-
PDF
- Split View
-
Views
-
Cite
Cite
Srinivasa Nithin Gopalsamy, Aditi Ramakrishnan, Mustaf M Shariff, Julie Gabel, Skyler Brennan, Cherie Drenzek, Monica M Farley, Robert P Gaynes, Emily J Cartwright, Brucellosis Initially Misidentified as Ochrobactrum anthropi Bacteremia: A Case Report and Review of the Literature, Open Forum Infectious Diseases, Volume 8, Issue 10, October 2021, ofab473, https://doi.org/10.1093/ofid/ofab473
Close - Share Icon Share
Abstract
Automated identification systems may misidentify Brucella, the causative agent of brucellosis, which may be re-emerging in the United States as the result of an expanding feral swine population. We present a case of Brucella suis likely associated with feral swine exposure that was misidentified as Ochrobactrum anthropi, a phylogenetic relative.
Brucellosis often manifests as a nonspecific febrile illness of varying duration, which can make diagnosis challenging, especially in nonendemic areas. Moreover, isolating Brucella in culture, the gold standard, requires biocontainment and highly skilled technical personnel to handle samples, while serologic tests, polymerase chain reaction (PCR)–based testing, and automated bacterial identification systems have suboptimal clinical sensitivity and specificity. We present a case of Brucella suis infection that was misidentified as Ochrobactrum anthropi bacteremia and review the related literature on misdiagnosis of brucellosis and its management implications. We also highlight an expanding epidemiologic risk factor for brucellosis and discuss treatment considerations and the risk of relapsed disease with delayed-onset complications, as demonstrated in this case.
CASE
A 36-year-old man presented after a recent hunting and fishing trip in Alaska. Two days into the trip, he developed fever, night sweats, dizziness, and generalized arthralgias with no focal joint pain. He had headaches and photosensitivity but no focal weakness, numbness, or changes in vision or hearing. An episode of presyncope 3 weeks later prompted him to pursue further evaluation. His medical history was notable for systemic lupus erythematosus with lupus nephritis in remission last treated 4 years ago with mycophenolate, hydroxychloroquine, and prednisone. His surgical history was notable for bilateral total hip replacements 2 years ago for avascular necrosis due to prolonged steroid use. He reported no cigarette smoking, alcohol, or illicit drug use. He was monogamous with his wife; they lived in rural Georgia.
On presentation, he had a temperature of 38.0°C, heart rate of 105 bpm, blood pressure of 108/72 mmHg, respiratory rate of 17, and oxygen saturation of 98% on room air. Physical examination was notable for diaphoresis, shotty cervical lymphadenopathy, and L1–2 spine tenderness. There was no photophobia, nuchal rigidity, focal neurologic deficits, or hip tenderness. Laboratory tests demonstrated leukopenia of 2.4 k/cm2 (42.2% segmented neutrophils, 45.5% lymphocytes, and 11.9% monocytes) with hemoglobin 13.9 g/dL and platelets 152 k/cm2. The alanine aminotransferase was 28 IU/L, aspartate aminotransferase 43 IU/L, and the C-reactive protein and erythrocyte sedimentation rate were elevated at 33.5 mg/L and 94 mm/h, respectively. The urinalysis demonstrated 2+ protein, 2 white blood cells per high power field, and <1 red blood cells per high power field. Lumbar and abdominal computed tomography imaging revealed splenomegaly and right L5 pars defect with hip visualization limited by streak artifact.
