-
PDF
- Split View
-
Views
-
Cite
Cite
Surya Chitra, Jordan Hinahara, Thomas F Goss, Kyle Gunter, Kenneth LaPensee, Health-Related Quality of Life as Measured by the 36-Item Short Form Survey Among Adults With Acute Bacterial Skin and Skin Structure Infections who Received Either Omadacycline or Linezolid in a Phase 3 Double-Blind, Double-Dummy Clinical Trial, Open Forum Infectious Diseases, Volume 8, Issue 10, October 2021, ofab459, https://doi.org/10.1093/ofid/ofab459
Close - Share Icon Share
Abstract
This analysis of data from a Phase 3 study of adults with acute bacterial skin and skin structure infections showed that successful oral treatment with omadacycline (n = 368) or linezolid (n = 367) was associated with improvement in health-related quality of life.
Omadacycline is an aminomethylcycline antibiotic that is approved for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and community-acquired bacterial pneumonia in adults [1–3]. Omadacycline has demonstrated activity against many common pathogens involved in ABSSSIs, including Staphylococcus aureus and Streptococcus pyogenes [4, 5]. The efficacy and safety of omadacycline for adults with ABSSSIs were established in 2 Phase 3 studies, OASIS-1 (NCT02378480) and OASIS-2 (NCT02877927) [6, 7]. The US Food and Drug Administration (FDA) stipulates that outcome measures for registrational studies should be “direct measures or established surrogates of how patients feel, function, or survive,” and these considerations have led to publication of FDA guidance for trials in ABSSSIs [8, 9]. Patient-reported outcomes (PROs) provide direct evidence of treatment benefit in that they directly measure how the patient feels or functions [10], and the 36-item Short Form Health Survey, version 2 (SF-36v2; Optum Inc., Minneapolis, MN, USA) [11, 12], a validated PRO health-related quality of life (HRQoL) measure [13], was prospectively incorporated in OASIS-2. The SF-36 has been included previously in study protocols to evaluate HRQoL in patients receiving antibiotic therapy for infections, including skin infections [14–16]. However, we are not aware of any studies of antibiotics that have demonstrated an association between clinical response (clinical success or clinical failure) and improvement in HRQoL, as measured by SF-36 score.
This post hoc exploratory analysis was performed to assess HRQoL outcomes as measured by the SF-36v2 in the OASIS-2 trial of patients randomized to oral omadacycline or oral linezolid treatment for ABSSSIs, overall and by clinical efficacy outcome.
METHODS
Study Design
OASIS-2 was a registrational Phase 3 clinical study comparing oral omadacycline with oral linezolid for treating adults with ABSSSIs. Clinical assessment of the infection site included the size of the primary lesion and microbiological assessment by gram stain and culture [7]. Patients were eligible for enrollment if they were aged ≥18 years, had a qualifying ABSSSI with primary lesion size ≥75 cm2, and had evidence of systemic inflammatory response within 24 hours before randomization [7]. Eligible patients were randomized 1:1 to receive either omadacycline or linezolid stratified by type of infection (wound infection, cellulitis/erysipelas, and major abscess) and receipt of a single antibiotic dose within 72 hours before dosing [7]. Dosing and administration requirements for the study drug are included in the Supplementary Data (Supplementary Table 1). Clinical assessment of the infection site included the size of the primary lesion and microbiological assessment by gram stain and culture [7].
Health-Related Quality of Life
Patients completed the SF-36v2 questionnaire at the screening (baseline) visit and the post-therapy evaluation (PTE) visit (occurring between days 14 and 28). The SF-36v2 comprises 36 items assessing overall physical and mental health, including questions measuring health across 8 domains (Physical Functioning, Role–Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role–Emotional, and Mental Health) and 2 psychometrically based component summary scores, a Physical Component Summary and Mental Component Summary [11, 12, 17]. Responses to each item within a domain are combined to generate a scaled score from 0 to 100, where 100 indicates the best possible health. The mapping of individual items to the overall scale structure is summarized in Supplementary Figure 1 [11, 12].
Patient Consent
All clinical trial participants provided written informed consent.
Data Analyses
Descriptive analyses of patient demographics and baseline characteristics were conducted. Results of the SF-36v2 were analyzed in accordance with established norm-based standards for the survey [12]. No prespecified analysis plan for these data was developed before initiation of the of study. A post hoc exploratory analysis was performed on the survey responses for the intent-to-treat (ITT) population (all randomized patients), overall and by clinical outcome. Due to the significant proportion of persons who inject drugs (PWID) in the OASIS-2 study, a subgroup analysis was also performed to assess the HRQoL impact of treatment in PWID.
