-
PDF
- Split View
-
Views
-
Cite
Cite
Zhongquan Liu, Huijie Huang, Teng Yue, Nahom Kiros Gebregziabher, Hui Gong, Peng Xu, Xiaoyue Dong, Yi Liu, Ziming Wu, Yan Guo, Tielin Ning, Long Li, Minna Zheng, Jie Yang, Jun Ma, Changping Li, Maohe Yu, Zhuang Cui, Standardly Trained Peer Volunteer–Led, Social App Recruiting–Based Human Immunodeficiency Virus Testing Strategy Using Rapid Testing Kits (SPARK) Among Men Who Have Sex With Men: A Population-Based Survey Evaluation, Open Forum Infectious Diseases, Volume 11, Issue 12, December 2024, ofae709, https://doi.org/10.1093/ofid/ofae709
Close - Share Icon Share
Abstract
Social app recruiting–based and peer-led testing strategies have been proven effective in increasing human immunodeficiency virus (HIV) testing among men who have sex with men (MSM), though their combination remains underevaluated. We aimed to assess the efficiency of a combined strategy named “standardly trained peer volunteer–led, social app recruiting–based HIV testing strategy using rapid testing kits” (SPARK).
Between March 2020 and December 2021, 177 trained peer volunteers tested 7256 eligible MSM testers. Volunteers primarily recruited testers to undergo HIV testing and counseling in social apps. Volunteers tested testers with HIV rapid antibody tests and interviewed testers while waiting for the results. Moreover, HIV testing data from other testing strategies, both pre- and postimplementation of SPARK, were collected to evaluate the capacity of SPARK to increase HIV testing.
During this study, MSM testers underwent 10 441 HIV tests; HIV testing volume increased 3-fold from 2020 to 2021. On average, each volunteer recruited 40.99 testers and facilitated 58.99 HIV tests. After SPARK implementation, HIV tests in 2021 increased 1-fold compared with those in 2019; especially for rural MSM testers, the number of HIV tests performed in 2020 and 2021 increased to 2.86 and 5.85 times, respectively, that in 2019. In spatial analysis, most testers sought geographical proximity volunteers for testing; similarly, most testers recruited were from volunteers’ own or nearby districts. More than 60% of HIV tests were performed outside of working hours on weekdays, regardless of whether the testers came from urban, periurban, or rural areas.
SPARK, an MSM-friendly, geographically accessible, and time-flexible testing strategy, has the potential to promote HIV testing among MSM.
Expanding human immunodeficiency virus (HIV) testing among men who have sex with men (MSM) is vital for achieving the first of the 95-95-95 targets [1]. Mainstream HIV testing strategies in China include voluntary counseling and testing, provider-initiated testing and counseling, and HIV self-testing (HIVST) [2]. Recently, innovative methods for promoting and distributing HIVST (eg, venue-based [3], social network–based [4], and internet-based [5] approaches) have been implemented to better reach MSM. Despite these efforts, only 49.7% of MSM in China had undergone HIV testing in 2020 [6]. Therefore, more effective HIV testing strategies are needed among MSM, especially “hard-to-reach” individuals.
Urbanization created more opportunities for homosexual interaction venues to thrive. Additionally, advancements in transportation have reduced interpersonal distances, while mobile phones and the internet have significantly expanded social networks, including those within the MSM community. The popularity of geosocial networking (GSN) and dating apps offers community-based organizations (CBOs) the chance to promote equity and prevent new HIV infections among MSM [7]. Moreover, technology-based recruitment methods can effectively reach many hard-to-reach individuals [8]. The use of GSN apps has significantly increased HIV test uptake by disseminating testing information to MSM living near testing sites in Beijing [9]. Several studies have explored HIVST via GSN apps, all of which have found it to be a practical and feasible complement to traditional methods [10–13].
However, these strategies may still retain the shortcomings of conventional venue-based HIV testing methods, such as limited coverage, and introduce new challenges for self-testers, including lack of experience in testing procedures and interpreting results [14], fear of a positive test result without immediate personal support [14], difficulties in linking to confirmatory tests and HIV care services, and low result recovery rates among negative self-testers [15]. Although significant gaps in evidence remain, social network–based strategies, community-based testing, HIV self-testing, and modifications to the traditional clinic-based model can effectively reach certain subsets of MSM [16].
Peer-led HIV testing has proven to be an effective strategy for increasing HIV testing rates. To effectively generate demand for HIV testing services, the World Health Organization (WHO) recommends evidence-based approaches such as peer-led interventions and digital platforms, video-based information, and online outreach [17]. Peer-led interventions significantly boosted HIV testing among MSM [18]. These interventions can build trust, effectively engage “hard-to-reach” individuals, offer MSM-friendly services, reduce discrimination and anxiety, and provide support for those who test positive [18, 19]. Research has shown that HIV rapid antibody testing conducted by trained peer volunteers in MSM-frequented venues results in higher rates of subsequent confirmatory tests and linkage to care than testing in China Center for Disease Control and Prevention (CDC) surveillance surveys [19]. While community-based HIV testing has been shown to increase testing rates by training community volunteers to provide interventions [20, 21], few studies have explored the significant potential of peer volunteers in scaling up HIV testing among MSM.
This study presents our experience with an innovative HIV testing uptake strategy called the “standardly trained peer volunteer–led, social app recruitment–based HIV testing strategy using rapid testing kits” (SPARK). This model involves CBOs recruiting and training MSM peer volunteers to mobilize HIV testing among MSM via social apps, particularly GSN. This article outlines the strategy's implementation, detailing its components and procedure. It also explores how the strategy promotes HIV testing among MSM and those MSM in different levels of urbanization.