On admission, blood cultures were obtained. He was placed on empiric vancomycin and piperacillin-tazobactam but remained febrile with a maximum temperature of 39.4°C 4 days into the admission. The infectious diseases service was consulted and recommended serologies for Coxiella, Tularemia, Brucella, leptospirosis, and serum PCR for West Nile virus. Given the absence of meningismus or focal neurologic symptoms, lumbar puncture was deferred. On hospital day 3, blood cultures began growing a gram-negative organism. The isolate grew on blood and chocolate agars but not on MacConkey agar. The VITEK 2 (bioMérieux, Durham, NC, USA) automated system identified the isolate as Ochrobactrum anthropi. Given ongoing fever and reported automated antimicrobial susceptibility data [1], the antibiotic regimen was changed to levofloxacin; susceptibility was later confirmed via E-test (bioMérieux, Durham, NC, USA). A transthoracic echocardiogram showed no vegetations, and his repeat blood cultures were without growth. The fevers resolved 2 days after the antibiotic change, and he was afebrile for 48 hours before discharge. His headache resolved and his arthralgias improved with no focal joint pain. He was discharged with a plan to complete a 2-week course of levofloxacin.
Following discharge, the Brucella immunoassay resulted in an IgM of 5.87 (negative if <0.8) and immunoglobulin G (IgG) of 1.92 with an antibody titer >1:1280 (Quest Diagnostics, San Juan Capistrano, CA, USA) [2]. The patient was contacted by the Georgia Department of Public Health (DPH) and reported field dressing a feral hog in Georgia and handling the raw meat without gloves. The DPH performed a Brucella microagglutination test (BMAT) that revealed a markedly elevated titer of 1:2560/1:320 reduced (IgG component), consistent with brucellosis.
On the subsequent clinic visit, 2 months after discharge, the patient reported return of fevers, night sweats, and polyarthralgias. He was started on therapy with a 6-week course of doxycycline and rifampin. Brucella serology studies at this time showed an IgM of 2.51 and IgG of 3.85. Three months after completing the antibiotic course, the patient developed acute-onset left hip pain after trying to catch a baseball. The pain was worse with weight-bearing and ambulation. X-ray showed a fixed prosthesis with no acute fracture, while magnetic resonance imaging revealed a joint effusion. The synovial fluid aspirate had 15 011 white blood cells/mm3 (56.7% neutrophils and 43% mononuclear cells) and grew Brucella on initial culture, and real-time PCR confirmed the isolate to be Brucella suis (Figure 1). The patient completed 4 weeks of gentamicin and restarted a prolonged course of doxycycline and rifampin. Notably, following completion of gentamicin, he reported difficulty with his balance that subsequently resolved in the absence of aminoglycoside therapy.
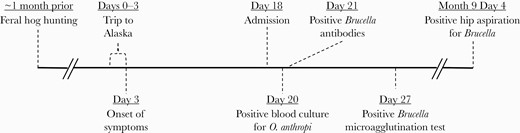
Timeline of patient’s course. For the diagnostic tests, the day denoted refers to the collection date rather than the result date. Distance between events on timeline not to scale.
DISCUSSION
This case highlights the limitations and pitfalls associated with the diagnosis and management of brucellosis, particularly in the context of misidentification as Ochrobactrum anthropi. O. anthropi and Brucella spp. are gram-negative organisms [3, 4] that are close phylogenetic relatives based on DNA, RNA, and protein analyses [4], resulting in significant cross-reactivity on Western blot [5] and overlay in 16S ribosomal RNA (rRNA) sequence signatures [5, 6]. With such similarities between the 2 species, diagnostic overlap is a concern [7, 8]. The VITEK 2 automated system, used in this case, and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry utilize a reference database to identify organisms. These automated systems are limited by the isolate-specific data available in the respective catalogues. Currently, only the VITEK 2 gram-negative bacteria card includes a Brucella species with B. melitensis [9], while Ochrobactrum anthropi is available routinely [9–11]. Table 1 summarizes the published reports of misidentification of Brucella species as O. anthropi with both older and updated automated systems. As Brucella is not regularly available on calorimetry or spectrometry databases, conventional culture techniques retain value. Though both Brucella and O. anthropi may grow on standard laboratory media including blood and chocolate agar, Brucella does not grow on MacConkey agar, while O. anthropi does. Thus, the American Society for Microbiology guidelines recommend that Brucella be identified by its growth patterns and state that automated systems have no role in the diagnosis of brucellosis [12].
