-
PDF
- Split View
-
Views
-
Cite
Cite
Elad Shemesh, Mahmood Abu Akel, Joy Feld, Devy Zisman, Nizar Hijaze, Ashraf Hamdan, Keren Zissman, Lupus myocarditis in an octogenarian patient—a case report, Oxford Medical Case Reports, Volume 2020, Issue 10, October 2020, omaa094, https://doi.org/10.1093/omcr/omaa094
Close - Share Icon Share
Abstract
Lupus myocarditis is a relatively rare manifestation of systemic lupus erythematosus. The majority of patients who experience myocardial involvement are females of young age. Here, we report a case of an 87-year-old male who was hospitalized because of perimyocarditis 2 weeks after undergoing percutaneous coronary intervention. Despite standard therapy his condition worsened, biomarkers and inflammation indices remained elevated, and pericardial effusion accumulated. The use of cardiac magnetic resonance (CMR) imaging, along with thorough history taking and testing for relevant antibodies allowed to establish the unusual diagnosis of lupus myocaridits. We demonstrate that lupus myocarditis may occur even in elderly males, as supported by characteristic CMR features.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disorder classically affecting young women. SLE can involve various systems, including the joints, kidneys, brain, skin and the heart [1]. Heart involvement may include the pericardium, myocardium, endocardium, heart valves and coronary arteries [2–4]. If the myocardium is indeed involved, high index of suspicion and prompt therapy are required, due to the risk of life-threatening events such as arrythmias, conduction abnormalities and heart failure [5].
We report an unusual late presentation of SLE myocarditis in an elderly patient who was admitted to the cardiac care unit due to weakness, chest pain and ST elevation MI.
CASE REPORT
An 87-year-old male, married and father of two, was hospitalized in the cardiac care unit because of fatigue and dyspnea 2 weeks after undergoing percutaneous coronary intervention to M3 coronary branch because of non-ST elevation myocardial infarction. His past medical history included ischemic heart disease (previous coronary intervention to the left anterior descending and the right coronary arteries in 2008), hypertension and dyslipidemia. Physical examination in the time of admission revealed blood pressure of 122/61 mm Hg, 69 bpm, core temperature of 36.5°C, signs of volume overload, including elevated jugular venous pressure and bilateral leg pitting edema. Examination of other systems, including the head, mouth, abdomen and skin did not demonstrate any further findings. Electrocardiographic recordings demonstrated a right bundle branch block pattern, along with ST segment elevation in the anterior wall territory (Fig. 1A). Troponin at admission was 4634 ng/dl (normal values: 0–14 ng/dl) (Table 1). An urgent cardiac catheterization was performed, in which all coronary arteries were evaluated as patent (Fig. 1B). On an echocardiographic exam a pericardial effusion was evident (Fig. 2). Therefore, the initial diagnosis was perimyocarditis. Indeed, C-reactive protein (CRP) at admission was high (6.86 mg/dl, normal values: 0.67–117 mg/dl) (Table 1). Treatment with high-dose aspirin and diuretics did not result in significant improvement: volume overload was still present, CRP levels remained high, and creatinine levels increased from a baseline of 1.22 mg/dl up to 2.13 mg/dl (Table 1). Further information regarding the route, doses and regiments of treatment are provided in Table 2. Notably, NT-proBNP peaked to nearly 30 000 pg/ml (normal values: 0–300 pg/ml) (Table 1). Due to impaired renal function attributed to cardiorenal interaction (Table 1), treatment was changed to high-dose steroids (Prednisone, 60 mg/day) (Table 2), and alternative diagnoses were explored: further history taking revealed that nearly 20 years ago he suffered from shortness of breath and was diagnosed with pleural effusion along with intermittent episodes of arthritis of the metacarpal phalangeal joints in both hands, skin rash consistent with subacute lupus erythematosus as confirmed by a skin biopsy SLE was then diagnosed, based on these features along with the presence of positive antinuclear antibody (ANA), anti-Smith and anti-RNP antibodies, thus fulfilling classification criteria for SLE [5], hydroxycholoroquine was initiated, leading to a quiescent state. He was lost to follow-up and did not take his medication regularly. Current evaluation ruled out any extracardiac symptoms or signs of a SLE flare. Laboratory evaluation revealed positive ANA with speckled pattern at a titter of 1:160 and elevated anti-Smith antibodies (3.9 AI, normal values: 0–0.9 AI) (Table 1). Finally, cardiac magnetic resonance (CMR) imaging was done (Fig. 3), demonstrating small left pleural effusion, moderate pericardial effusion, extensive late gadolinium (LGE) enhancement involving the midwall of the interventricular septum, epicardium of the inferolateral wall as well as the right ventricular free wall, hyperintense T2 signal indicating septal edema. Hence, the diagnosis of isolated SLE myocardial flare was established. The patient’s clinical condition improved while treated with high-dose prednisone, as supported by marked decrease in biomarker (troponin, NT-proBNP) and creatinine levels.
