-
PDF
- Split View
-
Views
-
Cite
Cite
Sin Jen Ong, Marissa Teo, Kiat Hon Lim, Su Pin Choo, Han Chong Toh, Rapamycin and Thalidomide Treatment of a Patient with Refractory Metastatic Gastroesophageal Adenocarcinoma: A Case Report, The Oncologist, Volume 15, Issue 9, September 2010, Pages 965–968, https://doi.org/10.1634/theoncologist.2010-0118
Close - Share Icon Share
Background
Aberrant activation of mammalian target of rapamycin (mTOR) has been found in malignancies including gastric cancer [1] and esophageal cancer [2]. A high level of phosphorylated mTOR (p-mTOR) expression is associated with a poor prognosis in gastric cancer patients. mTOR is a serine/threonine protein kinase and regulates protein translation, cell survival, and proliferation. The activation of IκB kinase β (IKKβ) results in deregulation of the tuberous sclerosis complex 1 (TSC1)–mTOR pathway. Rapamycin is a macrolide antibiotic derived from the fungus Streptomyces. In vitro studies have shown that it inhibits mTOR expression and impairs tumor cell survival and proliferation [2]. Tumor angiogenesis also plays a significant role in cancer growth and metastasis.
Case Report
We report the use of rapamycin and thalidomide in a 43-year-old Malay male with metastatic gastroesophageal carcinoma. In April 2005, he presented with dysphagia and vomiting. Investigations revealed a resectable gastroesophageal adenocarcinoma. He declined surgery then and opted for alternative therapy instead.
In August 2007, he returned with hematemesis, worsening dysphagia, and symptomatic anemia secondary to a larger gastroesophageal junction tumor. A computed tomography (CT) scan demonstrated new liver and left adrenal metastases. He was treated with argon plasma coagulation, palliative radiotherapy, and chemotherapy (capecitabine with oxaliplatin). He improved clinically with resolution of dysphagia, increased appetite, and increased weight. Six months later, the liver metastases had increased in size. Capecitabine and docetaxel were initiated but he tolerated the treatment poorly and declined further chemotherapy. A trial of rapamycin was started at 2 mg/day in March 2009. By then, his cancer antigen (CA) 19–9 level was 19,269 U/ml. This decreased to 16,216 U/ml 1 month later, and then thalidomide was added at 50 mg/day (Fig. 1).
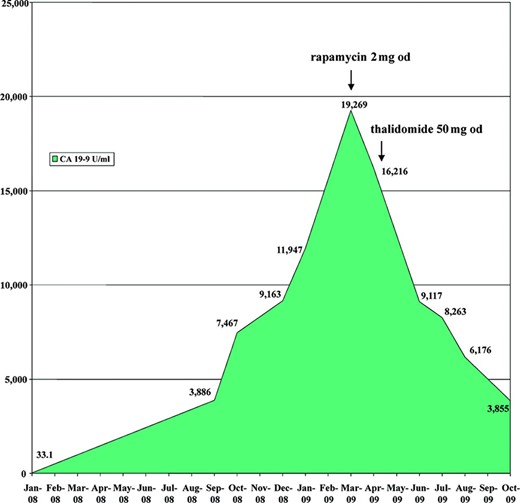
Trend of tumor marker cancer antigen (CA) 19-9.
Abbreviation: od, once daily.
Subsequent CT scans in May and July 2009 showed stable liver metastases with central necrosis. By August 2009, his CA 19–9 level had dropped significantly by about 70% to 6,176 U/ml and it had reached 3,855 U/ml by October 2009. The patient remained asymptomatic and tolerated the treatment well. His weight also increased by 4 kg. Both rapamycin and thalidomide were continued at the same doses and achieved good disease control before eventual progression 7 months later.
Immunohistochemistry was performed retrospectively on tissue taken before his treatment. This showed overexpression of phosphorylated AKT, phosphorylated IKKβ, p-mTOR (Fig. 2), and vascular endothelial growth factor receptor (VEGFR) (Fig. 3). Human epidermal growth factor receptor (HER)-2 staining was 2+ but the fluorescence in situ hybridization was negative.
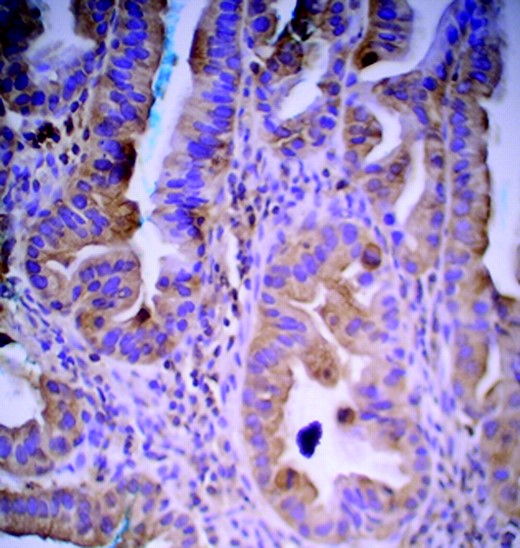
Cytoplasmic stains of phosphorylated mammalian target of rapamycin showing overexpression in 40% of adenocarcinoma cells (magnification, 20×).
