-
PDF
- Split View
-
Views
-
Cite
Cite
Katelyn A Pastick, Enock Kagimu, Joanna Dobbin, Kenneth Ssebambulidde, Jane Gakuru, Jack Milln, Betty Nakabuye, David B Meya, David R Boulware, Fiona V Cresswell, Nathan C Bahr, Pregnancy-Related Tuberculous Meningitis and Immune Reconstitution Inflammatory Syndrome: A Case Series and Systematic Review, Open Forum Infectious Diseases, Volume 9, Issue 10, October 2022, ofac513, https://doi.org/10.1093/ofid/ofac513
Close - Share Icon Share
Abstract
Tuberculosis is a leading cause of death among women of reproductive age. However, tuberculous meningitis, the most severe form of extrapulmonary tuberculosis, is rarely discussed in pregnancy despite this being a unique period of immune modulation that may predispose women to active disease.
We identified and described cases of tuberculous meningitis among pregnant or postpartum women screened during meningitis clinical trials in Uganda from 2018 to 2022. We conducted a systematic literature review via PubMed/Medline and Embase for all English-language publications from 1970 to 10 July 2022, to identify additional cases.
We identified 8 cases of pregnancy-related tuberculous meningitis in Ugandan women living with human immunodeficiency virus (HIV) and 40 additional cases via systematic literature review (none HIV-positive). Of all combined cases, 50% (24/48) were diagnosed postpartum; 50% (24/48) had initial onset during pregnancy, of which 38% (9/24) had worsening of symptoms or disease relapse following pregnancy cessation. Diagnosis was missed or delayed in 33% (16/48) of cases. For those with known outcomes, maternal mortality was 23% (11/48) and fetal/neonatal mortality was 30% (13/44). Of maternal survivors, 30% (11/37) had residual neurologic deficits.
The true incidence of tuberculous meningitis in pregnancy or the postpartum period is unclear but likely underappreciated. To date, nearly all published cases have occurred in HIV-negative or otherwise immunocompetent women. Given the well-described physiological immunosuppression during pregnancy and subsequent reconstitution postpartum, physicians must be aware of tuberculous meningitis and pregnancy-related immune reconstitution inflammatory syndrome, especially in countries with a high burden of tuberculosis and in women living with HIV.
Tuberculosis (TB) is one of the leading causes of death among women of reproductive age and is associated with preterm birth, intrauterine growth restriction, subsequent low birth weight, and a 6-fold increase in perinatal death [1]. Tuberculous meningitis (TB meningitis) accounts for 1%–2% of TB cases in individuals without human immunodeficiency virus (HIV), 4%–7% of all TB cases in people living with HIV, and around 15%–20% of HIV-associated meningitis diagnoses [2]. Late presentation and delayed diagnosis are key contributors to a high mortality rate (∼30% overall) [2]. The exact incidence of TB meningitis in pregnant women is unknown; however, 10%–50% of participants in published TB meningitis cohorts were women of reproductive age [3–7].
Pregnancy is a period of systemic immune modulation with a complex balance of immunosuppression to prevent rejection of the fetus and maintenance of the ability to fight off infections. Specific immunologic changes in pregnancy include an up-regulation of regulatory T cells and a switch from type 1 T-helper (Th1) cells and proinflammatory cytokines (eg, interleukin [IL]–12, interferon-γ, tumor necrosis factor [TNF]–α) to type 2 T-helper (Th2) (eg, IL-10) and type 3 T-helper (eg, TNF-β) cytokines, resulting in reduced cell-mediated immunity [8, 9]. These changes rapidly reverse following delivery with immune reconstitution, resulting in postpartum flares of rheumatologic or infectious diseases [8, 10, 11]. Importantly, Th2-predominant immune responses are associated with progression and severity of TB disease [12, 13]. These changes may ultimately increase the risk for TB (including TB meningitis) in pregnancy, and rapid postpartum immune reconstitution may contribute to worsening of TB symptoms and increase in postpartum diagnoses [14–16].
Immune reconstitution inflammatory syndrome (IRIS) in people living with HIV may describe the worsening or unmasking of a subclinical disease following antiretroviral therapy (ART) initiation (ie, unmasking IRIS) or symptom recurrence in a partially treated infection following ART initiation (ie, paradoxical IRIS) [17]. In HIV-associated TB meningitis, IRIS most frequently occurs 2–4 weeks after starting ART but can occur up to 3 months later and may occur in up to 50% of cases [17]. Importantly, paradoxical IRIS and similar reactions can affect HIV-negative persons following reversal of immunosuppressive conditions, such as cessation of pregnancy, medications, or start of TB therapy [18]. Cases of IRIS associated with pregnancy have been reported in HIV-positive women with Kaposi sarcoma [19, 20], toxoplasmosis [21], cryptococcosis [22–27], and in HIV-negative women with TB meningitis [28]. Yet to our knowledge, there have been no descriptions of pregnancy-related TB meningitis IRIS in women living with HIV to date. We describe the first 8 cases of HIV-associated TB meningitis in pregnancy and the postpartum period and report the findings of a systematic literature review evaluating overall outcomes.
METHODS
Case Series and Patient Consent Statement
From September 2018 to April 2022, persons with suspected meningitis were enrolled in 3 studies at Kiruddu National Referral Hospital in Kampala, Uganda. One cohort study focused on improving diagnostics and neurocognitive outcomes for those presenting with meningitis [29, 30]. The other 2 studies were phase 2 and 3 randomized controlled trials that compared high-dose rifampicin with standard-dose rifampicin [31, 32]. Institutional review board (IRB) approvals were obtained through the University of Minnesota, Mulago Hospital IRB and the London School of Hygiene and Tropical Medicine ethics committee; regulatory approval was obtained from the Uganda National Council of Science and Technology. All participants provided written informed consent prior to meningitis screening permitting collection of clinical outcomes. Pregnant and breastfeeding women were excluded from interventions but received diagnostic testing for TB meningitis and standard-of-care therapy.
Literature Review
A systematic literature review was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reports of TB meningitis associated with pregnancy. We searched PubMed/Medline and Embase using the Medical Subject Heading (MeSH) keywords “tuberculosis, meningeal” and “pregnancy” or “postpartum” or “peripartum” in PubMed/Medline and “mening*,” “tubercul*,” and “pregnan*,” “postpartum,” or “peripartum” in Embase for English-language manuscripts from 1970 to 10 July 2022. K. A. P. reviewed all title/abstracts for relevance. Publications unrelated to cases of TB meningitis in pregnancy or the postpartum period were excluded. References from included manuscripts were then searched for additional cases.
In the systematic literature review, symptom duration was defined using the highest value if a range was given. A delay in TB meningitis diagnosis and treatment for purposes of this manuscript was defined as ≥1 week of classic meningeal symptoms (ie, headache, altered mental status, fever, and/or neck stiffness) following initial presentation or as identified by the original authors.
