-
PDF
- Split View
-
Views
-
Cite
Cite
Patricia A. Tang, Daniel Y. C. Heng, Toni K. Choueiri, Impact of Body Composition on Clinical Outcomes in Metastatic Renal Cell Cancer, The Oncologist, Volume 16, Issue 11, November 2011, Pages 1484–1486, https://doi.org/10.1634/theoncologist.2011-0337
Close - Share Icon Share
Systemic therapy for metastatic renal cell cancer (mRCC) currently revolves around inhibition of angiogenesis through the vascular endothelial growth factor (VEGF) pathway or the mammalian target of rapamycin (mTOR) pathway. Unfortunately, there are no validated predictive factors that can accurately determine whether a patient will benefit from treatment with molecularly targeted agents. However, many groups have identified prognostic factors that provide insight into a patient's overall disease outcome independent of treatment [1]. These prognostic factors have been incorporated into multivariate models that stratify patients into poor, intermediate, and favorable risk groups. Commonly used models include the Memorial Sloan‐Kettering Cancer Center (MSKCC) criteria [2, 3], derived in the immunotherapy era, and the Heng criteria [4], derived from patients treated with novel anti‐VEGF therapies (Table 1).
Comparison of multivariate prognostic factor models in metastatic renal cell carcinoma
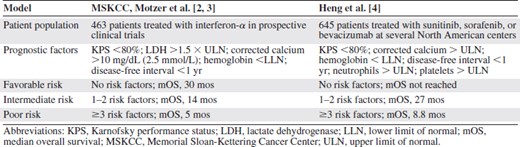
Comparison of multivariate prognostic factor models in metastatic renal cell carcinoma

Obesity is an established risk factor for developing RCC [5–8], and several studies suggested, perhaps counterintuitively, that a high body mass index (BMI) confers a survival advantage to patients undergoing nephrectomy [9, 10]. In the particular setting of mRCC and targeted therapy, the prognostic impact of obesity and body mass is not clear.
Potential mechanisms by which obesity could influence clinical outcomes include alterations in pharmocokinetics and drug concentrations as well as the presence of associated comorbidities such as diabetes and cardiovascular disease [11]. Obesity induces a “state of inflammation,” which results in elevations in tumor necrosis factor, interleukin (IL)‐1β, IL‐6, IL‐1 receptor antagonist, and C‐reactive protein [12]. Adipocytes produce multiple angiogenic factors such as VEGF and leptin [13]. Obesity can also activate the phosphoinositide 3‐kinase–Akt–mTOR pathway via reactive oxygen species [14] and hyperinsulinemia/insulin like growth factor [15]. Several groups (Table 2) have evaluated the association between obesity or high BMI and survival in mRCC patients. Choueiri et al. [16] found that a high BMI was independently associated with a longer overall survival (OS) time (hazard ratio [HR], 0.67; 95% confidence interval [CI], 0.49–0.91; p = .01) after adjusting for the Heng criteria in 475 mRCC patients treated with antiangiogenic therapy. Furthermore, patients with a normal BMI or low body surface area (BSA) had a shorter time to progression (TTP) and OS time than the obese group. However, BMI and BSA are relatively crude measurements of body composition.
Retrospective studies evaluating the impact of body composition on outcome in metastatic renal cell carcinoma patients treated with antiangiogenic therapies
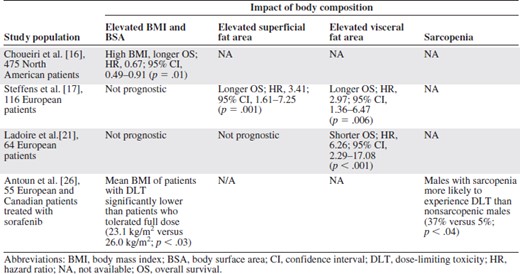
Retrospective studies evaluating the impact of body composition on outcome in metastatic renal cell carcinoma patients treated with antiangiogenic therapies

In the article that accompanies this commentary, Steffens et al. [17] evaluate the prognostic potential of four measures of body composition in 116 mRCC patients: BMI (kg/m2), BSA (m2), visceral fat area (VFA, in mm2), and superficial fat area (SFA, in mm2). Obesity was defined as a BMI ≥30 kg/m2 based on current World Health Organization standards or a BSA above the European average for men (1.98 m2) and women (1.74 m2) [18, 19]. Baseline VFA and SFA were calculated based on baseline computed tomography (CT) scans using the methods of Yoshizumi et al. [20]. Given the paucity of normative data on VFA and SFA, the threshold for obesity was arbitrarily defined as a value above the median observed in the patient cohort. Obesity was present in 19.8% of patients based on BMI and in 62.9% of patients based on BSA. On multivariate Cox regression analysis, including histological subtype and MSKCC status, there was no significant association between the progression‐free survival and OS and elevated BMI and BSA, the traditional definitions of obesity. However, elevated VFA and SFA were both independently associated with a longer progression‐free survival and OS time (VFA: HR, 2.97; 95% CI, 1.36–6.47; p = .006; SFA: HR, 3.41; 95% CI, 1.61–7.25; p = .001).