METHODS
Study Setting and Participants
This study was conducted in Tianjin, a mega-city in north China with about 14 million people. All 16 administrative districts in Tianjin were classified into urban, periurban, and rural (Supplementary Table 1). Traditional HIV testing strategies for MSM in Tianjin include venue-based HIV testing [22] and HIVST [23].
The study employed a population-based survey aimed at evaluating the effectiveness of a novel approach for promoting HIV testing among MSM. The project was planned to be executed between March 2020 and December 2030, but this study is based on data collected from March 2020 to December 2021.
The inclusion criteria are MSM who were male assigned at birth, aged ≥16 years, self-identified as homosexual or bisexual, confirmed as HIV negative, resided in Tianjin, and demonstrated a willingness to engage in HIV testing. A total of 7340 participants underwent 10 547 HIV tests. Among those participants, 84 participants were excluded and 7256 eligible participants were included in the final analysis (Supplementary Figure 1).
This study protocol was approved by the institutional review board of the Tianjin Center for Disease Control and Prevention (TJCDC). Each participant signed an informed consent form (Supplementary Methods).
The SPARK Strategy
The TJCDC and Tianjin Shenlan Public Health Counseling Service Center (hereafter, Shenlan), an MSM CBO, jointly designed and implemented the project. SPARK is an HIV testing strategy that features MSM peer volunteers and GSN apps. More details about the procedure of the project are shown in the Supplementary Methods.
Organizational Structure and Responsibilities
The project involves the below-mentioned entities including TJCDC, district-level CDCs, CBOs, and MSM volunteers (Figure 1). More details about the organizational structure and responsibilities in SPARK are described in the Supplementary Methods.
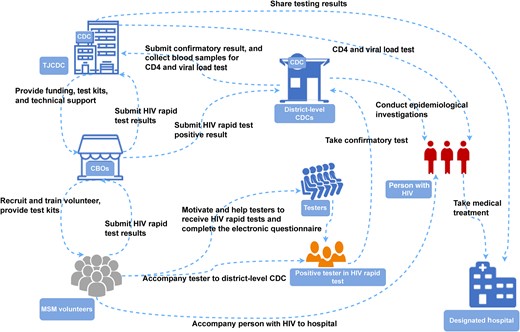
Organizational structure and responsibilities in standardly trained peer volunteer–led, social app recruiting–based HIV testing strategy using rapid testing kits (SPARK). Abbreviations: CBOs, community-based organizations; CDC, Center for Disease Control and Prevention; HIV, human immunodeficiency virus; MSM, men who have sex with men; TJCDC, Tianjin Center for Disease Control and Prevention.
Volunteer Recruitment, Screening, Training, and Management
The CBO, Shenlan, utilizes social networking platforms (official account, Blued, WeChat, QQ, etc) to advertise SPARK and recruit volunteers. Interested individuals can contact the CBO to apply for volunteer positions. Then, CBO screens potential MSM volunteers based on specific criteria, including male sex, sexual orientation (homosexual or bisexual), enthusiasm for HIV testing services, age ≥18 years, HIV negative, and long-term residency in Tianjin. Applicants are interviewed through both video and onsite interviews. In this context, they are assessed in terms of their HIV status, age, and physical and mental health. Applicants with a history of selling or using recreational drugs or those who have made offensive comments about society and the community are excluded to avoid potential punishments from regulatory authority and potential damage to the quality of testing results and questionnaire results. Applicants should also have a positive mental outlook and wear clean clothes. Strong communication skills and a keen willingness to participate in the program are preferred.
After recruitment, volunteers undergo comprehensive training covering various topics, including basic knowledge about HIV, the importance of participating in HIV prevention programs, operation of HIV rapid test kits, biosafety, peer education for persons with HIV, privacy protection, volunteer policies and regulations, mobilization strategies, counseling and survey interviewing skills, procedures, and management rules. Volunteers are issued a uniform volunteer work card after completing the interview, training, and assessment process, which includes a written HIV prevention knowledge test and practical tests (rapid HIV antibody testing, counseling skills, and survey interviewing techniques in simulated HIV testing and counseling [HTC] scenarios) conducted by at least 3 examiners. Newly certified volunteers must then recruit and test a minimum of 10 MSM participants in their probationary period, subject to ongoing quality control (Supplementary Methods). They are assigned to different groups based on their areas of residence.
Based on their areas of residence, volunteers were assigned to designated groups, with a minimum of 3 volunteers assigned to each district. The CBO assigned a full-time staff member with extensive testing and counseling experience as a supervisor responsible for contacting and managing volunteers daily. The group leader's role is to guide, coach, and support volunteers concerning identifying persons with HIV and assisting with follow-up referrals. Volunteers can arrange their schedules flexibly to recruit potential testers and conduct HIV testing services without disrupting their regular work and personal lives. Volunteers are required to undergo HIV testing every 3 months, and they can continue their work as long as their results remain negative. If a volunteer tests positive for HIV, they will withdraw from direct testing activities in SPARK but may participate in other HIV prevention activities based on their circumstances and preferences. Such activities may include community mobilization and health promotion, counseling, peer education, and care and support for people with HIV. Interested volunteers may also apply for full-time staff positions at CBO.