RESULTS
Case Series
Eight cases of TB meningitis associated with pregnancy were identified (Tables 1 and 2). One maternal death occurred; all fetuses survived to time of delivery with 1 pregnancy ongoing at time of publication. Long-term outcomes were unknown. CD4+ T-cell counts for cases 1–3 and 7–8 were unknown.
Systematic Review of Tuberculous Meningitis in Pregnancy and the Postpartum Period
| First Author, Publication Year [Reference] . | Case Number . | Age, y . | Pregnancy Status . | Symptom Durationa . | HIV and ART Status . | Maternal Outcome . | Fetal/Neonatal Outcome . |
|---|---|---|---|---|---|---|---|
| Pastick (current case series) | 1 | 29 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Died | Alive to delivery |
| 2 | 34 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 3 | 26 | Postpartum, <1 wk | 1 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 4 | 29 | Postpartum, 4 wk | 24 d | Positive (CD4 122 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 5 | 30 | Postpartum, 6 wk | NA | Positive (CD4 81 cells/µL), recent ART switch | Alive | Alive to delivery | |
| 6 | 25 | Postpartum, 12 wk | 60 d | Positive (CD4 93 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 7 | 38 | Postpartum, 8 wk | 60–90 d | Positive (unknown CD4), not on ART | Alive | Alive to delivery | |
| 8 | 28 | 28 wk | 14 d | Positive (unknown CD4), on ART | Alive | Alive, in utero | |
| Ye, 2019 [33] | 1 | 32 | 5–26 wk | 13 d | NA | Alive | Died |
| Namani, 2017 [34] | 2 | 25 | 24 wk | >14 d | Negative | Alive | Died, twins |
| Nakatani, 2017 [35] | 3 | 30 | 19 wk | 14 d | Negative | Alive, neurologic deficits | Stillbirth, 23 wk |
| Baidya, 2011 [36] | 4 | 26 | >37 wk | 14 d | NA | Alive | Alive, term delivery |
| Yeh, 2009 [37] | 5 | 18 | 23 wk | 21 d | Negative | Alive, neurologic deficits | Alive, delivered at 33 wk |
| Jana, 2008 [38] | 6 | 25 | 10 wk | 14–28 d | Negative | Alive, neurologic deficits | Died, intrauterine fetal death at 35 wk |
| Prevost, 1999 [39] | 7 | 23 | 23 wk | 7 d | Negative | Alive, neurologic deficits | Alive, delivered at 36 wk |
| Clark, 1986 [40] | 8 | 19 | 24–26 wk | 56 d | NA | Died | Alive, emergent cesarean delivery at 24–26 wk |
| Kingdom, 1989 [41] | 9 | 23 | 28 wk | 84 d | NA | Died, 6 d postpartum | Alive, delivered at 28 wk |
| 10 | 28 | Postpartum, <1 wk | 28 d | NA | Alive | Died, delivered at 28 wk | |
| 11 | 25 | Postpartum, 2 wk | 56 d | NA | Alive, neurologic deficits | Died, delivered at 28 wk | |
| 12 | 35 | Recent miscarriage 3 wk prior | 28 d | NA | Alive | Miscarriage at 12 wk | |
| Ray, 1997 [42] | 13 | 23 | Postpartum, <1 wk | 10.5 d | Negative | Died, day 15 | Alive, born at term |
| Liu, 2008 [43] | 14 | 29 | 14 wk | 16 d | Negative | Alive, neurologic deficits | Elective termination |
| Kutlu, 2007 [44] | 15 | 18 | 14 wk | NA | Negative | Alive | Alive, in utero |
| Chan, 2003 [45] | 16 | 35 | Postpartum, 4 wk | 2 d | NA | Alive, neurologic deficits | Alive, congenital TB delivered at 29 wk |
| Cheng, 2003 [28] | 17 | 35 | Postpartum, 4 wk | NA | Negative | Alive, neurologic deficits | Alive, delivered at 29 wk |
| Heywood, 1999 [46] | 18 | NA | Postpartum, 20 wk | NA | NA | Died 5 mo postpartum | Alive |
| 19 | NA | Pregnant, EGA not reported | NA | NA | Died | NA | |
| Jana, 1999 [47] | 20 | NA | Pregnant, EGA not reported | NA | NA | Alive | NA |
| 21 | NA | Pregnant | NA | NA | Alive | NA | |
| Ogawa, 1987 [48] | 22 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | At least 1 of 4 died | Two spontaneous abortions |
| 23 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 24 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 25 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| McIntyre, 1987 [49] | 26 | 23 | 26–30 wk | 39 d | NA | Died | Alive, delivered at 28 wk |
| Stands, 1977 [50] | 27 | 29 | 20 wk | 21 d | NA | Alive | Alive |
| Petrini, 1983 [51] | 28 | 32 | Postpartum, 7 wk | NA | NA | Alive | Died (congenital TB), delivered at 38 wk |
| Brandstetter, 1980 [52] | 29 | 24 | Postpartum, <1 wk | 5 d | NA | Alive | Alive, delivered at 39 wk |
| Chambers, 1975 [53] | 30 | 26 | Pregnant, EGA not reported | 7 d | NA | Died, day 9 | Alive |
| Gordon-Nesbitt, 1973 [54] | 31 | NA | Postpartum, 10 wk | NA | NA | Died, 10 wk postpartum | Alive, delivered at 39 wk, congenital TB |
| Golditch, 1971 [55] | 32 | 34 | 19 wk | NA | NA | Alive | Alive, delivered at 36 wk |
| Romero-Imbroda, 2020 [56] | 33 | 23 | Postpartum, not reported | 14 d | Negative | Alive | Alive |
| Saleh, 2013 [57] | 34 | 27 | Postpartum, 6 wk | 21 d | Negative | Alive | Alive |
| Shibolet, 1979 [58] | 35 | 21 | 16 wk | NA | NA | Died | Alive |
| Meyers, 1974 [59] | 36 | 26 | 33 wk | NA | NA | Alive | Alive, delivered at 35 wk |
| Wilson, 1973 [60] | 37 | 20 | 16 wk | NA | NA | Alive | Alive |
| Meregildo Rodriguez, 2020 [61] | 38 | 44 | Posttermination, 6 wk | 21 d | Negative | Alive | Clandestine abortion 6 wk prior |
| Smitha, 2013 [62] | 39 | 33 | 28 wk | NA | NAb | Alive | Alive, delivered preterm |
