-
PDF
- Split View
-
Views
-
Cite
Cite
Mario A Martinez, Ting-Yi Chen, Hosoon Choi, Munok Hwang, Dhammika Navarathna, Linhue Hao, Michael Gale, Gregory Camus, Hector E Ramirez, Chetan Jinadatha, Extended Remdesivir Infusion for Persistent Coronavirus Disease 2019 Infection, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac382, https://doi.org/10.1093/ofid/ofac382
Close - Share Icon Share
Abstract
Persistent severe acute respiratory syndrome coronavirus 2 infection is difficult to treat. Here, we report a case of 5-month persistent coronavirus disease 2019 in an immunocompromised patient who was successfully treated with 30 consecutive days of remdesivir. Prolonged remdesivir infusion with concurrent cycle threshold monitoring might provide a potential solution to cure these patients with difficult-to-treat infections.
Severe persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections have been reported in immunocompromised patients [1, 2]. However, the treatment strategies for these patients remain unclear and mainly consist of supportive care beyond the initial antiviral and immunotherapy [3]. Additionally, remdesivir was the only antiviral available in the inpatient setting. Monoclonal antibodies, nirmatrelvir and ritonavir, and molnupiravir are not approved for use in inpatient settings in the United States. Here we report a case of a patient with persistent coronavirus disease 2019 (COVID-19) proven by viral culture requiring a total of 5 hospital admissions. He was eventually treated with extended remdesivir infusion guided by nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) to prevent symptomatic recurrence.
CASE REPORT
A 44-year-old patient with underlying granulomatosis with polyangiitis and secondary hypogammaglobulinemia presented to our hospital in August 2020 with fever, chills, exertional dyspnea, and diarrhea. His comorbidities included hypogammaglobulinemia, chronic kidney disease, hypertension, obesity, deep venous thrombosis, and gout. At the time of admission, patient was taking allopurinol, warfarin, prednisone, and monthly intravenous immunoglobulin G (IVIG). Patient received rituximab for 2 years until 4 years prior to admission for granulomatosis. This was followed by mycophenolate and then high-dose prednisone with slow taper. At the time of admission for coronavirus disease 2019 (COVID-19), he was on prednisone 5 mg daily. COVID-19 was diagnosed by positive nasopharyngeal SARS-CoV-2 PCR on day 1. He received 5 days of remdesivir plus 10 days of dexamethasone and was discharged on room air. He presented with similar symptoms and was admitted from days 17 to 37. During that admission, he received convalescent plasma along with supportive care. He again developed fever, dyspnea, and hypoxia that required a third hospitalization from days 52 to 70. He had progressive chest imaging findings consistent with COVID-19 pneumonia. Extensive infectious diseases workup was all negative except elevated β-d-glucan. He was empirically treated 21 days for Pneumocystis pneumonia and discharged home on 2 L of oxygen and on prophylaxis with atovaquone. Five days later (day 75), he was admitted again (fourth admission day 75–103) with fever and worsening dyspnea, and repeat nasopharyngeal SARS-CoV-2 PCR remained positive. He received 10 days of remdesivir plus dexamethasone with significant improvement of clinical symptoms. On day 110, he presented with fever and dyspnea to the emergency department. Nasopharyngeal SARS-CoV-2 PCR was positive and SARS-CoV-2 immunoglobulin M and immunoglobulin G were negative.
Inhaled remdesivir has also been shown to reduce viral burden in a nonhuman primate model of SARS-CoV-2 infection [4]. Remdesivir has been shown to significantly reduce SARS-CoV-2 viral load in nasopharyngeal (NP) swabs in hospitalized patients with COVID-19 infection [5]. At this point, we ventured a strategy of extended remdesivir administration based partly on cycle threshold (Ct) value as a guide but primarily on the goal of negative nasopharyngeal SARS-CoV-2 PCR results, which we interpreted as a marker of complete eradication of viable virus during his fifth admission (days 110–142). He received a total of 30 days of remdesivir until he had repeatedly tested negative for nasopharyngeal SARS-CoV-2 PCR twice 24 hours apart. The patient did receive dexamethasone, convalescent plasma, and IVIG treatment throughout the course of illness and hospitalization but never received other immunomodulators such as tocilizumab or JAK inhibitors. The clinical course and administration of remdesivir are shown in Figure 1A. The Ct value during remdesivir infusion was monitored (Figure 1B). The patient was monitored for side effects throughout the duration of treatment with daily examinations, and 2–3 times weekly complete blood count and comprehensive metabolic profile. After the completion of treatment we did monthly telephonic visits with the patient for 3 months and then quarterly. The only minor side effect reported was some hair thinning, which reversed quickly. We are unsure if that was due to a medication side effect or overall chronic illness from prolonged infection. The patient was successfully cured with 30 days of remdesivir (without dexamethasone) using this strategy and, at 12-month follow-up, there is no indication of recurrence of symptoms (Table 1).