Implementation Process
Volunteers utilize their personal social networks and social software platforms to mobilize MSM to receive face-to-face counseling and testing. Volunteers would also create officially certified accounts in GSN apps and other social apps. Then, they would send private messages to nearby users (Supplementary Figure 2), publish HTC information in their profiles (Supplementary Figure 3) and timeline posts (Supplementary Figure 4), or promote HTC through live streaming. Once a person expressed willingness to participate in HTC (Supplementary Figures 2 and 5), the volunteer would determine a location for testing with the person. The testing times and locations are mutually agreed upon by both parties, and it can be anywhere convenient for the person being tested, such as cars and parks.
Standardized SPARK kits were provided by the TJCDC. The kits were printed with a quick response code to access the electronic questionnaire and included an HIV rapid antibody test kit and other items (Supplementary Methods). The primary test conducted is the Diagnostic Kit for Antibody to HIV (1 + 2) (Colloidal Gold). All used supplies are collected in a medical waste disposal box and submitted to the CDC for centralized disposal. Participants could choose between 2 testing options: (1) having a volunteer collect a fingertip blood sample for a rapid antibody test (lay provider HIV testing); or (2) having a volunteer supervise and guide them while they conducted an onsite HIVST and interpreted the results (directly assisted HIVST).
Testers who test HIV positive are referred to the district-level CDC, where a 5-mL ethylenediaminetetraacetic acid anticoagulant blood sample is taken for further testing. The confirmatory test (Western blot test or nucleic acid test) is conducted at the district-level CDC, while CD4 T-cell counts and viral load tests are performed at the TJCDC. Volunteers accompany the rapid test–reactive participants throughout this process to ensure they receive the necessary tests. Full-time staff members from CBOs are responsible for accompanying confirmed cases to medical institutions to initiate antiviral treatment.
While participants wait to receive their test results, volunteers offer counseling services and assist them in completing the electronic questionnaire (Supplementary Text 1). An electronic questionnaire on Wenjuanxing is accessed by scanning the quick response code printed on the standard kit with a smartphone. Subsequently, the tester completes the electronic questionnaire.
Details about quality control and data management are described in the Supplementary Methods.
Data Collection and Statistical Analysis
Details about data collection and the statistical analysis are described in the Supplementary Methods.
RESULTS
Overview of the Implementation of SPARK
During the first 22 months of SPARK implementation, 177 volunteers recruited 7256 testers, who underwent 10 441 HIV tests. The monthly number of volunteers in SPARK consistently increased. The number of active volunteers grew from 1 initially to 67 in December 2020 and 152 in December 2021. Typically, each volunteer conducted about 6 tests per month. On average, each volunteer successfully recruited 40.99 testers (7256/177) and facilitated 58.99 HIV tests (10 441/177). The monthly tests in SPARK showed a fluctuating upward trend. In 2020, 2314 tests were performed (192 from March to June and 2122 from July to December). In 2021, 8127 tests (3971 from January to June and 4156 from July to December) were conducted, nearly 4 times the number in the first year, averaging approximately 677 tests per month (Figure 2).
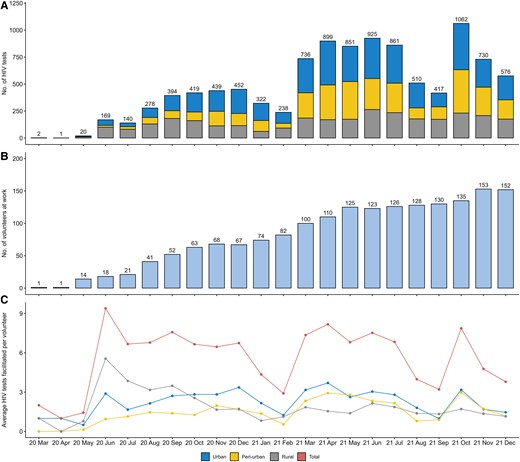
Monthly counts of human immunodeficiency virus (HIV) tests (A), volunteers at work (B), and average HIV tests facilitated per volunteer (C) from March 2020 to December 2021.
By the end of 2021, 268 of 304 HIV-reactive testers (88.16%) had undergone further confirmatory testing, and all tested positive for HIV; of the 268 testers with HIV, 242 (90.30%) received antiretroviral therapy (ART) (Figure 3).
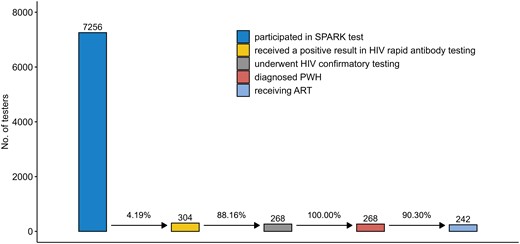
Human immunodeficiency virus (HIV) testing and treatment cascade. Of 7256 testers, 268 were finally confirmed as persons with HIV. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PWH, people with human immunodeficiency virus; SPARK, standardly trained peer volunteer–led, social app recruiting–based HIV testing strategy using rapid testing kits.
Characteristics of Participants in SPARK
The median age of the 7256 participants was 30 years (interquartile range, 24–37 years). Approximately 84% of participants were recruited through GSN apps. Approximately 36% had never undergone HIV testing, and 41.19%, 32.26%, and 26.54% resided in urban areas, periurban areas, and rural areas, respectively.