| Samson Ejiji, 2013 [63] | 40 | 38 | 27 wk | 84 d | Negative | Alive, neurologic deficits | Alive, in utero |
| First Author, Publication Year [Reference] . | Case Number . | Age, y . | Pregnancy Status . | Symptom Durationa . | HIV and ART Status . | Maternal Outcome . | Fetal/Neonatal Outcome . |
|---|---|---|---|---|---|---|---|
| Pastick (current case series) | 1 | 29 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Died | Alive to delivery |
| 2 | 34 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 3 | 26 | Postpartum, <1 wk | 1 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 4 | 29 | Postpartum, 4 wk | 24 d | Positive (CD4 122 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 5 | 30 | Postpartum, 6 wk | NA | Positive (CD4 81 cells/µL), recent ART switch | Alive | Alive to delivery | |
| 6 | 25 | Postpartum, 12 wk | 60 d | Positive (CD4 93 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 7 | 38 | Postpartum, 8 wk | 60–90 d | Positive (unknown CD4), not on ART | Alive | Alive to delivery | |
| 8 | 28 | 28 wk | 14 d | Positive (unknown CD4), on ART | Alive | Alive, in utero | |
| Ye, 2019 [33] | 1 | 32 | 5–26 wk | 13 d | NA | Alive | Died |
| Namani, 2017 [34] | 2 | 25 | 24 wk | >14 d | Negative | Alive | Died, twins |
| Nakatani, 2017 [35] | 3 | 30 | 19 wk | 14 d | Negative | Alive, neurologic deficits | Stillbirth, 23 wk |
| Baidya, 2011 [36] | 4 | 26 | >37 wk | 14 d | NA | Alive | Alive, term delivery |
| Yeh, 2009 [37] | 5 | 18 | 23 wk | 21 d | Negative | Alive, neurologic deficits | Alive, delivered at 33 wk |
| Jana, 2008 [38] | 6 | 25 | 10 wk | 14–28 d | Negative | Alive, neurologic deficits | Died, intrauterine fetal death at 35 wk |
| Prevost, 1999 [39] | 7 | 23 | 23 wk | 7 d | Negative | Alive, neurologic deficits | Alive, delivered at 36 wk |
| Clark, 1986 [40] | 8 | 19 | 24–26 wk | 56 d | NA | Died | Alive, emergent cesarean delivery at 24–26 wk |
| Kingdom, 1989 [41] | 9 | 23 | 28 wk | 84 d | NA | Died, 6 d postpartum | Alive, delivered at 28 wk |
| 10 | 28 | Postpartum, <1 wk | 28 d | NA | Alive | Died, delivered at 28 wk | |
| 11 | 25 | Postpartum, 2 wk | 56 d | NA | Alive, neurologic deficits | Died, delivered at 28 wk | |
| 12 | 35 | Recent miscarriage 3 wk prior | 28 d | NA | Alive | Miscarriage at 12 wk | |
| Ray, 1997 [42] | 13 | 23 | Postpartum, <1 wk | 10.5 d | Negative | Died, day 15 | Alive, born at term |
| Liu, 2008 [43] | 14 | 29 | 14 wk | 16 d | Negative | Alive, neurologic deficits | Elective termination |
| Kutlu, 2007 [44] | 15 | 18 | 14 wk | NA | Negative | Alive | Alive, in utero |
| Chan, 2003 [45] | 16 | 35 | Postpartum, 4 wk | 2 d | NA | Alive, neurologic deficits | Alive, congenital TB delivered at 29 wk |
| Cheng, 2003 [28] | 17 | 35 | Postpartum, 4 wk | NA | Negative | Alive, neurologic deficits | Alive, delivered at 29 wk |
| Heywood, 1999 [46] | 18 | NA | Postpartum, 20 wk | NA | NA | Died 5 mo postpartum | Alive |
| 19 | NA | Pregnant, EGA not reported | NA | NA | Died | NA | |
| Jana, 1999 [47] | 20 | NA | Pregnant, EGA not reported | NA | NA | Alive | NA |
| 21 | NA | Pregnant | NA | NA | Alive | NA | |
| Ogawa, 1987 [48] | 22 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | At least 1 of 4 died | Two spontaneous abortions |
| 23 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 24 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 25 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| McIntyre, 1987 [49] | 26 | 23 | 26–30 wk | 39 d | NA | Died | Alive, delivered at 28 wk |
| Stands, 1977 [50] | 27 | 29 | 20 wk | 21 d | NA | Alive | Alive |
| Petrini, 1983 [51] | 28 | 32 | Postpartum, 7 wk | NA | NA | Alive | Died (congenital TB), delivered at 38 wk |
| Brandstetter, 1980 [52] | 29 | 24 | Postpartum, <1 wk | 5 d | NA | Alive | Alive, delivered at 39 wk |
| Chambers, 1975 [53] | 30 | 26 | Pregnant, EGA not reported | 7 d | NA | Died, day 9 | Alive |
| Gordon-Nesbitt, 1973 [54] | 31 | NA | Postpartum, 10 wk | NA | NA | Died, 10 wk postpartum | Alive, delivered at 39 wk, congenital TB |
| Golditch, 1971 [55] | 32 | 34 | 19 wk | NA | NA | Alive | Alive, delivered at 36 wk |
| Romero-Imbroda, 2020 [56] | 33 | 23 | Postpartum, not reported | 14 d | Negative | Alive | Alive |
| Saleh, 2013 [57] | 34 | 27 | Postpartum, 6 wk | 21 d | Negative | Alive | Alive |
| Shibolet, 1979 [58] | 35 | 21 | 16 wk | NA | NA | Died | Alive |
| Meyers, 1974 [59] | 36 | 26 | 33 wk | NA | NA | Alive | Alive, delivered at 35 wk |
| Wilson, 1973 [60] | 37 | 20 | 16 wk | NA | NA | Alive | Alive |
| Meregildo Rodriguez, 2020 [61] | 38 | 44 | Posttermination, 6 wk | 21 d | Negative | Alive | Clandestine abortion 6 wk prior |
| Smitha, 2013 [62] | 39 | 33 | 28 wk | NA | NAb | Alive | Alive, delivered preterm |
| Samson Ejiji, 2013 [63] | 40 | 38 | 27 wk | 84 d | Negative | Alive, neurologic deficits | Alive, in utero |
Abbreviations: ART, antiretroviral therapy; EGA, estimated gestational age; HIV, human immunodeficiency virus; NA, not available or unspecified; TB, tuberculosis.
Approximate.
Unable to obtain full manuscript.