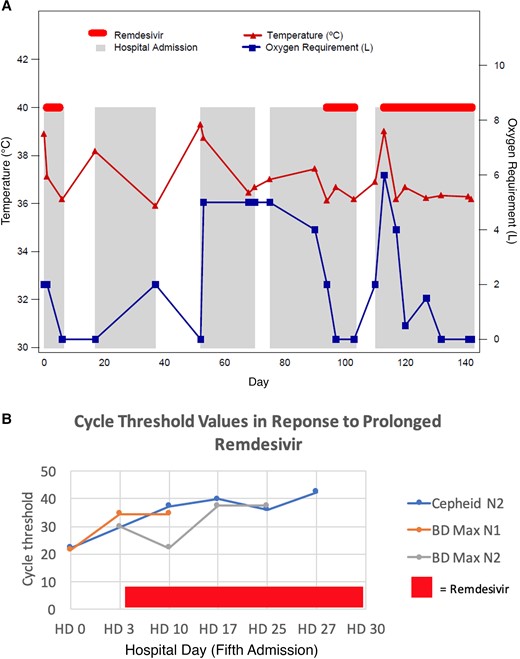
Clinical response to standard and extended infusions of remdesivir with corresponding cycle threshold values. Abbreviation: HD, hospital day.
Admission Details, Clinical Presentation, Radiological Features, and Treatment
| Admission . | Day . | COVID-19 Treatment . | Other Treatment . | Status . | Oxygen Requirement . | Relevant Laboratory Tests . | Radiology . |
|---|---|---|---|---|---|---|---|
| 1 | 0–7 | D1–5 remdesivir D1–10 dexamethasone | Ceftriaxone + azithromycin | AD | NC 2L | CRP: 3.3 mg/dL | D0 CXR: subtle ground glass opacities in the LUL and RML |
| HOS | NC 2L | … | … | ||||
| DIS | RA | … | … | ||||
| 2 | 18–37 | D23 convalescent plasma | Broad-spectrum antibacterial antibiotics followed by atovaquone for presumed PJP | AD | RA | CRP: 5.3 mg/dL | D18 CXR: consolidating infiltrates in LML |
| HOS | NC 2–5L | CRP: 11.9 mg/dL | D22 CT chest: worsening airspace disease with large LUL and LLL ground glass opacities | ||||
| DIS | NC 2L | … | D34 CT chest: interval worsening of diffuse ground glass opacities throughout the right lung. Findings are consistent with history of COVID-19 pneumonia. New subpleural consolidation in the RLL. Improved but residual ground glass opacities in the left lung | ||||
| 3 | 52–70 | Cefepime + levofloxacin + atovaquone prophylaxis | AD | RA | CRP: 17.2 mg/dL | D52 CXR: consolidation and atelectasis of the lower lungs bilaterally and ground glass densities at the LML | |
| HOS | NC 5L | CRP: 9.5 mg/dL | D62 CT chest: difference in distribution of the nonspecific scattered ground glass opacities of the lungs | ||||
| DIS | NC 5L | … | … | ||||
| 4 | 75–103 | D94–103 remdesivir Dexamethasone | Vancomycin Cefepime followed by ampicillin + sulbactam for Escherichia coli cultured from sputum | AD | NC 5L | CRP: 17.9 mg/dL | D75 CT chest: persistence of extensive bilateral pulmonary ground glass density |
| HOS | NC 5L-HF NC-RA | … | D90 CT chest: extensive bilateral ground glass infiltrates with some mixed interval change | ||||
| DIS | RA | … | … | ||||
| 5 | 110–142 | D111 convalescent plasma D113–142 remdesivir | AD | NC 6L | CRP: 9.2 mg/dL | D111 CT chest: redemonstration of extensive bilateral ground glass opacities with some mixed interval change but an overall similar imaging pattern to the prior study | |
| HOS | NC2L-HF NC-RA | CRP: 1.7 mg/dL | D125 CXR: ground glass opacities of the lungs bilaterally have decreased from prior study (taken on D105) | ||||
| DIS | RA | CRP: 0.8 mg/dL at 12 mo after discharge | D141 CXR: lung fields appear essentially clear on current study. Appearance suggests resolved bilateral pneumonia |
| Admission . | Day . | COVID-19 Treatment . | Other Treatment . | Status . | Oxygen Requirement . | Relevant Laboratory Tests . | Radiology . |
|---|---|---|---|---|---|---|---|
| 1 | 0–7 | D1–5 remdesivir D1–10 dexamethasone | Ceftriaxone + azithromycin | AD | NC 2L | CRP: 3.3 mg/dL | D0 CXR: subtle ground glass opacities in the LUL and RML |
| HOS | NC 2L | … | … | ||||
| DIS | RA | … | … | ||||
| 2 | 18–37 | D23 convalescent plasma | Broad-spectrum antibacterial antibiotics followed by atovaquone for presumed PJP | AD | RA | CRP: 5.3 mg/dL | D18 CXR: consolidating infiltrates in LML |
| HOS | NC 2–5L | CRP: 11.9 mg/dL | D22 CT chest: worsening airspace disease with large LUL and LLL ground glass opacities | ||||
| DIS | NC 2L | … | D34 CT chest: interval worsening of diffuse ground glass opacities throughout the right lung. Findings are consistent with history of COVID-19 pneumonia. New subpleural consolidation in the RLL. Improved but residual ground glass opacities in the left lung | ||||
| 3 | 52–70 | Cefepime + levofloxacin + atovaquone prophylaxis | AD | RA | CRP: 17.2 mg/dL | D52 CXR: consolidation and atelectasis of the lower lungs bilaterally and ground glass densities at the LML | |