Participants in rural areas had the lowest prior HIV testing rate (54.57%) compared to those in periurban (65.23%) and urban (68.45%) areas (P < .001). The HIV prevalence rate among rural participants was 4.57% (88/1926), while it was 3.25% (76/2341) among periurban participants and 3.48% (104/2989) among urban participants (P = .054). Rural participants were more likely to not use condoms during their last intercourse or to use condoms less frequently (P < .001). More details are shown in Table 1.
| Characteristics . | Overall (n = 7256) . | Urban (n = 2989) . | Periurban (n = 2341) . | Rural (n = 1926) . | P Value . |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 30.0 (24.00–37.00) | 30.00 (25.00–36.00) | 27.00 (22.00–34.00) | 33.00 (26.00–40.00) | <.001 |
| College education | 4327 (59.63) | 1909 (63.87) | 1570 (67.07) | 848 (44.03) | <.001 |
| Marital status | <.001 | ||||
| Unmarried | 4954 (68.27) | 2239 (74.91) | 1789 (76.42) | 926 (48.08) | |
| Married | 2090 (28.80) | 683 (22.85) | 500 (21.36) | 907 (47.09) | |
| Other | 212 (2.92) | 67 (2.24) | 52 (2.22) | 93 (4.83) | |
| Employed | 5479 (75.51) | 2369 (79.26) | 1470 (62.79) | 1640 (85.15) | <.001 |
| Self-identified as bisexual | 1172 (16.15) | 299 (10.00) | 369 (15.76) | 504 (26.17) | <.001 |
| Recruitment channela | <.001 | ||||
| GSN apps | 5226 (83.91) | 2179 (82.91) | 1814 (84.02) | 1233 (85.57) | |
| Other social apps | 549 (8.82) | 204 (7.76) | 170 (7.87) | 175 (12.14) | |
| Friends | 446 (7.16) | 240 (9.13) | 175 (8.11) | 31 (2.15) | |
| Physical places | 7 (0.11) | 5 (0.19) | 0 (0.00) | 2 (0.14) | |
| Used condom during the last intercourse | 5381 (74.16) | 2294 (76.75) | 1758 (75.10) | 1329 (69.00) | <.001 |
| Condom use frequencyb | <.001 | ||||
| Never | 379 (6.09) | 94 (3.58) | 82 (3.80) | 203 (14.09) | |
| Sometimes | 2567 (41.24) | 1146 (43.66) | 897 (41.55) | 524 (36.36) | |
| Always | 3279 (52.67) | 1385 (52.76) | 1180 (54.65) | 714 (49.55) | |
| No. of sexual partnersc | <.001 | ||||
| 0 | 942 (15.13) | 437 (16.65) | 326 (15.10) | 179 (12.42) | |
| 1–5 | 5050 (81.12) | 2037 (77.60) | 1787 (82.77) | 1226 (85.08) | |
| 6–10 | 164 (2.63) | 107 (4.08) | 38 (1.76) | 19 (1.32) | |
| >10 | 69 (1.11) | 44 (1.68) | 8 (0.37) | 17 (1.18) | |
| Used other HIV risk reduction strategiesd | 1258 (20.21) | 432 (16.46) | 557 (25.80) | 269 (18.67) | <.001 |
| Knowledge of PEPe | 4539 (72.92) | 2038 (77.64) | 1719 (79.62) | 782 (54.27) | <.001 |
| Ever had an HIV test | 4624 (63.73) | 2046 (68.45) | 1527 (65.23) | 1051 (54.57) | <.001 |
| Referral services | 3020 (41.63) | 1341 (44.86) | 1187 (50.70) | 495 (25.70) | <.001 |
| Test times in SPARK ≥2 | 2034 (28.03) | 804 (26.90) | 579 (24.73) | 651 (33.80) | <.001 |
| Retested by the initial volunteerf | 1403 (68.98) | 549 (68.28) | 339 (58.55) | 515 (79.11) | <.001 |
| Positive by HIV rapid antibody test | 304 (4.19) | 116 (3.88) | 89 (3.80) | 99 (5.14) | .052 |
| Underwent HIV confirmatory testingg | 268 (88.16) | 104 (89.66) | 76 (85.39) | 88 (88.89) | .620 |
| Person with HIV | 268 (3.69) | 104 (3.48) | 76 (3.25) | 88 (4.57) | .054 |
| ARTh | 242 (90.30) | 93 (89.42) | 70 (92.11) | 79 (89.77) | .820 |
| CD4 count, cells/μLh, median (IQR) | 291.00 (189.00–470.53) | 291.34 (198.00–510.78) | 285.00 (184.50–487.03) | 293.56 (180.00–393.00) | .630 |
| HIV RNA, copies/mLh, median (IQR) | 18 304.00 (5712.00–50170.00) | 15 608.00 (6176.00–39756.00) | 20 336.00 (4952.00–57544.00) | 25 961.00 (7324.00–53048.00) | .460 |
| Late HIV diagnosish | 151 (56.34) | 54 (51.92) | 48 (63.16) | 49 (55.68) | .320 |
| Presentation with advanced HIV diseaseh | 71 (26.49) | 27 (25.96) | 21 (27.63) | 23 (26.14) | .970 |