The change from baseline for each scaled score was analyzed using an analysis of covariance model with treatment group as a fixed effect and baseline scaled score as a covariate. Least-squares mean differences, corresponding 95% CIs, and P values were obtained using this model.
RESULTS
In total, 735 patients were enrolled in OASIS-2, and demographics and baseline characteristics in the ITT population were broadly similar between the 2 treatment groups, although a history of diabetes mellitus was reported in a higher percentage of patients in the linezolid group (8.7%, vs 4.1% in the omadacycline group) (Supplementary Table 2). Overall, most patients were male (462/735 [63%]) and White (668/735 [91%]). Injection drug use was the most common comorbid condition among OASIS-2 patients, and 69% of the ABSSSIs in the study were reported to be related to injection drug use [7].
Of the 735 patients enrolled in the study, 719 (361 in the linezolid group and 358 in the omadacycline group) fully completed the survey at baseline and 632 (316 in both groups) fully completed the survey at PTE. The lower number of completed surveys at PTE was due to loss to follow-up as well as incomplete surveys. Among all patients, irrespective of clinical outcome, baseline scores (data not shown) and changes from baseline to PTE follow-up in SF-36v2 mean scores were similar between the omadacycline and linezolid groups (Figure 1; Supplementary Table 3). Scores improved across all domains for the omadacycline group, and for all domains except General Health and Role–Emotional for the linezolid group (Figure 1; Supplementary Table 3). No statistically significant difference in the changes in mean domain scores or component summary scores was seen between omadacycline and linezolid in the ITT population (Figure 1). The Physical Component Summary score showed a statistically significant improvement from baseline for both treatment groups (Figure 1). At baseline, the SF-36 scores for both treatment groups were consistently lower than normative scores for the US population, most notably for the Bodily Pain domain (Supplementary Figure 2) [12]. When treatment groups were combined, among both PWID and non-PWID there was a substantial improvement from baseline in the Physical Component Summary score, similar to the overall patient population (Supplementary Table 3).
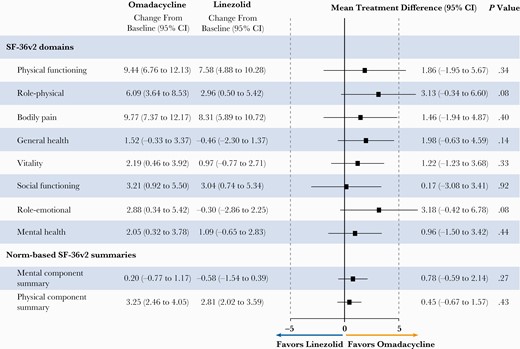
Changes in SF-36v2 domain and component summary scores from baseline to post-therapy evaluation follow-up in patients in the omadacycline and linezolid treatment groups, irrespective of clinical outcome.a All values are for the intent-to-treat population and are reported as mean (95% CI). Post-therapy evaluation follow-up occurred at 7–14 days after the last dose of study treatment. aSF-36v2 scores at baseline and post-therapy evaluation follow-up are shown in Supplementary Table 3. Abbreviation: SF-36v2, 36-item Short Form Health Survey, version 2.
An additional analysis was performed for data pooled across treatment groups for the effect by clinical outcome (Table 1). In patients who had clinical success, regardless of treatment group, statistically significant improvements from baseline to PTE were reported in 6 of the 8 domains of the SF-36v2 survey and the Physical Component Summary score (Table 1). There was a small, nonsignificant decrease in the Mental Component Summary score from baseline to follow-up in patients with clinical success (Table 1). Patients with clinical failure had similar baseline scores (data not shown) as those with clinical success; however, no significant changes in SF-36v2 scores from baseline to PTE were seen in the pooled group of patients who had clinical failure (Table 1).