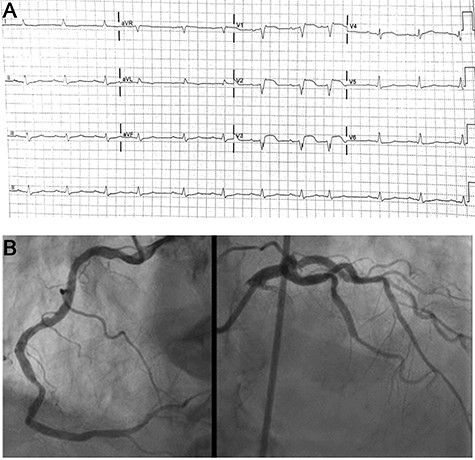
(A) ECG recordings at admission. ST elevation in v1-v3 leads; (B) Coronary angiography demonstrating patent coronary arteries without significant disease.
| Lab test . | Admission . | Day 5 . | Day 10 . | Day 16—discharge . |
|---|---|---|---|---|
| Creatinine (mg/dl) | 1.97 | 2.13 | 1.32 | 1.38 |
| CRP (mg/dl) | 5.3 | 1.29 | 0.8 | 0.41 |
| Troponin (ng/dl) | 4634 | 2750 | 1052 | |
| NT-pro-BNP (pg/ml) | 30 922 | 25 504 | ||
| Hemoglobin (g/dl) | 8.6 | 9.9 | 11 | 10.8 |
| White blood cells (k/ul) | 5.31 | 7.58 | 11.72 | 11.4 |
| Lab test . | Admission . | Day 5 . | Day 10 . | Day 16—discharge . |
|---|---|---|---|---|
| Creatinine (mg/dl) | 1.97 | 2.13 | 1.32 | 1.38 |
| CRP (mg/dl) | 5.3 | 1.29 | 0.8 | 0.41 |
| Troponin (ng/dl) | 4634 | 2750 | 1052 | |
| NT-pro-BNP (pg/ml) | 30 922 | 25 504 | ||
| Hemoglobin (g/dl) | 8.6 | 9.9 | 11 | 10.8 |
| White blood cells (k/ul) | 5.31 | 7.58 | 11.72 | 11.4 |
| Lab test . | Admission . | Day 5 . | Day 10 . | Day 16—discharge . |
|---|---|---|---|---|
| Creatinine (mg/dl) | 1.97 | 2.13 | 1.32 | 1.38 |
| CRP (mg/dl) | 5.3 | 1.29 | 0.8 | 0.41 |
| Troponin (ng/dl) | 4634 | 2750 | 1052 | |
| NT-pro-BNP (pg/ml) | 30 922 | 25 504 | ||
| Hemoglobin (g/dl) | 8.6 | 9.9 | 11 | 10.8 |
| White blood cells (k/ul) | 5.31 | 7.58 | 11.72 | 11.4 |
| Lab test . | Admission . | Day 5 . | Day 10 . | Day 16—discharge . |
|---|---|---|---|---|
| Creatinine (mg/dl) | 1.97 | 2.13 | 1.32 | 1.38 |
| CRP (mg/dl) | 5.3 | 1.29 | 0.8 | 0.41 |
| Troponin (ng/dl) | 4634 | 2750 | 1052 | |
| NT-pro-BNP (pg/ml) | 30 922 | 25 504 | ||
| Hemoglobin (g/dl) | 8.6 | 9.9 | 11 | 10.8 |
| White blood cells (k/ul) | 5.31 | 7.58 | 11.72 | 11.4 |
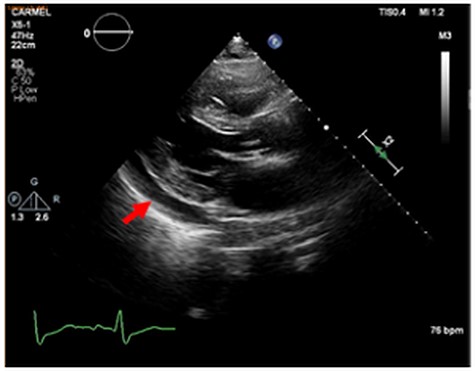
Echocardiographic study, long axis view. Red arrow marks pericardial effusion.