| HOS | NC 5L | CRP: 9.5 mg/dL | D62 CT chest: difference in distribution of the nonspecific scattered ground glass opacities of the lungs | ||||
| DIS | NC 5L | … | … | ||||
| 4 | 75–103 | D94–103 remdesivir Dexamethasone | Vancomycin Cefepime followed by ampicillin + sulbactam for Escherichia coli cultured from sputum | AD | NC 5L | CRP: 17.9 mg/dL | D75 CT chest: persistence of extensive bilateral pulmonary ground glass density |
| HOS | NC 5L-HF NC-RA | … | D90 CT chest: extensive bilateral ground glass infiltrates with some mixed interval change | ||||
| DIS | RA | … | … | ||||
| 5 | 110–142 | D111 convalescent plasma D113–142 remdesivir | AD | NC 6L | CRP: 9.2 mg/dL | D111 CT chest: redemonstration of extensive bilateral ground glass opacities with some mixed interval change but an overall similar imaging pattern to the prior study | |
| HOS | NC2L-HF NC-RA | CRP: 1.7 mg/dL | D125 CXR: ground glass opacities of the lungs bilaterally have decreased from prior study (taken on D105) | ||||
| DIS | RA | CRP: 0.8 mg/dL at 12 mo after discharge | D141 CXR: lung fields appear essentially clear on current study. Appearance suggests resolved bilateral pneumonia |
Abbreviations: AD, admission; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CXR, chest radiograph; D, day; DIS, on discharge; HF, high flow; HOS, during hospitalization; LLL, left lower lobe; LUL, left upper lobe; NC, nasal canula; PJP, Pneumocystis jirovecii pneumonia; RA, room air; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Admission Details, Clinical Presentation, Radiological Features, and Treatment
| Admission . | Day . | COVID-19 Treatment . | Other Treatment . | Status . | Oxygen Requirement . | Relevant Laboratory Tests . | Radiology . |
|---|---|---|---|---|---|---|---|
| 1 | 0–7 | D1–5 remdesivir D1–10 dexamethasone | Ceftriaxone + azithromycin | AD | NC 2L | CRP: 3.3 mg/dL | D0 CXR: subtle ground glass opacities in the LUL and RML |
| HOS | NC 2L | … | … | ||||
| DIS | RA | … | … | ||||
| 2 | 18–37 | D23 convalescent plasma | Broad-spectrum antibacterial antibiotics followed by atovaquone for presumed PJP | AD | RA | CRP: 5.3 mg/dL | D18 CXR: consolidating infiltrates in LML |
| HOS | NC 2–5L | CRP: 11.9 mg/dL | D22 CT chest: worsening airspace disease with large LUL and LLL ground glass opacities | ||||
| DIS | NC 2L | … | D34 CT chest: interval worsening of diffuse ground glass opacities throughout the right lung. Findings are consistent with history of COVID-19 pneumonia. New subpleural consolidation in the RLL. Improved but residual ground glass opacities in the left lung | ||||
| 3 | 52–70 | Cefepime + levofloxacin + atovaquone prophylaxis | AD | RA | CRP: 17.2 mg/dL | D52 CXR: consolidation and atelectasis of the lower lungs bilaterally and ground glass densities at the LML | |
| HOS | NC 5L | CRP: 9.5 mg/dL | D62 CT chest: difference in distribution of the nonspecific scattered ground glass opacities of the lungs | ||||
| DIS | NC 5L | … | … | ||||
| 4 | 75–103 | D94–103 remdesivir Dexamethasone | Vancomycin Cefepime followed by ampicillin + sulbactam for Escherichia coli cultured from sputum | AD | NC 5L | CRP: 17.9 mg/dL | D75 CT chest: persistence of extensive bilateral pulmonary ground glass density |
| HOS | NC 5L-HF NC-RA | … | D90 CT chest: extensive bilateral ground glass infiltrates with some mixed interval change | ||||
| DIS | RA | … | … | ||||
| 5 | 110–142 | D111 convalescent plasma D113–142 remdesivir | AD | NC 6L | CRP: 9.2 mg/dL | D111 CT chest: redemonstration of extensive bilateral ground glass opacities with some mixed interval change but an overall similar imaging pattern to the prior study | |
| HOS | NC2L-HF NC-RA | CRP: 1.7 mg/dL | D125 CXR: ground glass opacities of the lungs bilaterally have decreased from prior study (taken on D105) | ||||
| DIS | RA | CRP: 0.8 mg/dL at 12 mo after discharge | D141 CXR: lung fields appear essentially clear on current study. Appearance suggests resolved bilateral pneumonia |