Changes in SF-36v2 Domain and Component Summary Scores From Baseline to Post-therapy Evaluation (Overall and by Clinical Outcome): Pooled Data
| Quality of Life Outcome . | Clinical Outcome . | . |
|---|---|---|
| . | Patients With Clinical Success . | Patients With Clinical Failure . |
| SF-36v2 domains | ||
| Physical Functioning | 8.85 (6.47 to 11.23) n = 603 | 4.38 (−3.66 to 12.41) n = 32 |
| Role–Physical | 4.77 (2.62 to 6.92) n = 604 | 4.89 (−1.88 to 11.65) n = 32 |
| Bodily Pain | 9.47 (7.5 to 11.44) n = 604 | 0.22 (−9.23 to 9.67) n = 32 |
| General Health | 0.43 (−1.02 to 1.87) n = 577 | 2.92 (−3.65 to 9.48) n = 31 |
| Vitality | 1.49 (0.1 to 2.89) n = 604 | 4.11 (−0.53 to 8.74) n = 32 |
| Social Functioning | 3.23 (1.3 to 5.16) n = 603 | 5.86 (−1.56 to 13.28) n = 32 |
| Role–Emotional | 1.68 (−0.43 to 3.79) n = 604 | −3.11 (−11.84 to 5.61) n = 32 |
| Mental Health | 1.67 (0.28 to 3.06) n = 604 | 2.34 (−3 to 7.68) n = 32 |
| Norm-based SF-36v2 summaries | ||
| Mental Component Summary | −0.18 (−0.96 to 0.6) n = 576 | 1.04 (−1.67 to 3.75) n = 31 |
| Physical Component Summary | 3.12 (2.45 to 3.78) n = 576 | 1.75 (−0.8 to 4.31) n = 31 |
| Quality of Life Outcome . | Clinical Outcome . | . |
|---|---|---|
| . | Patients With Clinical Success . | Patients With Clinical Failure . |
| SF-36v2 domains | ||
| Physical Functioning | 8.85 (6.47 to 11.23) n = 603 | 4.38 (−3.66 to 12.41) n = 32 |
| Role–Physical | 4.77 (2.62 to 6.92) n = 604 | 4.89 (−1.88 to 11.65) n = 32 |
| Bodily Pain | 9.47 (7.5 to 11.44) n = 604 | 0.22 (−9.23 to 9.67) n = 32 |
| General Health | 0.43 (−1.02 to 1.87) n = 577 | 2.92 (−3.65 to 9.48) n = 31 |
| Vitality | 1.49 (0.1 to 2.89) n = 604 | 4.11 (−0.53 to 8.74) n = 32 |
| Social Functioning | 3.23 (1.3 to 5.16) n = 603 | 5.86 (−1.56 to 13.28) n = 32 |
| Role–Emotional | 1.68 (−0.43 to 3.79) n = 604 | −3.11 (−11.84 to 5.61) n = 32 |
| Mental Health | 1.67 (0.28 to 3.06) n = 604 | 2.34 (−3 to 7.68) n = 32 |
| Norm-based SF-36v2 summaries | ||
| Mental Component Summary | −0.18 (−0.96 to 0.6) n = 576 | 1.04 (−1.67 to 3.75) n = 31 |
| Physical Component Summary | 3.12 (2.45 to 3.78) n = 576 | 1.75 (−0.8 to 4.31) n = 31 |
Results are shown for pooled treatment groups (omadacycline and linezolid). All values are for the intent-to-treat population and are reported as mean (95% CI), together with the numbers of patients for each domain/summary. Post-therapy evaluation follow-up occurred at 7–14 days after the last dose of study treatment.
Abbreviation: SF-36v2, 36-item Short Form Health Survey, version 2.
