, AstraZeneca (C/A); GlaxoSmithKline, Genentech/Roche (RF). The other authors indicated no financial relationships.