| Admission . | Day . | COVID-19 Treatment . | Other Treatment . | Status . | Oxygen Requirement . | Relevant Laboratory Tests . | Radiology . |
|---|---|---|---|---|---|---|---|
| 1 | 0–7 | D1–5 remdesivir D1–10 dexamethasone | Ceftriaxone + azithromycin | AD | NC 2L | CRP: 3.3 mg/dL | D0 CXR: subtle ground glass opacities in the LUL and RML |
| HOS | NC 2L | … | … | ||||
| DIS | RA | … | … | ||||
| 2 | 18–37 | D23 convalescent plasma | Broad-spectrum antibacterial antibiotics followed by atovaquone for presumed PJP | AD | RA | CRP: 5.3 mg/dL | D18 CXR: consolidating infiltrates in LML |
| HOS | NC 2–5L | CRP: 11.9 mg/dL | D22 CT chest: worsening airspace disease with large LUL and LLL ground glass opacities | ||||
| DIS | NC 2L | … | D34 CT chest: interval worsening of diffuse ground glass opacities throughout the right lung. Findings are consistent with history of COVID-19 pneumonia. New subpleural consolidation in the RLL. Improved but residual ground glass opacities in the left lung | ||||
| 3 | 52–70 | Cefepime + levofloxacin + atovaquone prophylaxis | AD | RA | CRP: 17.2 mg/dL | D52 CXR: consolidation and atelectasis of the lower lungs bilaterally and ground glass densities at the LML | |
| HOS | NC 5L | CRP: 9.5 mg/dL | D62 CT chest: difference in distribution of the nonspecific scattered ground glass opacities of the lungs | ||||
| DIS | NC 5L | … | … | ||||
| 4 | 75–103 | D94–103 remdesivir Dexamethasone | Vancomycin Cefepime followed by ampicillin + sulbactam for Escherichia coli cultured from sputum | AD | NC 5L | CRP: 17.9 mg/dL | D75 CT chest: persistence of extensive bilateral pulmonary ground glass density |
| HOS | NC 5L-HF NC-RA | … | D90 CT chest: extensive bilateral ground glass infiltrates with some mixed interval change | ||||
| DIS | RA | … | … | ||||
| 5 | 110–142 | D111 convalescent plasma D113–142 remdesivir | AD | NC 6L | CRP: 9.2 mg/dL | D111 CT chest: redemonstration of extensive bilateral ground glass opacities with some mixed interval change but an overall similar imaging pattern to the prior study | |
| HOS | NC2L-HF NC-RA | CRP: 1.7 mg/dL | D125 CXR: ground glass opacities of the lungs bilaterally have decreased from prior study (taken on D105) | ||||
| DIS | RA | CRP: 0.8 mg/dL at 12 mo after discharge | D141 CXR: lung fields appear essentially clear on current study. Appearance suggests resolved bilateral pneumonia |
Abbreviations: AD, admission; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CXR, chest radiograph; D, day; DIS, on discharge; HF, high flow; HOS, during hospitalization; LLL, left lower lobe; LUL, left upper lobe; NC, nasal canula; PJP, Pneumocystis jirovecii pneumonia; RA, room air; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
VIRAL CULTURE, WHOLE GENOME SEQUENCING, AND REMDESIVIR EFFECTIVENESS ESSAY
SARS-CoV-2 isolates from NP specimens were sent for culture, proven to be viable, and subjected to whole genome sequencing (WGS) (Supplementary Materials and Methods). Unfortunately, the day 0 NP specimen was not available for sequencing. WGS revealed the virus’ lineage was B.1.369 and contained the P314L mutation in the RNA-dependent RNA polymerase (RdRP) gene, encoded at ORF1b. B.1.369 belongs to the same phylogenetic cluster as the Iota (B.1.526), Beta (B.1.351), and Mu (B.1.621) variants (Supplementary Table 1Aand Supplementary Figure 1A). This correlates well with the time these viruses emerged in the population. The likelihood that the sequenced viruses were different on day 0 (ie, reinfection vs persistent infection) because of missing sample is unlikely because it was within 90 days of the first presentation. We believe the chances of reinfection within 90 days during that phase of the pandemic and probably even today are highly unlikely. The guidance from the Centers for Disease Control and Prevention at that time was