This is in stark contrast to the results of Ladoire et al. [21], who evaluated the prognostic impact of BMI, SFA, and VFA in French patients with mRCC. The same definition of obesity was used as in the German cohort (BMI >30 kg/m2, SFA above the median, VFA above the median using the methods of Yoshizumi et al. [20]). The French cohort had mean baseline SFA and VFA similar to those of the German group, but more patients had a poor performance status (20 of 113 with a Karnofsky performance status score <80). On multivariate analysis, including the MSKCC group, high VFA was associated with a significantly shorter TTP and OS time (HR, 6.26; 95% CI, 2.29–17.08; p < .001) in patients treated with antiangiogenic drugs (n = 59), but not in patients treated with cytokines. BMI and SFA were not prognostic. Ladoire et al. [21] suggested that high VFA was a predictive factor because it was associated with worse outcomes for patients treated with antiangiogenic therapy but not cytokines.
Another potentially important aspect of body composition is sarcopenia, or skeletal muscle wasting. In a single‐institution study of patients with advanced lung and gastrointestinal malignancies, the concurrent presence of sarcopenia and obesity was associated with a worse OS outcome (HR, 4.2; 95% CI, 2.4–7.2; p .0001) than in nonsarcopenic obese patients [22]. The impact of sarcopenia on long‐term outcomes in mRCC patients is unknown. Limited data are available from a subset of mRCC patients who participated in the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET), a randomized trial of sorafenib compared with placebo after failure of standard therapy [23]. Sarcopenia was present in 72% of patients with a BMI <25 kg/m2 and in 34% of patients with a BMI > 25 kg/m2 [24]. Treatment with sorafenib led to a significant loss of skeletal muscle at 12 months (8.0% decrease; p < .01), compared with placebo. The skeletal muscle loss was postulated to be a result of the downstream effects of mTOR inhibition from sorafenib based on preclinical models [25]. Dose‐limiting toxicities were most common in sarcopenic underweight patients (BMI <25 kg/m2) and least common in patients who were not sarcopenic and/or had a BMI >25 kg/m2 [26]. Despite the small sample size, these data suggest that sarcopenia exacerbates sorafenib‐induced toxicities. Sorafenib, in turn, exacerbates skeletal muscle loss, creating a vicious circle in this setting, leading to worse clinical outcomes.
It is also worth mentioning that the better prognosis in obese patients observed by Choueiri et al. [16] and Steffens et al. [17] may be related to better tolerability of targeted agents that are dosed independently of body weight. However, intuitively, a major concern with obese patients is underdosing.
The routine use of CT to assess therapeutic response provides an unparalleled opportunity to precisely quantify body composition. Superior outcomes were associated with elevated BMI and BSA in the study of Choueiri et al. [16] and with elevated SFA or VFA in the study of Steffens et al. [17]; conversely, poorer outcomes were linked with elevated VFA in the study of Ladoire et al. [21]. This may be a result of an imbalance in prognostic factors that were not controlled for and/or an unknown interaction or effect modifier more prevalent in one study than in another. Both Ladoire et al. [21] and Steffens et al. [17] performed their respective multivariate analyses controlling for the MSKCC criteria, which were validated in clinical trial patients in the immunotherapy era [2, 3]. Given the widely disparate results, the prognostic value of body composition (BMI, BSA, SFA, VFA, and sarcopenia) should be evaluated in a larger patient population to validate threshold values for SFA, VFA, and sarcopenia. Such studies will help determine whether body composition provides further refinement of the current prognostic models and pave the way for better prognostication.
Editor's Note: See the accompanying article, “Does obesity influence the prognosis of metastatic renal cell carcinoma in patients treated with vascular endothelial growth factor‐targeted therapy?” by S. Steffens, V. Grünwald, K.I. Ringe, et al., on pages 1565–1571 of this issue.
(C/A), consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Patricia A. Tang, Toni K. Choueiri, Daniel Y.C. Heng
Collection and/or assembly of data: Patricia A. Tang
Data analysis and interpretation: Patricia A. Tang, Toni K. Choueiri, Daniel Y.C. Heng
Manuscript writing: Patricia A. Tang, Toni K. Choueiri, Daniel Y.C. Heng
Final approval of manuscript: Patricia A. Tang, Toni K. Choueiri, Daniel Y.C. Heng
References
Author notes
Disclosures: Patricia A. Tang: None; Daniel Y. C. Heng: None; Toni K. Choueiri: Pfizer, Aveo, GlaxoSmithKline, Novartis (C/A).