| Characteristics . | Overall (n = 7256) . | Urban (n = 2989) . | Periurban (n = 2341) . | Rural (n = 1926) . | P Value . |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 30.0 (24.00–37.00) | 30.00 (25.00–36.00) | 27.00 (22.00–34.00) | 33.00 (26.00–40.00) | <.001 |
| College education | 4327 (59.63) | 1909 (63.87) | 1570 (67.07) | 848 (44.03) | <.001 |
| Marital status | <.001 | ||||
| Unmarried | 4954 (68.27) | 2239 (74.91) | 1789 (76.42) | 926 (48.08) | |
| Married | 2090 (28.80) | 683 (22.85) | 500 (21.36) | 907 (47.09) | |
| Other | 212 (2.92) | 67 (2.24) | 52 (2.22) | 93 (4.83) | |
| Employed | 5479 (75.51) | 2369 (79.26) | 1470 (62.79) | 1640 (85.15) | <.001 |
| Self-identified as bisexual | 1172 (16.15) | 299 (10.00) | 369 (15.76) | 504 (26.17) | <.001 |
| Recruitment channela | <.001 | ||||
| GSN apps | 5226 (83.91) | 2179 (82.91) | 1814 (84.02) | 1233 (85.57) | |
| Other social apps | 549 (8.82) | 204 (7.76) | 170 (7.87) | 175 (12.14) | |
| Friends | 446 (7.16) | 240 (9.13) | 175 (8.11) | 31 (2.15) | |
| Physical places | 7 (0.11) | 5 (0.19) | 0 (0.00) | 2 (0.14) | |
| Used condom during the last intercourse | 5381 (74.16) | 2294 (76.75) | 1758 (75.10) | 1329 (69.00) | <.001 |
| Condom use frequencyb | <.001 | ||||
| Never | 379 (6.09) | 94 (3.58) | 82 (3.80) | 203 (14.09) | |
| Sometimes | 2567 (41.24) | 1146 (43.66) | 897 (41.55) | 524 (36.36) | |
| Always | 3279 (52.67) | 1385 (52.76) | 1180 (54.65) | 714 (49.55) | |
| No. of sexual partnersc | <.001 | ||||
| 0 | 942 (15.13) | 437 (16.65) | 326 (15.10) | 179 (12.42) | |
| 1–5 | 5050 (81.12) | 2037 (77.60) | 1787 (82.77) | 1226 (85.08) | |
| 6–10 | 164 (2.63) | 107 (4.08) | 38 (1.76) | 19 (1.32) | |
| >10 | 69 (1.11) | 44 (1.68) | 8 (0.37) | 17 (1.18) | |
| Used other HIV risk reduction strategiesd | 1258 (20.21) | 432 (16.46) | 557 (25.80) | 269 (18.67) | <.001 |
| Knowledge of PEPe | 4539 (72.92) | 2038 (77.64) | 1719 (79.62) | 782 (54.27) | <.001 |
| Ever had an HIV test | 4624 (63.73) | 2046 (68.45) | 1527 (65.23) | 1051 (54.57) | <.001 |
| Referral services | 3020 (41.63) | 1341 (44.86) | 1187 (50.70) | 495 (25.70) | <.001 |
| Test times in SPARK ≥2 | 2034 (28.03) | 804 (26.90) | 579 (24.73) | 651 (33.80) | <.001 |
| Retested by the initial volunteerf | 1403 (68.98) | 549 (68.28) | 339 (58.55) | 515 (79.11) | <.001 |
| Positive by HIV rapid antibody test | 304 (4.19) | 116 (3.88) | 89 (3.80) | 99 (5.14) | .052 |
| Underwent HIV confirmatory testingg | 268 (88.16) | 104 (89.66) | 76 (85.39) | 88 (88.89) | .620 |
| Person with HIV | 268 (3.69) | 104 (3.48) | 76 (3.25) | 88 (4.57) | .054 |
| ARTh | 242 (90.30) | 93 (89.42) | 70 (92.11) | 79 (89.77) | .820 |
| CD4 count, cells/μLh, median (IQR) | 291.00 (189.00–470.53) | 291.34 (198.00–510.78) | 285.00 (184.50–487.03) | 293.56 (180.00–393.00) | .630 |
| HIV RNA, copies/mLh, median (IQR) | 18 304.00 (5712.00–50170.00) | 15 608.00 (6176.00–39756.00) | 20 336.00 (4952.00–57544.00) | 25 961.00 (7324.00–53048.00) | .460 |
| Late HIV diagnosish | 151 (56.34) | 54 (51.92) | 48 (63.16) | 49 (55.68) | .320 |
| Presentation with advanced HIV diseaseh | 71 (26.49) | 27 (25.96) | 21 (27.63) | 23 (26.14) | .970 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; GSN, geosocial networking; HIV, human immunodeficiency virus; IQR, interquartile range; PEP, postexposure prophylaxis; SPARK, standardly trained peer volunteer–led, social app recruiting–based HIV testing strategy using rapid testing kits.
aMissing = 1028.
bMissing = 1031.
cMissing = 1031.
dMissing = 1031.
eMissing = 1031.
fOnly those who tested in SPARK more than once, n = 2034.
gOnly those who tested positive in HIV rapid antibody test, n = 304.
hOnly those who were identified as person with HIV, n = 268.