Cases of brucellosis initially misdiagnosed as Ochrobactrum anthropi infection
| Case . | O. anthropi Identification Method . | Brucella Antibody . | AgglutinationTest . | PCR . |
|---|---|---|---|---|
| Elsaghir et al., 2003 [28] | API 20NE | Positive | Positive | B. melitensis |
| Horvat et al., 2011 [7] | RapID NF Plus | B. suis | ||
| Carrington et al., 2012 [8] | RapID NF Plus VITEK 2 | Positive | B. suis | |
| Vila et al., 2016 [29] | VITEK 2 | Positive | Positive | B. suis |
| Trêpa et al, 2018 [30] | VITEK MS | Positive | B. melitensis | |
| Poonawala et al., 2018 [31] | VITEK MS | Positive | B. melitensis | |
| Khaliulina Ushakova et al., 2020 [32] | MALDI-TOF MS Brukera | Positive | ||
| Current case | VITEK 2 | Positive | Positive | B. suis |
| Case . | O. anthropi Identification Method . | Brucella Antibody . | AgglutinationTest . | PCR . |
|---|---|---|---|---|
| Elsaghir et al., 2003 [28] | API 20NE | Positive | Positive | B. melitensis |
| Horvat et al., 2011 [7] | RapID NF Plus | B. suis | ||
| Carrington et al., 2012 [8] | RapID NF Plus VITEK 2 | Positive | B. suis | |
| Vila et al., 2016 [29] | VITEK 2 | Positive | Positive | B. suis |
| Trêpa et al, 2018 [30] | VITEK MS | Positive | B. melitensis | |
| Poonawala et al., 2018 [31] | VITEK MS | Positive | B. melitensis | |
| Khaliulina Ushakova et al., 2020 [32] | MALDI-TOF MS Brukera | Positive | ||
| Current case | VITEK 2 | Positive | Positive | B. suis |
Identification method refers to the modality used that initially identified the isolate as O. anthropi. Agglutination test includes serum agglutination test (including tube, plate, Rose-Bengal and Wright tests) and Brucella microagglutination test. PCR testing includes 16S rRNA sequencing.
Abbreviation: PCR, polymerase chain reaction.
aLater changed to B. melitensis
Cases of brucellosis initially misdiagnosed as Ochrobactrum anthropi infection
| Case . | O. anthropi Identification Method . | Brucella Antibody . | AgglutinationTest . | PCR . |
|---|---|---|---|---|
| Elsaghir et al., 2003 [28] | API 20NE | Positive | Positive | B. melitensis |
| Horvat et al., 2011 [7] | RapID NF Plus | B. suis | ||
| Carrington et al., 2012 [8] | RapID NF Plus VITEK 2 | Positive | B. suis | |
| Vila et al., 2016 [29] | VITEK 2 | Positive | Positive | B. suis |
| Trêpa et al, 2018 [30] | VITEK MS | Positive | B. melitensis | |
| Poonawala et al., 2018 [31] | VITEK MS | Positive | B. melitensis | |
| Khaliulina Ushakova et al., 2020 [32] | MALDI-TOF MS Brukera | Positive | ||
| Current case | VITEK 2 | Positive | Positive | B. suis |
| Case . | O. anthropi Identification Method . | Brucella Antibody . | AgglutinationTest . | PCR . |
|---|---|---|---|---|
| Elsaghir et al., 2003 [28] | API 20NE | Positive | Positive | B. melitensis |
| Horvat et al., 2011 [7] | RapID NF Plus | B. suis | ||
| Carrington et al., 2012 [8] | RapID NF Plus VITEK 2 | Positive | B. suis | |
| Vila et al., 2016 [29] | VITEK 2 | Positive | Positive | B. suis |
| Trêpa et al, 2018 [30] | VITEK MS | Positive | B. melitensis | |
| Poonawala et al., 2018 [31] | VITEK MS | Positive | B. melitensis | |
| Khaliulina Ushakova et al., 2020 [32] | MALDI-TOF MS Brukera | Positive | ||
| Current case | VITEK 2 | Positive | Positive | B. suis |
Identification method refers to the modality used that initially identified the isolate as O. anthropi. Agglutination test includes serum agglutination test (including tube, plate, Rose-Bengal and Wright tests) and Brucella microagglutination test. PCR testing includes 16S rRNA sequencing.