not to recollect NP specimen in a recent positive as it likely to be positive; hence, day 18 NP specimen was not collected. An in vitro remdesivir susceptibility assay demonstrated that our patient’s isolate (B.1.369) was effectively inhibited by remdesivir, thus showing that the P314L nsp12/RdRp mutation seen by WGS had no effect on remdesivir activity (Supplementary Table 1Band Supplementary Figure 1B and 1C). No other nsp12/RdRp substitution was identified (Supplementary Table 1B). In a previous case of persistent SARS-CoV-2 viremia in an immunodeficient patient, a 76-year-old woman with a postrituximab B-cell immunodeficiency for whom remdesivir therapy failed, the authors found a new RdRP mutation, D484Y, that did not exist pretreatment. However, the lack of susceptibility testing did not conclusively prove association of treatment failure and resistance because of the reported new point mutation [6]. Another report did identify a new point mutation nsp12 E802D during treatment of a patient with acquired B-cell deficiency who had developed an indolent persistent SARS-CoV-2 infection and failed remdesivir therapy but ultimately responded after treatment with casirivimab-imdevimab. In vitro experiments confirmed an approximately 6-fold increase in remdesivir inhibitory concentration 50 (IC50) associated with the mutation, which also resulted in a fitness cost in the absence of remdesivir [7]. The same E802D mutation was previously identified in an in vitro remdesivir resistance selection experiment, and found to confer an approximately 2.5-fold increase in IC50 to the drug [8]. In our case, we were able to extend remdesivir treatment for a total of 30 days in this immunocompromised patient with persistent COVID-19 to overcome the lack of sustained virologic response to standard duration of 10 days and achieved clinical cure. We did not observe any side effects or laboratory abnormalities due to the extension. Remdesivir was (and still is) the only antiviral treatment option for hospitalized patients shaping this therapeutic decision for our patient. This is the first case report of extended remdesivir infusion beyond the standard 10 days of recommended therapy by the manufacturer. This supports further study of extended remdesivir therapy in immunocompromised patients with persistent infection and symptoms, as an alternative treatment management along with other reported option of monoclonal antibodies [7, 9]. Our patient has remained symptom-free at 12 months of follow-up.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. A. M. and T.-Y.C. contributed to data collection and manuscript writing. D. N., H. C., and M. H. contributed to polymerase chain reaction (PCR) and whole genome sequencing (WGS). L. H. and M. G. contributed to viral culture and remdesivir effective assay. G. C. contributed to technical review and manuscript writing, C. J. contributed to PCR, WGS, and manuscript writing.
Acknowledgments. The authors thank Maricel De Mesa, PhD, for helping in formatting the figures.
Patient consent. A signed informed consent from the patient was obtained, and the institutional review board of the Central Texas Veterans Health Care System, Temple, Texas, exempted this case report as per local policy.
Financial support. This work was supported by a grant from the Office of Research and Development, Department of Veterans Affairs as part of funding for VASeqCURE (C. J., principal investigator), which in turn received funding from the American Rescue Plan Act funds and with additional support from the Central Texas Veterans Health Care System. L. H. and M. G. are supported by the National Institutes of Health (grant number AI151698).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Potential conflicts of interest. G. C. is an employee and shareholder of Gilead Sciences. All other authors report no potential conflicts.





Comments