| Characteristics . | Overall (n = 7256) . | Urban (n = 2989) . | Periurban (n = 2341) . | Rural (n = 1926) . | P Value . |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 30.0 (24.00–37.00) | 30.00 (25.00–36.00) | 27.00 (22.00–34.00) | 33.00 (26.00–40.00) | <.001 |
| College education | 4327 (59.63) | 1909 (63.87) | 1570 (67.07) | 848 (44.03) | <.001 |
| Marital status | <.001 | ||||
| Unmarried | 4954 (68.27) | 2239 (74.91) | 1789 (76.42) | 926 (48.08) | |
| Married | 2090 (28.80) | 683 (22.85) | 500 (21.36) | 907 (47.09) | |
| Other | 212 (2.92) | 67 (2.24) | 52 (2.22) | 93 (4.83) | |
| Employed | 5479 (75.51) | 2369 (79.26) | 1470 (62.79) | 1640 (85.15) | <.001 |
| Self-identified as bisexual | 1172 (16.15) | 299 (10.00) | 369 (15.76) | 504 (26.17) | <.001 |
| Recruitment channela | <.001 | ||||
| GSN apps | 5226 (83.91) | 2179 (82.91) | 1814 (84.02) | 1233 (85.57) | |
| Other social apps | 549 (8.82) | 204 (7.76) | 170 (7.87) | 175 (12.14) | |
| Friends | 446 (7.16) | 240 (9.13) | 175 (8.11) | 31 (2.15) | |
| Physical places | 7 (0.11) | 5 (0.19) | 0 (0.00) | 2 (0.14) | |
| Used condom during the last intercourse | 5381 (74.16) | 2294 (76.75) | 1758 (75.10) | 1329 (69.00) | <.001 |
| Condom use frequencyb | <.001 | ||||
| Never | 379 (6.09) | 94 (3.58) | 82 (3.80) | 203 (14.09) | |
| Sometimes | 2567 (41.24) | 1146 (43.66) | 897 (41.55) | 524 (36.36) | |
| Always | 3279 (52.67) | 1385 (52.76) | 1180 (54.65) | 714 (49.55) | |
| No. of sexual partnersc | <.001 | ||||
| 0 | 942 (15.13) | 437 (16.65) | 326 (15.10) | 179 (12.42) | |
| 1–5 | 5050 (81.12) | 2037 (77.60) | 1787 (82.77) | 1226 (85.08) | |
| 6–10 | 164 (2.63) | 107 (4.08) | 38 (1.76) | 19 (1.32) | |
| >10 | 69 (1.11) | 44 (1.68) | 8 (0.37) | 17 (1.18) | |
| Used other HIV risk reduction strategiesd | 1258 (20.21) | 432 (16.46) | 557 (25.80) | 269 (18.67) | <.001 |
| Knowledge of PEPe | 4539 (72.92) | 2038 (77.64) | 1719 (79.62) | 782 (54.27) | <.001 |
| Ever had an HIV test | 4624 (63.73) | 2046 (68.45) | 1527 (65.23) | 1051 (54.57) | <.001 |
| Referral services | 3020 (41.63) | 1341 (44.86) | 1187 (50.70) | 495 (25.70) | <.001 |
| Test times in SPARK ≥2 | 2034 (28.03) | 804 (26.90) | 579 (24.73) | 651 (33.80) | <.001 |
| Retested by the initial volunteerf | 1403 (68.98) | 549 (68.28) | 339 (58.55) | 515 (79.11) | <.001 |
| Positive by HIV rapid antibody test | 304 (4.19) | 116 (3.88) | 89 (3.80) | 99 (5.14) | .052 |
| Underwent HIV confirmatory testingg | 268 (88.16) | 104 (89.66) | 76 (85.39) | 88 (88.89) | .620 |
| Person with HIV | 268 (3.69) | 104 (3.48) | 76 (3.25) | 88 (4.57) | .054 |
| ARTh | 242 (90.30) | 93 (89.42) | 70 (92.11) | 79 (89.77) | .820 |
| CD4 count, cells/μLh, median (IQR) | 291.00 (189.00–470.53) | 291.34 (198.00–510.78) | 285.00 (184.50–487.03) | 293.56 (180.00–393.00) | .630 |
| HIV RNA, copies/mLh, median (IQR) | 18 304.00 (5712.00–50170.00) | 15 608.00 (6176.00–39756.00) | 20 336.00 (4952.00–57544.00) | 25 961.00 (7324.00–53048.00) | .460 |
| Late HIV diagnosish | 151 (56.34) | 54 (51.92) | 48 (63.16) | 49 (55.68) | .320 |
| Presentation with advanced HIV diseaseh | 71 (26.49) | 27 (25.96) | 21 (27.63) | 23 (26.14) | .970 |
| Characteristics . | Overall (n = 7256) . | Urban (n = 2989) . | Periurban (n = 2341) . | Rural (n = 1926) . | P Value . |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 30.0 (24.00–37.00) | 30.00 (25.00–36.00) | 27.00 (22.00–34.00) | 33.00 (26.00–40.00) | <.001 |
| College education | 4327 (59.63) | 1909 (63.87) | 1570 (67.07) | 848 (44.03) | <.001 |
| Marital status | <.001 | ||||
| Unmarried | 4954 (68.27) | 2239 (74.91) | 1789 (76.42) | 926 (48.08) | |
| Married | 2090 (28.80) | 683 (22.85) | 500 (21.36) | 907 (47.09) | |
| Other | 212 (2.92) | 67 (2.24) | 52 (2.22) | 93 (4.83) | |
| Employed | 5479 (75.51) | 2369 (79.26) | 1470 (62.79) | 1640 (85.15) | <.001 |
| Self-identified as bisexual | 1172 (16.15) | 299 (10.00) | 369 (15.76) | 504 (26.17) | <.001 |
| Recruitment channela | <.001 | ||||