Abbreviation: PCR, polymerase chain reaction.
aLater changed to B. melitensis
Given the microbiological challenges in diagnosing brucellosis, an awareness of its local epidemiology may help maintain clinical suspicion. Four Brucella species can cause human disease: B. melitensis (isolated from sheep, goats, camels, and buffalo), B. abortus (from cattle), B. canis (from dogs), and B. suis (from swine) [3, 13]. While B. suis has been eradicated from the American commercial swine population [14], it is endemic among feral swine [15–17]. The geographic distribution of brucellosis, particularly B. suis, may be expanding with the increasing population and geographic distribution of feral swine in the United States. Feral swine are described as the most destructive invasive species nationally and are most prominent in the South [18, 19]. There are an estimated 4–6 million feral swine in the United States [20], with 200 000–600 000 reported in Georgia alone [21]. From 2010 to 2019, 42 probable and confirmed cases of brucellosis were reported to the Georgia DPH. Of the 26 confirmed cases, B. suis was the causative organism in 21. In at least 16 of the 21, feral swine exposure was documented. Hunting and field dressing feral swine is a growing sport that is aimed at controlling the feral swine population but is increasingly identified as a risk factor for B. suis infection in the United States [22].
As with diagnosing brucellosis, subsequent management is challenging. Monotherapy is not recommended, and an extended antibiotic duration is often necessary. The preferred regimens are doxycycline combined with an aminoglycoside or rifampin. While this patient did not have meningismus, encephalopathy, myelitis, radiculitis, or neuritis, such neurologic complications should prompt cerebrospinal fluid evaluation and may warrant the addition of ceftriaxone or trimethoprim-sulfamethoxazole [3]. Although the aminoglycoside-containing regimen is associated with less treatment failure [23], doxycycline and rifampin are often selected due to their availability and convenience [24]. The patient’s vestibular dysfunction symptoms also highlight the potential toxicity with aminoglycosides, though this is generally self-limited, as in this case [3]. Despite appropriate therapy, continued diligence is required as a course of brucellosis may involve treatment relapse, particularly among patients with positive blood cultures [25], within 6 months of therapy [26]. Relapse may manifest atypically with late-declaring organ complications; hardware such as a prosthetic joint is at high risk in the setting of bacteremia [27].
This case underscores the initial and long-term challenges of diagnosing and managing brucellosis. A thorough history is imperative, including recent and remote occupational and nonoccupational exposures, with a contemporary understanding of the epidemiology. Clinicians must maintain an elevated index of suspicion and utilize conventional microbiologic tools as automated identification systems may not suffice, as exemplified by the misidentification of Brucella species as Ochrobactrum anthropi. Upon diagnosing brucellosis, providers must be wary of relapsed or complicated disease with metastatic foci of infection, including organ and joint involvement, particularly in the presence of hardware.
Acknowledgments
Financial support. TL1TR002382 provided protected time for S. N. Gopalsamy.
Potential conflicts of interest. All authors report no potential conflicts of interest relevant to this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This manuscript does not include factors necessitating patient consent.
Author contributions. Srinivasa Nithin Gopalsamy, MD—drafting manuscript, producing table and figure. Aditi Ramakrishnan, MD—drafting and revising manuscript. Mustaf M. Shariff, MD—drafting and revising manuscript. Julie Gabel, DVM, MPH—drafting and revising manuscript. Skyler Brennan, MPH—drafting and revising manuscript. Cherie Drenzek, DVM, MS—drafting and revising manuscript. Monica M. Farley, MD—drafting and revising manuscript. Robert P. Gaynes, MD—drafting and revising manuscript. Emily J. Cartwright, MD—drafting, revising, and submitting manuscript.





Comments