Systematic Review of Tuberculous Meningitis in Pregnancy and the Postpartum Period
| First Author, Publication Year [Reference] . | Case Number . | Age, y . | Pregnancy Status . | Symptom Durationa . | HIV and ART Status . | Maternal Outcome . | Fetal/Neonatal Outcome . |
|---|---|---|---|---|---|---|---|
| Pastick (current case series) | 1 | 29 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Died | Alive to delivery |
| 2 | 34 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 3 | 26 | Postpartum, <1 wk | 1 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 4 | 29 | Postpartum, 4 wk | 24 d | Positive (CD4 122 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 5 | 30 | Postpartum, 6 wk | NA | Positive (CD4 81 cells/µL), recent ART switch | Alive | Alive to delivery | |
| 6 | 25 | Postpartum, 12 wk | 60 d | Positive (CD4 93 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 7 | 38 | Postpartum, 8 wk | 60–90 d | Positive (unknown CD4), not on ART | Alive | Alive to delivery | |
| 8 | 28 | 28 wk | 14 d | Positive (unknown CD4), on ART | Alive | Alive, in utero | |
| Ye, 2019 [33] | 1 | 32 | 5–26 wk | 13 d | NA | Alive | Died |
| Namani, 2017 [34] | 2 | 25 | 24 wk | >14 d | Negative | Alive | Died, twins |
| Nakatani, 2017 [35] | 3 | 30 | 19 wk | 14 d | Negative | Alive, neurologic deficits | Stillbirth, 23 wk |
| Baidya, 2011 [36] | 4 | 26 | >37 wk | 14 d | NA | Alive | Alive, term delivery |
| Yeh, 2009 [37] | 5 | 18 | 23 wk | 21 d | Negative | Alive, neurologic deficits | Alive, delivered at 33 wk |
| Jana, 2008 [38] | 6 | 25 | 10 wk | 14–28 d | Negative | Alive, neurologic deficits | Died, intrauterine fetal death at 35 wk |
| Prevost, 1999 [39] | 7 | 23 | 23 wk | 7 d | Negative | Alive, neurologic deficits | Alive, delivered at 36 wk |
| Clark, 1986 [40] | 8 | 19 | 24–26 wk | 56 d | NA | Died | Alive, emergent cesarean delivery at 24–26 wk |
| Kingdom, 1989 [41] | 9 | 23 | 28 wk | 84 d | NA | Died, 6 d postpartum | Alive, delivered at 28 wk |
| 10 | 28 | Postpartum, <1 wk | 28 d | NA | Alive | Died, delivered at 28 wk | |
| 11 | 25 | Postpartum, 2 wk | 56 d | NA | Alive, neurologic deficits | Died, delivered at 28 wk | |
| 12 | 35 | Recent miscarriage 3 wk prior | 28 d | NA | Alive | Miscarriage at 12 wk | |
| Ray, 1997 [42] | 13 | 23 | Postpartum, <1 wk | 10.5 d | Negative | Died, day 15 | Alive, born at term |
| Liu, 2008 [43] | 14 | 29 | 14 wk | 16 d | Negative | Alive, neurologic deficits | Elective termination |
| Kutlu, 2007 [44] | 15 | 18 | 14 wk | NA | Negative | Alive | Alive, in utero |
| Chan, 2003 [45] | 16 | 35 | Postpartum, 4 wk | 2 d | NA | Alive, neurologic deficits | Alive, congenital TB delivered at 29 wk |
| Cheng, 2003 [28] | 17 | 35 | Postpartum, 4 wk | NA | Negative | Alive, neurologic deficits | Alive, delivered at 29 wk |
| Heywood, 1999 [46] | 18 | NA | Postpartum, 20 wk | NA | NA | Died 5 mo postpartum | Alive |
| 19 | NA | Pregnant, EGA not reported | NA | NA | Died | NA | |
| Jana, 1999 [47] | 20 | NA | Pregnant, EGA not reported | NA | NA | Alive | NA |
| 21 | NA | Pregnant | NA | NA | Alive | NA | |
| Ogawa, 1987 [48] | 22 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | At least 1 of 4 died | Two spontaneous abortions |
| 23 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 24 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 25 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| McIntyre, 1987 [49] | 26 | 23 | 26–30 wk | 39 d | NA | Died | Alive, delivered at 28 wk |
| Stands, 1977 [50] | 27 | 29 | 20 wk | 21 d | NA | Alive | Alive |
| Petrini, 1983 [51] | 28 | 32 | Postpartum, 7 wk | NA | NA | Alive | Died (congenital TB), delivered at 38 wk |
| Brandstetter, 1980 [52] | 29 | 24 | Postpartum, <1 wk | 5 d | NA | Alive | Alive, delivered at 39 wk |
| Chambers, 1975 [53] | 30 | 26 | Pregnant, EGA not reported | 7 d | NA | Died, day 9 | Alive |
| Gordon-Nesbitt, 1973 [54] | 31 | NA | Postpartum, 10 wk | NA | NA | Died, 10 wk postpartum | Alive, delivered at 39 wk, congenital TB |
| Golditch, 1971 [55] | 32 | 34 | 19 wk | NA | NA | Alive | Alive, delivered at 36 wk |
| Romero-Imbroda, 2020 [56] | 33 | 23 | Postpartum, not reported | 14 d | Negative | Alive | Alive |
| Saleh, 2013 [57] | 34 | 27 | Postpartum, 6 wk | 21 d | Negative | Alive | Alive |
| Shibolet, 1979 [58] | 35 | 21 | 16 wk | NA | NA | Died | Alive |
| Meyers, 1974 [59] | 36 | 26 | 33 wk | NA | NA | Alive | Alive, delivered at 35 wk |
| Wilson, 1973 [60] | 37 | 20 | 16 wk | NA | NA | Alive | Alive |
| Meregildo Rodriguez, 2020 [61] | 38 | 44 | Posttermination, 6 wk | 21 d | Negative | Alive | Clandestine abortion 6 wk prior |
| Smitha, 2013 [62] | 39 | 33 | 28 wk | NA | NAb | Alive | Alive, delivered preterm |
| Samson Ejiji, 2013 [63] | 40 | 38 | 27 wk | 84 d | Negative | Alive, neurologic deficits | Alive, in utero |
| First Author, Publication Year [Reference] . | Case Number . | Age, y . | Pregnancy Status . | Symptom Durationa . | HIV and ART Status . | Maternal Outcome . | Fetal/Neonatal Outcome . |
|---|---|---|---|---|---|---|---|
| Pastick (current case series) | 1 | 29 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Died | Alive to delivery |
| 2 | 34 | Postpartum, 4 wk | 14 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 3 | 26 | Postpartum, <1 wk | 1 d | Positive (unknown CD4), not on ART | Alive, neurologic deficits | Alive to delivery | |
| 4 | 29 | Postpartum, 4 wk | 24 d | Positive (CD4 122 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 5 | 30 | Postpartum, 6 wk | NA | Positive (CD4 81 cells/µL), recent ART switch | Alive | Alive to delivery | |
| 6 | 25 | Postpartum, 12 wk | 60 d | Positive (CD4 93 cells/µL), recent diagnosis, on ART | Alive | Alive to delivery | |
| 7 | 38 | Postpartum, 8 wk | 60–90 d | Positive (unknown CD4), not on ART | Alive | Alive to delivery | |
| 8 | 28 | 28 wk | 14 d | Positive (unknown CD4), on ART | Alive | Alive, in utero | |