| GSN apps | 5226 (83.91) | 2179 (82.91) | 1814 (84.02) | 1233 (85.57) | |
| Other social apps | 549 (8.82) | 204 (7.76) | 170 (7.87) | 175 (12.14) | |
| Friends | 446 (7.16) | 240 (9.13) | 175 (8.11) | 31 (2.15) | |
| Physical places | 7 (0.11) | 5 (0.19) | 0 (0.00) | 2 (0.14) | |
| Used condom during the last intercourse | 5381 (74.16) | 2294 (76.75) | 1758 (75.10) | 1329 (69.00) | <.001 |
| Condom use frequencyb | <.001 | ||||
| Never | 379 (6.09) | 94 (3.58) | 82 (3.80) | 203 (14.09) | |
| Sometimes | 2567 (41.24) | 1146 (43.66) | 897 (41.55) | 524 (36.36) | |
| Always | 3279 (52.67) | 1385 (52.76) | 1180 (54.65) | 714 (49.55) | |
| No. of sexual partnersc | <.001 | ||||
| 0 | 942 (15.13) | 437 (16.65) | 326 (15.10) | 179 (12.42) | |
| 1–5 | 5050 (81.12) | 2037 (77.60) | 1787 (82.77) | 1226 (85.08) | |
| 6–10 | 164 (2.63) | 107 (4.08) | 38 (1.76) | 19 (1.32) | |
| >10 | 69 (1.11) | 44 (1.68) | 8 (0.37) | 17 (1.18) | |
| Used other HIV risk reduction strategiesd | 1258 (20.21) | 432 (16.46) | 557 (25.80) | 269 (18.67) | <.001 |
| Knowledge of PEPe | 4539 (72.92) | 2038 (77.64) | 1719 (79.62) | 782 (54.27) | <.001 |
| Ever had an HIV test | 4624 (63.73) | 2046 (68.45) | 1527 (65.23) | 1051 (54.57) | <.001 |
| Referral services | 3020 (41.63) | 1341 (44.86) | 1187 (50.70) | 495 (25.70) | <.001 |
| Test times in SPARK ≥2 | 2034 (28.03) | 804 (26.90) | 579 (24.73) | 651 (33.80) | <.001 |
| Retested by the initial volunteerf | 1403 (68.98) | 549 (68.28) | 339 (58.55) | 515 (79.11) | <.001 |
| Positive by HIV rapid antibody test | 304 (4.19) | 116 (3.88) | 89 (3.80) | 99 (5.14) | .052 |
| Underwent HIV confirmatory testingg | 268 (88.16) | 104 (89.66) | 76 (85.39) | 88 (88.89) | .620 |
| Person with HIV | 268 (3.69) | 104 (3.48) | 76 (3.25) | 88 (4.57) | .054 |
| ARTh | 242 (90.30) | 93 (89.42) | 70 (92.11) | 79 (89.77) | .820 |
| CD4 count, cells/μLh, median (IQR) | 291.00 (189.00–470.53) | 291.34 (198.00–510.78) | 285.00 (184.50–487.03) | 293.56 (180.00–393.00) | .630 |
| HIV RNA, copies/mLh, median (IQR) | 18 304.00 (5712.00–50170.00) | 15 608.00 (6176.00–39756.00) | 20 336.00 (4952.00–57544.00) | 25 961.00 (7324.00–53048.00) | .460 |
| Late HIV diagnosish | 151 (56.34) | 54 (51.92) | 48 (63.16) | 49 (55.68) | .320 |
| Presentation with advanced HIV diseaseh | 71 (26.49) | 27 (25.96) | 21 (27.63) | 23 (26.14) | .970 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; GSN, geosocial networking; HIV, human immunodeficiency virus; IQR, interquartile range; PEP, postexposure prophylaxis; SPARK, standardly trained peer volunteer–led, social app recruiting–based HIV testing strategy using rapid testing kits.
aMissing = 1028.
bMissing = 1031.
cMissing = 1031.
dMissing = 1031.
eMissing = 1031.
fOnly those who tested in SPARK more than once, n = 2034.
gOnly those who tested positive in HIV rapid antibody test, n = 304.
hOnly those who were identified as person with HIV, n = 268.
Compared to testers from other testing models, participants in this study were more likely to be younger and living in rural areas and less likely to have had prior HIV testing. More details are shown in Supplementary Table 2.
Pre- and Postimplementation of SPARK in Tianjin
Postimplementation of SPARK, HIV tests increased notably. Monthly HIV tests from January 2020 to July 2020 displayed a downward trend compared to the previous year. However, after 6 months of SPARK implementation, there was a significant upturn in monthly HIV tests compared to the previous year. HIV tests in 2021 (second year of SPARK implementation) doubled compared to 2019. Starting from February 2021, SPARK contributed to approximately half of the monthly HIV tests conducted in Tianjin. Moreover, HIV testing in rural areas in 2020 and 2021 was 2.86 (1228/429) and 5.85 (2516/429) times higher, respectively, compared to 2019. Following its implementation, SPARK accounted for approximately 80% of HIV tests conducted in rural areas for most months. Similar trends were observed in periurban and urban areas (Figure 4).