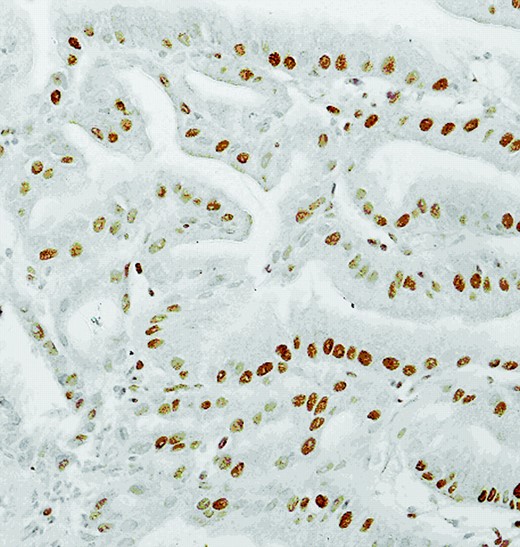
Nuclear stains of vascular endothelial growth factor receptor showing overexpression in 60% of adenocarcinoma cells (magnification, 20×).
Discussion
Overexpression of mTOR presents a potential role for its inhibition by rapamycin in the management of gastroesophageal cancer. Bile acid and a low pH environment were found to activate IKKβ, with subsequent phosphorylation and suppression of TSC1. This then leads to activation of the mTOR pathway in esophageal adenocarcinoma [2].
A retrospective review of 108 cases of esophageal cancer showed aberrant p-mTOR expression in 25% of specimens. There was an association with a lesser degree of differentiation but not with tumor location, tumor–node–metastasis stage, lymph node metastases, or expression of the proliferation marker Ki-67 [3]. On the other hand, Yu et al. [1] evaluated 1,072 cases of gastric cancer and found a p-mTOR overexpression rate of 46.5%. This was associated with lymph node metastases, advanced-stage disease, and a shorter median survival time at every stage. In fact, strong expression of p-mTOR was reported in up to 64% of diffuse-type and 60% of intestinal-type gastric cancers [4].
A phase II trial involving everolimus (an oral inhibitor of mTOR) monotherapy in 53 patients with previously treated metastatic gastric cancer showed a disease control rate of 56% and median progression-free survival interval of 2.7 months. The treatment was generally well tolerated and the median overall survival time was 10.1 months [5].
Thalidomide is a known inhibitor of angiogenesis [6] and an immunomodulator. It inhibited tumor growth and reduced microvessel density in mice implanted with esophageal cancer cells that expressed VEGF and/or basic fibroblast growth factor [7]. Thalidomide may also improve lean body mass and weight loss [8].
The combination of an antiangiogenic agent, bevacizumab, and rapamycin was shown to reduce tumor growth in mouse models of hepatocellular carcinoma more significantly than either bevacizumab or rapamycin alone [9]. Similarly, the combination of rapamycin and a thalidomide analog in the treatment of multiple myeloma (MM) patients showed much synergism. This combination overcame both resistance to conventional chemotherapy in MM cell lines and the protective effects on tumor cells by interleukin-6, insulin-like growth factor-1, and bone marrow stromal cells [10].
Given the evidence of potential synergy, an antiangiogenic agent was considered, and thalidomide, rather than bevacizumab, was chosen because of cost concerns. To our knowledge, there are no known clinical benefits of thalidomide monotherapy in gastric cancer, aside from the apparent dose-dependent effects on recurrent bleeding in one case of gastric cancer [11].
Rapamycin is known to upregulate HER-2 expression paradoxically via mTOR complex 2 activation [12], whereas trastuzumab, a monoclonal antibody against HER-2, leads to a longer median overall survival time when added to chemotherapy in HER-2+ advanced gastric cancer patients [13]. Treatment of mice bearing HER-2+ mammary tumors with both trastuzumab and rapamycin was more effective than treatment with either alone [14]. However, positive HER-2 expression was not detected in that patient, which is consistent with about 90% of our local gastric cancer series (unpublished data), a frequency lower than in the ToGA trial.
In our patient, the tumor marker CA 19–9 responded markedly after starting rapamycin, and the downward trend has continued so far. The beneficial effect may, however, be confounded or consolidated by the later addition of thalidomide. Scan evidence of disease progression was apparent only after approximately 7 months of treatment.
Prior staining of biomarkers could have identified potential responders to targeted agents and, in the future, could permit better individualization of treatment.
Conclusion
The combination of rapamycin and thalidomide in our patient with metastatic gastroesophageal carcinoma demonstrated disease stability associated with a significant tumor marker response and improved clinical quality of life. This suggests a potential for further evaluation of mTOR inhibition with an antiangiogenic agent in gastroesophageal carcinomas upon identification of the appropriate biomarkers.
Author Contributions
Conception/Design: Han Chong Toh
Provision of study material or patients: Han Chong Toh
Collection and/or assembly of data: Sin Jen Ong, Marissa Teo, Kiat Hon Lim
Data analysis and interpretation: Sin Jen Ong, Marissa Teo, Kiat Hon Lim
Manuscript writing: Sin Jen Ong, Su Pin Choo
Final approval of manuscript: Han Chong Toh
References
Author notes
Disclosures: Sin Jen Ong: None; Marissa Teo: None; Kiat Hon Lim: None; Su Pin Choo: None; Han Chong Toh: None.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors or independent peer reviewers.