| Ye, 2019 [33] | 1 | 32 | 5–26 wk | 13 d | NA | Alive | Died |
| Namani, 2017 [34] | 2 | 25 | 24 wk | >14 d | Negative | Alive | Died, twins |
| Nakatani, 2017 [35] | 3 | 30 | 19 wk | 14 d | Negative | Alive, neurologic deficits | Stillbirth, 23 wk |
| Baidya, 2011 [36] | 4 | 26 | >37 wk | 14 d | NA | Alive | Alive, term delivery |
| Yeh, 2009 [37] | 5 | 18 | 23 wk | 21 d | Negative | Alive, neurologic deficits | Alive, delivered at 33 wk |
| Jana, 2008 [38] | 6 | 25 | 10 wk | 14–28 d | Negative | Alive, neurologic deficits | Died, intrauterine fetal death at 35 wk |
| Prevost, 1999 [39] | 7 | 23 | 23 wk | 7 d | Negative | Alive, neurologic deficits | Alive, delivered at 36 wk |
| Clark, 1986 [40] | 8 | 19 | 24–26 wk | 56 d | NA | Died | Alive, emergent cesarean delivery at 24–26 wk |
| Kingdom, 1989 [41] | 9 | 23 | 28 wk | 84 d | NA | Died, 6 d postpartum | Alive, delivered at 28 wk |
| 10 | 28 | Postpartum, <1 wk | 28 d | NA | Alive | Died, delivered at 28 wk | |
| 11 | 25 | Postpartum, 2 wk | 56 d | NA | Alive, neurologic deficits | Died, delivered at 28 wk | |
| 12 | 35 | Recent miscarriage 3 wk prior | 28 d | NA | Alive | Miscarriage at 12 wk | |
| Ray, 1997 [42] | 13 | 23 | Postpartum, <1 wk | 10.5 d | Negative | Died, day 15 | Alive, born at term |
| Liu, 2008 [43] | 14 | 29 | 14 wk | 16 d | Negative | Alive, neurologic deficits | Elective termination |
| Kutlu, 2007 [44] | 15 | 18 | 14 wk | NA | Negative | Alive | Alive, in utero |
| Chan, 2003 [45] | 16 | 35 | Postpartum, 4 wk | 2 d | NA | Alive, neurologic deficits | Alive, congenital TB delivered at 29 wk |
| Cheng, 2003 [28] | 17 | 35 | Postpartum, 4 wk | NA | Negative | Alive, neurologic deficits | Alive, delivered at 29 wk |
| Heywood, 1999 [46] | 18 | NA | Postpartum, 20 wk | NA | NA | Died 5 mo postpartum | Alive |
| 19 | NA | Pregnant, EGA not reported | NA | NA | Died | NA | |
| Jana, 1999 [47] | 20 | NA | Pregnant, EGA not reported | NA | NA | Alive | NA |
| 21 | NA | Pregnant | NA | NA | Alive | NA | |
| Ogawa, 1987 [48] | 22 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | At least 1 of 4 died | Two spontaneous abortions |
| 23 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 24 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| 25 | NA | Postpartum, 6 wk to 5 mo | NA | Negative | … | … | |
| McIntyre, 1987 [49] | 26 | 23 | 26–30 wk | 39 d | NA | Died | Alive, delivered at 28 wk |
| Stands, 1977 [50] | 27 | 29 | 20 wk | 21 d | NA | Alive | Alive |
| Petrini, 1983 [51] | 28 | 32 | Postpartum, 7 wk | NA | NA | Alive | Died (congenital TB), delivered at 38 wk |
| Brandstetter, 1980 [52] | 29 | 24 | Postpartum, <1 wk | 5 d | NA | Alive | Alive, delivered at 39 wk |
| Chambers, 1975 [53] | 30 | 26 | Pregnant, EGA not reported | 7 d | NA | Died, day 9 | Alive |
| Gordon-Nesbitt, 1973 [54] | 31 | NA | Postpartum, 10 wk | NA | NA | Died, 10 wk postpartum | Alive, delivered at 39 wk, congenital TB |
| Golditch, 1971 [55] | 32 | 34 | 19 wk | NA | NA | Alive | Alive, delivered at 36 wk |
| Romero-Imbroda, 2020 [56] | 33 | 23 | Postpartum, not reported | 14 d | Negative | Alive | Alive |
| Saleh, 2013 [57] | 34 | 27 | Postpartum, 6 wk | 21 d | Negative | Alive | Alive |
| Shibolet, 1979 [58] | 35 | 21 | 16 wk | NA | NA | Died | Alive |
| Meyers, 1974 [59] | 36 | 26 | 33 wk | NA | NA | Alive | Alive, delivered at 35 wk |
| Wilson, 1973 [60] | 37 | 20 | 16 wk | NA | NA | Alive | Alive |
| Meregildo Rodriguez, 2020 [61] | 38 | 44 | Posttermination, 6 wk | 21 d | Negative | Alive | Clandestine abortion 6 wk prior |
| Smitha, 2013 [62] | 39 | 33 | 28 wk | NA | NAb | Alive | Alive, delivered preterm |
| Samson Ejiji, 2013 [63] | 40 | 38 | 27 wk | 84 d | Negative | Alive, neurologic deficits | Alive, in utero |
Abbreviations: ART, antiretroviral therapy; EGA, estimated gestational age; HIV, human immunodeficiency virus; NA, not available or unspecified; TB, tuberculosis.
Approximate.
Unable to obtain full manuscript.
Cerebrospinal Fluid Results for 8 Cases of Human Immunodeficiency Virus and Pregnancy-Related Tuberculous Meningitis
| CSF Parameters . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Case 8 . |
|---|---|---|---|---|---|---|---|---|
| Opening pressure, mm H2O (normal <250) | NA | 400 | 15 | 200 | 80 | 280 | 70 | 170 |
| White blood cells, cells/µL | 625 (83% lymphocytes) | 275 (78% lymphocytes) | 165 (85% lymphocytes) | 310 (85% lymphocytes) | <5 | <5 | <5 | 635 (83% lymphocytes) |
| Protein, mg/dL | 187 | 147 | 103 | 184 | 90 | 79 | 29 | 138 |
| Glucose, mmol/L | NA | <1.1 | <1.1 | <1.1 | 45 | Unknown | 59 | Low |
| Lactate, mmol/L | NA | 12.3 | NA | NA | 2.2 | Unknown | 2.4 | 5.5 |
| AFB stain | − | − | − | − | − | − | − | − |
| CSF GeneXpert MTB/RIF Ultra | + | − | + | + (trace) | − | + | − | − |
| Urine GeneXpert MTB/RIF Ultra | − | − | − | − | − | − | − | − |
| Urine LAM | − | − | − | − | + | +/−a | − | + |
| CSF culture | + | − | + | − | NA | NA | NA | NA |
| CSF Parameters . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Case 8 . |
|---|---|---|---|---|---|---|---|---|
| Opening pressure, mm H2O (normal <250) | NA | 400 | 15 | 200 | 80 | 280 | 70 | 170 |
| White blood cells, cells/µL | 625 (83% lymphocytes) | 275 (78% lymphocytes) | 165 (85% lymphocytes) | 310 (85% lymphocytes) | <5 | <5 | <5 | 635 (83% lymphocytes) |
| Protein, mg/dL | 187 | 147 | 103 | 184 | 90 | 79 | 29 | 138 |
| Glucose, mmol/L | NA | <1.1 | <1.1 | <1.1 | 45 | Unknown | 59 | Low |
| Lactate, mmol/L | NA | 12.3 | NA | NA | 2.2 | Unknown | 2.4 | 5.5 |
| AFB stain | − | − | − | − | − | − | − | − |
| CSF GeneXpert MTB/RIF Ultra | + | − | + | + (trace) | − | + | − | − |
| Urine GeneXpert MTB/RIF Ultra | − | − | − | − | − | − | − | − |
| Urine LAM | − | − | − | − | + | +/−a | − | + |
| CSF culture | + | − | + | − | NA | NA | NA | NA |
Abbreviations: −, negative; +, positive; AFB, acid-fast bacilli; CSF, cerebrospinal fluid; LAM, lipoarabinomannan; NA, not performed or not available.