Changes in SF-36v2 Domain and Component Summary Scores From Baseline to Post-therapy Evaluation (Overall and by Clinical Outcome): Pooled Data
| Quality of Life Outcome . | Clinical Outcome . | . |
|---|---|---|
| . | Patients With Clinical Success . | Patients With Clinical Failure . |
| SF-36v2 domains | ||
| Physical Functioning | 8.85 (6.47 to 11.23) n = 603 | 4.38 (−3.66 to 12.41) n = 32 |
| Role–Physical | 4.77 (2.62 to 6.92) n = 604 | 4.89 (−1.88 to 11.65) n = 32 |
| Bodily Pain | 9.47 (7.5 to 11.44) n = 604 | 0.22 (−9.23 to 9.67) n = 32 |
| General Health | 0.43 (−1.02 to 1.87) n = 577 | 2.92 (−3.65 to 9.48) n = 31 |
| Vitality | 1.49 (0.1 to 2.89) n = 604 | 4.11 (−0.53 to 8.74) n = 32 |
| Social Functioning | 3.23 (1.3 to 5.16) n = 603 | 5.86 (−1.56 to 13.28) n = 32 |
| Role–Emotional | 1.68 (−0.43 to 3.79) n = 604 | −3.11 (−11.84 to 5.61) n = 32 |
| Mental Health | 1.67 (0.28 to 3.06) n = 604 | 2.34 (−3 to 7.68) n = 32 |
| Norm-based SF-36v2 summaries | ||
| Mental Component Summary | −0.18 (−0.96 to 0.6) n = 576 | 1.04 (−1.67 to 3.75) n = 31 |
| Physical Component Summary | 3.12 (2.45 to 3.78) n = 576 | 1.75 (−0.8 to 4.31) n = 31 |
| Quality of Life Outcome . | Clinical Outcome . | . |
|---|---|---|
| . | Patients With Clinical Success . | Patients With Clinical Failure . |
| SF-36v2 domains | ||
| Physical Functioning | 8.85 (6.47 to 11.23) n = 603 | 4.38 (−3.66 to 12.41) n = 32 |
| Role–Physical | 4.77 (2.62 to 6.92) n = 604 | 4.89 (−1.88 to 11.65) n = 32 |
| Bodily Pain | 9.47 (7.5 to 11.44) n = 604 | 0.22 (−9.23 to 9.67) n = 32 |
| General Health | 0.43 (−1.02 to 1.87) n = 577 | 2.92 (−3.65 to 9.48) n = 31 |
| Vitality | 1.49 (0.1 to 2.89) n = 604 | 4.11 (−0.53 to 8.74) n = 32 |
| Social Functioning | 3.23 (1.3 to 5.16) n = 603 | 5.86 (−1.56 to 13.28) n = 32 |
| Role–Emotional | 1.68 (−0.43 to 3.79) n = 604 | −3.11 (−11.84 to 5.61) n = 32 |
| Mental Health | 1.67 (0.28 to 3.06) n = 604 | 2.34 (−3 to 7.68) n = 32 |
| Norm-based SF-36v2 summaries | ||
| Mental Component Summary | −0.18 (−0.96 to 0.6) n = 576 | 1.04 (−1.67 to 3.75) n = 31 |
| Physical Component Summary | 3.12 (2.45 to 3.78) n = 576 | 1.75 (−0.8 to 4.31) n = 31 |
Results are shown for pooled treatment groups (omadacycline and linezolid). All values are for the intent-to-treat population and are reported as mean (95% CI), together with the numbers of patients for each domain/summary. Post-therapy evaluation follow-up occurred at 7–14 days after the last dose of study treatment.
Abbreviation: SF-36v2, 36-item Short Form Health Survey, version 2.
DISCUSSION
There is much interest in the “voice” of the patient in medical decision-making processes [18, 19], and one gap in current registrational ABSSSI studies is understanding what is most important to the patient [8]. Reduction in lesion size is used as a surrogate end point for registrational studies, substituting for an end point that is a direct measure of how a patient feels [10]. PROs can increase clarity and precision when interpreting end points for clinical meaningfulness [10]. Although it is likely to have been an assumption in previous studies, we believe that the current study is the first to report an observed difference for improvement in HRQoL, as measured by the SF-36v2, in patients who received antibiotic treatment for ABSSSI and had clinical success, as opposed to those with failure. As omadacycline and linezolid are both highly efficacious antibiotics in ABSSSIs, these data indicate that effective clinical treatment of ABSSSIs can be expected to improve HRQoL in this patient population. Baseline domain scores were lower than the US normative population, suggesting substantial QOL burden in ABSSSI before treatment [12]. While there were slight numerical improvements in summary scores for the clinical failure group from baseline to follow-up, no statistically significant change in HRQoL scale scores was observed in this group, potentially due to a type II error given the small number of patients in that group. To better understand how patients feel and whether this is associated with their clinical outcome, consideration of patient-reported factors such as HRQoL should be explored further in future clinical trials.