Length, doses and routes of colchecine, aspirin, prednisone and diuretics during hospitalization
| Drug . | Route . | Initiation date and dose at initiation . | Changes made . | Length of treatment during hospitalization . |
|---|---|---|---|---|
| Colchicine | Oral | AD+1; 0.5 mg | None | 15 days |
| Aspirin | Oral | AD+1; 2400 mg | Stopped after 4 days due to deterioration in renal function | 4 days |
| Prednisone | Oral | AD+4; 60 mg | None | 12 days |
| Fusid | IV | AD+3; 60 mg | Dose increased to 80 mg on AD+4, to 120 mg on AD+7, and then decreased back to 60 mg on AD+11 | 12 days |
| Dopamine | IV | AD+4; 4mcg/kg/min | None | 8 days |
| Drug . | Route . | Initiation date and dose at initiation . | Changes made . | Length of treatment during hospitalization . |
|---|---|---|---|---|
| Colchicine | Oral | AD+1; 0.5 mg | None | 15 days |
| Aspirin | Oral | AD+1; 2400 mg | Stopped after 4 days due to deterioration in renal function | 4 days |
| Prednisone | Oral | AD+4; 60 mg | None | 12 days |
| Fusid | IV | AD+3; 60 mg | Dose increased to 80 mg on AD+4, to 120 mg on AD+7, and then decreased back to 60 mg on AD+11 | 12 days |
| Dopamine | IV | AD+4; 4mcg/kg/min | None | 8 days |
AD, admission day; IV, intravenous.
Length, doses and routes of colchecine, aspirin, prednisone and diuretics during hospitalization
| Drug . | Route . | Initiation date and dose at initiation . | Changes made . | Length of treatment during hospitalization . |
|---|---|---|---|---|
| Colchicine | Oral | AD+1; 0.5 mg | None | 15 days |
| Aspirin | Oral | AD+1; 2400 mg | Stopped after 4 days due to deterioration in renal function | 4 days |
| Prednisone | Oral | AD+4; 60 mg | None | 12 days |
| Fusid | IV | AD+3; 60 mg | Dose increased to 80 mg on AD+4, to 120 mg on AD+7, and then decreased back to 60 mg on AD+11 | 12 days |
| Dopamine | IV | AD+4; 4mcg/kg/min | None | 8 days |
| Drug . | Route . | Initiation date and dose at initiation . | Changes made . | Length of treatment during hospitalization . |
|---|---|---|---|---|
| Colchicine | Oral | AD+1; 0.5 mg | None | 15 days |
| Aspirin | Oral | AD+1; 2400 mg | Stopped after 4 days due to deterioration in renal function | 4 days |
| Prednisone | Oral | AD+4; 60 mg | None | 12 days |
| Fusid | IV | AD+3; 60 mg | Dose increased to 80 mg on AD+4, to 120 mg on AD+7, and then decreased back to 60 mg on AD+11 | 12 days |
| Dopamine | IV | AD+4; 4mcg/kg/min | None | 8 days |
AD, admission day; IV, intravenous.
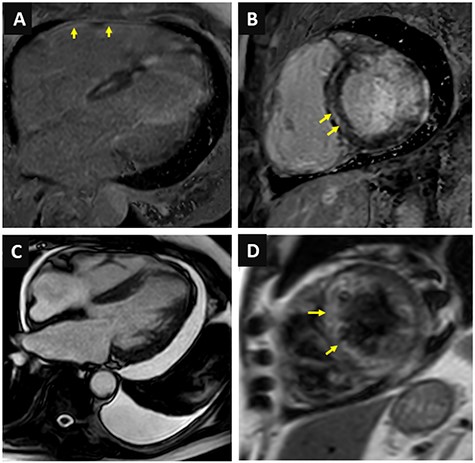
Cardiac magnetic resonance imaging. (A) Late gadolinium enhancement of the right ventricular free wall; (B) Mid-wall septal late gadolinium enhancement; (C) Moderate pericardial effusion and small left-sided pleural effusion; (D) High signal intensity in T2 weighted images.
DISCUSSION
Previous reports showed that prevalence of lupus myocarditis is 5–10% of patients [3, 4], and mortality may be as high as 10.3% according to a large case series from Paris [6]. Although other studies report that concomitant lupus activity in various organ occurs in 97% of the patients with lupus myocarditis [7], our report provides a rare evidence that lupus myocarditis may be a sole manifestation. Clearly, given the rarity of myocardial involvement, diagnosis is elusive. Previously, endomyocardial biopsy was considered a gold standard [8] but, given its poor predictive value and potential complications and its invasive nature, other modalities have emerged. Of note, CMR can be used to detect myocardial involvement in SLE. Specific patterns that were reported in previous reports of patients with SLE myocarditis included T2 signal abnormalities, decreased cardiac function, LGE [9]. These features were also evident in our patient (Fig. 3), and together with elevated biomarkers and positive antibodies suggested that SLE is the most probable diagnosis, although the absence of myocardial biopsy precluded a definitive answer.
We contend that this report is of particular importance given the exceptional age and gender of the patient at diagnosis and the presentation at the time of the flare, which was limited to the heart. Furthermore, this report highlights the important role of CMR in diagnosing SLE myocarditis.
In conclusion, this report demonstrates that SLE myocarditis may complicate octogenarian patients as an isolated manifestation. CMR is a relevant and important diagnostic method for SLE myocarditis, with the potential to decrease the need for tissue biopsies.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICAL APPROVAL
Not applicable.
CONSENT
Written consent obtained.
GUARANTOR
E.S. is nominated as the manuscript’s guarantor.
ACKNOWLEDGMENT
This case report had no source of funding.