Positive Fuji LAM, negative Alere LAM.
Cerebrospinal Fluid Results for 8 Cases of Human Immunodeficiency Virus and Pregnancy-Related Tuberculous Meningitis
| CSF Parameters . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Case 8 . |
|---|---|---|---|---|---|---|---|---|
| Opening pressure, mm H2O (normal <250) | NA | 400 | 15 | 200 | 80 | 280 | 70 | 170 |
| White blood cells, cells/µL | 625 (83% lymphocytes) | 275 (78% lymphocytes) | 165 (85% lymphocytes) | 310 (85% lymphocytes) | <5 | <5 | <5 | 635 (83% lymphocytes) |
| Protein, mg/dL | 187 | 147 | 103 | 184 | 90 | 79 | 29 | 138 |
| Glucose, mmol/L | NA | <1.1 | <1.1 | <1.1 | 45 | Unknown | 59 | Low |
| Lactate, mmol/L | NA | 12.3 | NA | NA | 2.2 | Unknown | 2.4 | 5.5 |
| AFB stain | − | − | − | − | − | − | − | − |
| CSF GeneXpert MTB/RIF Ultra | + | − | + | + (trace) | − | + | − | − |
| Urine GeneXpert MTB/RIF Ultra | − | − | − | − | − | − | − | − |
| Urine LAM | − | − | − | − | + | +/−a | − | + |
| CSF culture | + | − | + | − | NA | NA | NA | NA |
| CSF Parameters . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Case 8 . |
|---|---|---|---|---|---|---|---|---|
| Opening pressure, mm H2O (normal <250) | NA | 400 | 15 | 200 | 80 | 280 | 70 | 170 |
| White blood cells, cells/µL | 625 (83% lymphocytes) | 275 (78% lymphocytes) | 165 (85% lymphocytes) | 310 (85% lymphocytes) | <5 | <5 | <5 | 635 (83% lymphocytes) |
| Protein, mg/dL | 187 | 147 | 103 | 184 | 90 | 79 | 29 | 138 |
| Glucose, mmol/L | NA | <1.1 | <1.1 | <1.1 | 45 | Unknown | 59 | Low |
| Lactate, mmol/L | NA | 12.3 | NA | NA | 2.2 | Unknown | 2.4 | 5.5 |
| AFB stain | − | − | − | − | − | − | − | − |
| CSF GeneXpert MTB/RIF Ultra | + | − | + | + (trace) | − | + | − | − |
| Urine GeneXpert MTB/RIF Ultra | − | − | − | − | − | − | − | − |
| Urine LAM | − | − | − | − | + | +/−a | − | + |
| CSF culture | + | − | + | − | NA | NA | NA | NA |
Abbreviations: −, negative; +, positive; AFB, acid-fast bacilli; CSF, cerebrospinal fluid; LAM, lipoarabinomannan; NA, not performed or not available.
Positive Fuji LAM, negative Alere LAM.
Case 1
A 29-year-old woman presented 1 month postpartum following spontaneous vaginal delivery with a 2-week history of headaches and fevers, 5 days of visual changes, and 3 days of altered mental status. She also had night sweats and weight loss. Examination showed meningism, nuchal rigidity, and a Glasgow Coma Scale (GCS) score of 14/15. She had been diagnosed with HIV 6 years prior but was no longer taking ART and had not been in HIV care for 4 years. Cerebrospinal fluid (CSF) obtained via lumbar puncture (LP) confirmed TB meningitis via GeneXpert MTB/RIF Ultra (Cepheid, Sunnyvale, California). Sadly, the patient died after 3 days of standard TB meningitis treatment of rifampicin, isoniazid, ethambutol, and pyrazinamide with dexamethasone.
Case 2
A 34-year-old woman presented 1 month postpartum after a spontaneous vaginal delivery with a 2-week history of reduced appetite and altered mental status. She was admitted to a psychiatric hospital initially diagnosed with postpartum psychosis and was treated with haloperidol. Following a positive HIV test, she was diagnosed with HIV-induced psychosis. She then developed a headache and dizziness with a decline in GCS score and was transferred to the national referral hospital where antibiotics were started. She later developed meningism and a cranial nerve VI palsy. An LP was performed on day 3 of hospitalization (day 21 of symptoms) with an opening pressure of 400 mm H2O. Probable TB meningitis was putatively diagnosed given low CSF glucose (<1.1 mmol/L), elevated protein (147 mg/dL), and lymphocyte-predominant pleocytosis (total 275 cells/µL, 78% lymphocytes). The patient had a negative CSF cryptococcal antigen, GeneXpert MTB/RIF Ultra, acid-fast bacilli (AFB) stain, urine GeneXpert, and urine Alere lipoarabinomannan (LAM) (Alere, Waltham, Massachusetts). Head computed tomography (CT) showed an acute basal ganglia infarct and hydrocephalus. The patient was started on standard TB meningitis therapy and was discharged on day 16 with significant residual neurological deficits.
Case 3
A 26-year-old woman living with HIV presented 5 days postpartum with fevers and altered mental status. She had been diagnosed with HIV and pulmonary TB 18 and 4 months prior to admission but was reportedly nonadherent to ART and the continuation phase of rifampicin and isoniazid. She was initially diagnosed with postpartum psychosis. During hospitalization, she developed neck pain, seizures, and cranial nerve VI–VII palsies and experienced a subsequent GCS decline. Antibiotics were started due to concern for retained placental tissue. She underwent an LP on day 21 of symptoms with an opening pressure of 15 mm H2O and positive CSF GeneXpert MTB/RIF Ultra. She was started on standard TB meningitis therapy and phenytoin. The patient's consciousness level improved but right-sided hemiplegia persisted; she was discharged after 12 days of TB therapy.
Case 4
A 29-year-old woman became unwell following spontaneous vaginal delivery with nonspecific weakness, weight loss, and diaphoresis. She was found to be HIV positive with a CD4+ count of 122 cells/µL and was immediately started on ART. She continued to deteriorate and eventually presented to the hospital with a 24-day history of headache and 2–4 days of confusion and visual changes. She was cachexic and had nuchal rigidity, a cranial nerve VI palsy, and paraplegia with a GCS score of 14/15. CSF was obtained via LP with an opening pressure of 200 mm H2O and was GeneXpert MTB/RIF Ultra positive. She was started on standard TB meningitis therapy and was discharged 17 days after admission.