OASIS-2 demonstrated that the oral antibiotics omadacycline and linezolid can treat significant ABSSSI (omadacycline: median lesion size [interquartile range {IQR}], 322 [198–495] cm²; linezolid: median lesion size [IQR], 294 [190–462] cm²) without hospitalization [7]. Clinical failure rates reported with omadacycline and linezolid were relatively low, at 7% and 9%, respectively [7]. Given the findings of the current analysis, that patients with clinical failure had a nonsignificant improvement in quality of life, real-world use studies and future guidelines should incorporate this knowledge, along with available efficacy, safety, antibiotic resistance rates, and economic data, to provide empiric treatment recommendations that allow many patients to receive appropriate therapy and minimize intravenous therapy and hospitalizations when possible.
PWID are a vulnerable population at high risk of ABSSSIs [20, 21]; PWID constituted a substantial proportion of the patients in OASIS-2 [7]. In the current analysis, PWID in both treatment groups showed improvement in HRQoL scores, which suggests that they can benefit from efficacious antibiotic therapy. However, as the number of patients with clinical failure was small, it was not possible to perform an analysis of changes in HRQoL by clinical outcome for the PWID subpopulation. Incorporating PROs in future studies may provide additional insights.
Several limitations should be considered when interpreting these data. There is no FDA-recommended HRQoL measure for ABSSSI treatment. General HRQoL questionnaires such as SF-36v2 are widely used and well validated, but the impact of antibiotic treatment on patient-reported HRQoL in ABSSSI is not. Furthermore, there is no universally accepted definition of what constitutes a clinically meaningful difference in HRQoL research for ABSSSI; this should be defined in future studies. It is unclear whether an HRQoL tool such as the SF-36v2, which uses a 4-week look-back period, is the most appropriate instrument for an acute illness like ABSSSI. The acute SF-36v2 form uses a 1-week recall period, which may be more appropriate for acute infections [11]. The OASIS-2 study was not specifically designed or powered to assess HRQoL outcomes, and the analyses reported here were post hoc exploratory analyses; given the small number of patients with clinical failure, further analyses for covariates could not be performed. Possible contributors to treatment failure and lower HRQoL could extend beyond antibiotic selection and resistance to include patient comorbidities, disease severity, surgical interventions, baseline lesion size, or lower baseline HRQoL [22]. Future studies should include relevant multivariate analyses to account for any confounding factors in the data. It would also be worthwhile to examine in a larger patient population whether absolute baseline SF-36v2 scores were similar in patients with clinical success and patients with clinical failure, or if there were any differences. Additionally, the patients’ preinfection HRQoL scores were unknown; it may be useful to determine in future studies if HRQoL scores are restored after treatment. The data used for the current analysis were obtained in the environment of a Phase 3 study in which these and other possible contributory factors were monitored and controlled. The differences in patient-reported, treatment-associated HRQoL identified here warrant further investigation, particularly in real-world studies, including when treating ABSSSI with antibiotics to which causative pathogens, such as S. pyogenes and S. aureus, have notable resistance and documented failure rates.
CONCLUSIONS
This exploratory analysis of HRQoL data collected in the OASIS-2 Phase 3 clinical trial suggests that, in patients with ABSSSI, those who achieve clinical success have a significant improvement in HRQoL and those with clinical failure do not. As stipulated in FDA guidance [8], clinical studies should aim to measure how a patient feels and functions, and patient-reported factors such as HRQoL may be useful to capture the “voice” of the patient, in addition to surrogate outcomes such as lesion size, hospital readmission rates, and safety.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This study was funded by Paratek Pharmaceuticals, Inc. Medical editorial assistance, funded by Paratek Pharmaceuticals, Inc., was provided by Felicity Leigh, PhD, of Innovative Strategic Communications, Inc. The authors thank the participants and investigators involved in the OASIS-2 study.
Potential conflicts of interest. Surya Chitra and Kyle Gunter are employees of Paratek Pharmaceuticals. Kenneth LaPensee was an employee of Paratek Pharmaceuticals at the time of this analysis. Thomas Goss and Jordan Hinahara are employees of Boston Healthcare Associates, which received consulting fees from Paratek Pharmaceuticals. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Surya Chitra performed the statistical analysis of the HRQoL study data. Kenneth LaPensee, Thomas Goss, Jordan Hinahara, and Kyle Gunter contributed to the interpretation of the study data and writing and editing of this manuscript.
Patient consent. All clinical trial participants provided written informed consent.
Data availability. The primary study results have been published in Lancet Infectious Diseases [7] and are available at ClinicalTrials.gov (NCT02877927) and in the OASIS-2 clinical study report (Clinical Study Report: PTK0796-ABSI-16301).





Comments