Case 5
A 30-year-old woman living with HIV for >10 years recently switched ART due to a nonsuppressed HIV viral load on second-line ART and suboptimal adherence with a CD4+ count of 81 cells/µL. She was admitted to the hospital 6 weeks postpartum in status epilepticus with reports of high-grade fevers and a cough. Following resolution of seizures, she remained confused with severe meningism and a cranial nerve VII palsy. Serum and CSF cryptococcal antigen tests were negative. Her LP revealed an opening pressure of 80 mm H2O and noninflammatory CSF, with a negative GeneXpert MTB/RIF Ultra, but positive urine Alere LAM (grade 4+). Her chest radiograph was consistent with miliary TB. She was treated for probable TB meningitis with standard therapy. After 14 days of hospitalization, she was discharged without any significant residual neurologic deficits.
Case 6
A 25-year-old woman with newly diagnosed HIV (CD4+ T-cell count of 93 cells/µL), presented to the hospital 3 months postpartum with a 2-month history of stiff neck, confusion, right-sided weakness, fevers, and cough in the setting of recent ART initiation 2 weeks prior. She had severe meningism, a cranial nerve VII palsy, and right-sided hemiparesis with a GCS score of 11/15. An LP revealed an opening pressure of 280 mm H2O and noninflammatory findings, but positive CSF Xpert MTB/RIF Ultra and urine FujiLAM (Fujifilm SILVAMP TB-LAM, Fujifilm, Japan). Urine Alere LAM and Xpert MTB/RIF Ultra were negative. A head CT demonstrated multiple infarcts in the left caudate and thalamic area with meningeal enhancement. She was treated for definite TB meningitis and possible postpartum IRIS with standard TB meningitis treatment. Her ART was held during hospitalization. She was discharged without neurologic impairment.
Case 7
A 38-year-old woman with HIV who had been nonadherent to ART for 6 months presented to the hospital 2 months postpartum with 2–3 months of fever, cough, and weight loss. She was found to have lower limb weakness, meningism with a positive Kernig sign, and a GCS score of 13/15. An LP revealed an opening pressure of 70 mm H2O and demonstrated minimal inflammation with a negative CSF Xpert MTB/RIF Ultra. Urine TB tests were negative. A chest radiograph demonstrated hilar adenopathy with bilateral opacities and tracheal deviation. She was treated for presumptive TB meningitis with standard therapy and discharged without neurologic impairment.
Case 8
A 28-year-old woman with HIV on ART for 4 years presented at 28 weeks of gestation with 2 weeks of headache and photophobia, 1 week of fevers, and 4 days of confusion. She had a positive Kernig sign and a GCS score of 15/15. An LP revealed an opening pressure of 170 mm H2O in addition to neutrophilic pleocytosis, hypoglycorrhachia, elevated protein, and CSF lactate. CSF cryptococcal antigen, Gram stain, AFB stain, and Xpert MTB/RIF Ultra were negative. A urine Xpert MTB/RIF Ultra was negative; however, urine TB-LAM was positive. The patient was subsequently treated for presumptive TB meningitis and possible bacterial meningitis with standard therapy and was discharged without any neurologic deficits.
Systematic Literature Review
Of 72 titles/abstracts identified, 42 were excluded (18 were unavailable in English, 15 were unrelated to TB meningitis, and 9 were unrelated to pregnancy) (Figure 1). The remaining 30 articles underwent full text review, and their citations revealed an additional 2 articles related to cases of TB meningitis associated with pregnancy. Among these 32 articles, a total of 40 cases of TB meningitis in pregnancy and the postpartum period were identified (Table 1) [28, 33–63].
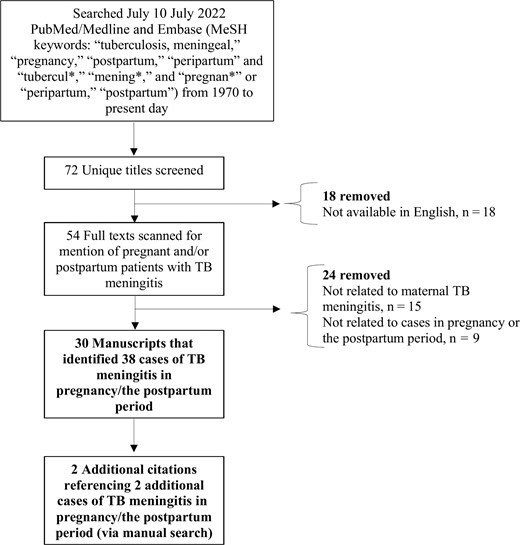
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of literature search. Abbreviations: MeSH, Medical Subject Heading; TB, tuberculous.
All Combined Cases
Of all combined cases of TB meningitis associated with pregnancy in this case series and systematic literature review (n = 48), the median age was 27 years (interquartile range [IQR], 23.5–32 years) for those with age provided (39/48). Half of the cases (24/48 [50%]) occurred during pregnancy: 1 in the first trimester (1–12 weeks), 16 in the second trimester (13–28 weeks), and 3 in the third trimester (29 weeks–delivery), and 4 had undisclosed or indeterminate estimated gestational age. The remainder (24/48 [50%]) occurred either in the postpartum period (n = 20) or following a miscarriage or pregnancy termination (n = 4) (Supplementary Figure 1). Nine of the 24 cases that first occurred during pregnancy (38%) were reported to have worsening of symptoms or relapse of disease following pregnancy loss or delivery [35, 37, 39, 46, 49, 50, 53, 58, 59], 3 of which were reported as due to medication nonadherence or patient lost to follow-up [46, 58, 59].
Of the previously reported cases, 58% (23/40) did not report HIV status, and 43% (17/40) reported the patient was HIV negative or immunocompetent. The median duration of symptoms was 16 days (IQR, 14–28 days) among those reported (31/48 [65%]). A misdiagnosis or possible delay in diagnosis was reported in 33% of cases (16/48), including 2 herein [33, 38, 39, 41–43, 49, 51, 52, 59, 63]. Maternal mortality was 23% (11/48) and fetal/neonatal mortality was 30% (13/44). Of those women who survived, 30% (11/37) were reported to have residual neurologic deficits. Three of the cases described within (cases 4–6) had recent ART initiation, which may have predisposed them to postpartum ART-related IRIS.
DISCUSSION
Data pertaining to TB meningitis outcomes in pregnancy and peripartum period are sparse despite TB being a leading cause of death among women of reproductive age. This is the first case series and first systematic review of pregnancy-related TB meningitis in women living with HIV/AIDS. Beyond the 8 cases described here, we identified only 40 additional cases of TB meningitis associated with pregnancy, all of which occurred in women living without HIV. TB meningitis presentations were variable in nature and diagnoses were frequently delayed—often not occurring until the postpartum period. This finding, while likely multifactorial in nature, is consistent with prior studies and may highlight the potential role of postpartum immune reconstitution or paradoxical reactions in those living with or without HIV and TB in pregnancy [15, 64].
Pregnant women may be at an increased risk for various infections, including TB, due to the reduction of Th1 immunity and predominance of Th2 immune responses. The reversal of proinflammatory Th1 cytokines postpartum may permit women to mount a stronger immune response to underlying disease or latent infections, which may result in IRIS and underlie the mechanism of postpartum disease flares of some autoimmune conditions (eg, Graves’ disease) [64, 65]. In our case series, we described several cases of possible HIV-associated postpartum TB meningitis IRIS and found that numerous published cases also occurred or worsened during the postpartum period in women without HIV. This finding is consistent with reports of pulmonary TB, where acute symptom worsening has been documented postpartum [15, 64, 66–69]. While this cannot be entirely associated with immune reconstitution alone, a case series of TB in pregnancy found that 76% (22/29) of cases diagnosed postpartum had no symptoms during pregnancy [28]. Furthermore, other studies have found higher rates of TB in pregnant or postpartum women compared to nonpregnant women [15, 16], highlighting the need for additional work in this area.
We found maternal mortality from TB meningitis in this case series and systematic review to be higher than that of HIV-negative nonpregnant persons (23% vs 17%) [70]. While this finding is limited due to presumed publication bias, overall small sample size, and inadequate ability and/or attempts to detect TB meningitis, the information is nonetheless a helpful starting point. We also found that a third of women who survived had some degree of residual neurologic deficits. This finding is similar to the percentage of adults with physical disabilities as a result of TB meningitis (30% vs 32%) [70]; however, these data may not have been systematically captured. Pregnant and postpartum women may be at increased risk for TB meningitis–associated neurological deficits in part due to thromboembolic phenomenon such as arterial stroke or central venous sinus thrombosis with increased coagulability and propensity for clot formation due to pregnancy.
Early diagnosis and treatment of TB meningitis and associated IRIS is paramount in improving outcomes [71]. In this study, we found that over a third of all cases had a delay in diagnosis. Delays in both symptom recognition and diagnostic turnaround time were key barriers. Diagnostic delay may occur as nonspecific symptoms such as fatigue and poor appetite may be attributed to pregnancy itself, or incorrectly attributed to obstetric complications such as postpartum psychosis or puerperal sepsis (as highlighted in our case series). Delays in tuberculous diagnostic test results combined with inadequate test sensitivities further complicated/delayed diagnoses. None of the existing CSF tests for TB meningitis have high enough negative predictive values to rule out disease [72]; thus, providers must rely on multiple test results, maintain a high index of suspicion, and treat if the clinical picture is suggestive regardless of the results (see case 2). Diagnosis of TB meningitis IRIS is also difficult [17]; clinicians must focus on the symptom onset in relation to the immune reconstitution—be this due to ART initiation or cessation of pregnancy.
Importantly, the treatment of choice for pregnant women with TB meningitis does not presently differ from that of nonpregnant people. All patients should receive rifampicin, isoniazid, pyrazinamide, and ethambutol for 2 months followed by rifampicin and isoniazid for 7–10 months. Pyrazinamide in pregnancy is controversial in the United States but is recommended by the World Health Organization for treatment of pregnant persons and is utilized for the first 2 months of treatment in nonpregnant persons with TB meningitis [73]. Overall, standard TB medications are thought to be generally safe in pregnancy [74], but carry an increased risk of hepatitis, peripheral neurotoxicity with isoniazid, and rare reports of congenital abnormalities or hemorrhagic disease with rifampicin. Streptomycin should be avoided due to fetal ototoxicity [65]. Moxifloxacin and other fluoroquinolones are often avoided due to concern for arthropathy in animals; however, there are a few small studies and case reports of fluoroquinolones being safely used in pregnancy [75–77]. For women living with HIV diagnosed with TB meningitis, providers must carefully consider when to initiate ART. In nonpregnant persons, ART is usually delayed until after 4–8 weeks of TB treatment initiation given the increased risk of adverse events in patients who have shorter duration of time between TB therapy and ART [78, 79]. In pregnant women, providers must assess the risk of possible ART-associated IRIS and the risk of mother-to-child HIV transmission, where there is a clear imperative to start ART immediately.
In people with TB meningitis–associated IRIS, higher-dose corticosteroids are recommended in addition to antimycobacterial therapy. Short courses of antenatal corticosteroids (ie, ≤10 days) are frequently utilized to promote fetal lung maturity in women at high risk for preterm delivery and in treatment of coronavirus disease 2019. However, prolonged use has been associated with negative fetal/neonatal outcomes [80]. Providers therefore should weigh the risks and benefits of the utilization of fluorinated steroids (eg, dexamethasone), which have higher rates of placental transfer, as opposed to nonfluorinated steroids (eg, prednisolone) in pregnant patients with TB meningitis who may require prolonged steroid courses [81, 82]. Other immunosuppressive agents can be considered in select cases, but safety in pregnancy must be considered as, for instance, thalidomide (used selectively in pediatric TB meningitis) would not be an option due to teratogenicity [17, 83]. It is vital to note that there are no research studies on which to base TB meningitis treatment recommendations in pregnancy, as this population has been systematically excluded from clinical research on the topic, including within our own trials described above. Many questions remain regarding whether pregnant patients should be managed in the same way as nonpregnant adults given the multiple pregnancy-related physiological changes.
Our case series and systematic review has several limitations. First, we did not assess publications related to general TB in pregnancy, which may have contained additional cases of meningitis. Second, our report cannot establish the true incidence of TB meningitis in pregnancy or the postpartum period. Third, while we noted a high proportion of cases presenting postpartum, this may be due to a multitude of factors such as the patient being present in postpartum care, potentially without engagement in care prior to birth, as opposed to theorized immune reconstitution alone. Additional considerations should be made for screening for latent TB infection in pregnancy particularly for those living with HIV and in endemic areas as is encouraged by the World Health Organization [74]. Potential benefits from screening and TB preventive treatment in pregnancy have been described [84, 85] and trials evaluating this in pregnancy have been completed [86–88], including a safety pharmacokinetic study of a 3-month regimen of isoniazid and rifapentine [89].
CONCLUSIONS
Pregnant women may be at increased risk for TB and TB meningitis due to various immunologic changes and immune reconstitution postpartum. We found limited literature on TB meningitis associated with pregnancy despite TB being a leading cause of death in women of reproductive age. With unacceptably high rates of mortality and neurologic morbidity among pregnant or recently pregnant women as described in this study, providers should maintain a high index of suspicion and consider screening for TB during pregnancy, particularly in endemic areas. Additional descriptive epidemiological work examining both disease burden and clinical outcomes of TB and other advanced opportunistic infections associated with pregnancy are needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial Support. This research is supported by the National Institute of Neurological Disorders and Stroke (award number K23NS110470 to N. C. B.); the Fogarty International Center (grant number R01NS086312); and the National Institute of Allergy and Infectious Diseases (grant number R01AI162786 to D. R. B.). F. V. C. is supported by a UK National Institute for Health and Care Research Academic Clinical Lectureship (award number CL-2020-27-001).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Potential conflicts of interest. The authors: No reported conflicts.





Comments