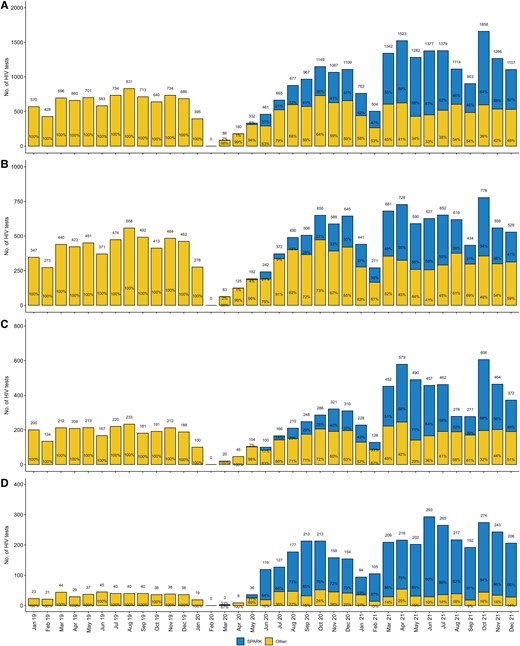
Monthly counts of human immunodeficiency virus tests among men who have sex with men (MSM) (A) and urban MSM (B), periurban MSM (C), and rural MSM (D) from January 2019 to December 2021 in Tianjin. Abbreviations: HIV, human immunodeficiency virus; SPARK, standardly trained peer volunteer–led, social app–based HIV testing strategy using rapid testing kits
Post the introduction of SPARK, there was a widespread increase in the number of HIV tests conducted across all 16 districts of Tianjin, particularly in rural districts (Supplementary Figures 6–21).
Spatial Analysis
Regarding the distribution of volunteers, compared to urban/periurban areas, there were fewer volunteers in rural areas (Supplementary Figure 22). As for the distribution of testers, the number of testers in most rural districts (four-fifths) was less than 300 (Supplementary Figure 23).
Despite having fewer volunteers and testers in rural districts, three-fifths of rural districts had per-volunteer recruitment numbers for testers that exceeded the overall average observed in SPARK (40.99), while only three-sevenths of urban districts and 1 of 4 periurban districts had per-volunteer recruitment numbers for testers higher than the overall average observed in SPARK (Supplementary Figure 24).
For testers from each district, most of them sought volunteers from their/nearby districts (Supplementary Figures 25–40) for testing; in particular, testers in rural districts primarily remained within their located districts for testing (Supplementary Figures 36–40).
For volunteers from each district, most testers they recruited were from their district or nearby districts (Supplementary Figures 41–56); for volunteers from rural districts, >79% of testers they recruited were from their local districts (Supplementary Figures 52–56).
Testing Time of HIV Tests
Regarding the timing of HIV tests, 36.60%, 32.58%, and 30.82% occurred on weekends or holidays, nonworking hours on workdays, and working hours on workdays, respectively; whether in urban, periurban, or rural MSM testers, >60% of HIV tests occurred on weekends or holidays, or nonworking hours on workdays (Supplementary Figure 57).
DISCUSSION
The WHO guidelines emphasize evidence-based demand-creation strategies, updated counseling messages, the promotion of HIV self-testing, and social network–based testing approaches for key populations [24]. Furthermore, a review highlights the benefits of peer- and community-led HIV responses, suggesting that prevention programs aiming for people at higher risk of HIV acquisition to follow such approaches whenever possible [25].
Recently, the WHO recommended social network–based approaches to HIV testing for key populations, including peer recruitment, distribution of HIV self-testing kits, use of digital and social media tools, and anonymous methods to protect confidentiality [26]. One study also strongly advises the use of CBOs and peer-to-peer communication approaches to enhance health outcomes by promoting trust, security, and privacy among MSM [27].
This study aimed to evaluate the effect of a new innovative approach to voluntary HIV rapid test promoting strategy meant to create demand and mend the drawbacks of conventional institutional and self-testing approaches. By eliminating concerns related to physical access, privacy, lack of skill, and lack of immediate support, the project improved voluntary HIV test uptake among MSM in Tianjin. The key future lies in the involvement of peer volunteers in addressing all of the above-mentioned recommendations. The study showed that SPARK significantly increased the number of HIV tests and maintained a high monthly testing volume as the number of active volunteers continued to grow each month. In its second year, SPARK's testing numbers surpassed those achieved in the third year by a GSN-based voluntary HIV counseling and testing strategy conducted in Beijing [9]. This indicates that there is room for improvement in the existing social app- or social network–based testing strategies to enhance demand for testing and actual testing volumes.
Peer volunteers in SPARK demonstrated, through social apps and livestream programs, a higher capacity for creating demand and mobilizing HIV testing among the MSM population compared to the indexes in a secondary distribution strategy study (average recruited HIV testers: 40.99 per volunteer vs 0.85 per index; average performed HIV tests: 58.99 per volunteer vs 0.90 per index) [4]. Peer volunteers in SPARK could reach more potential testers from the social app users, whereas indexes in the secondary distribution strategy could only recruit potential testers from their immediate social networks. Additionally, trained volunteers may have a stronger confidence and motivation to recruit testers than indexes, as volunteerism increases the sense of ownership that translates into positive behaviors [28]. Moreover, HIV tests among MSM in Tianjin increased, while tests using traditional methods declined (likely due to coronavirus disease 2019) [29]. The increased could be attributed to SPARK.
Barriers such as geographical distance, time constraints, and societal stigmatization impede MSM access to HIV prevention and testing [30–32]. A study among sexual minorities found that distance to travel to receive a HIV test is associated with reduced HIV testing in the past year, suggesting that greater travel distances and living in rural areas limit access to HIV testing [30]. SPARK is found to be particularly effective at overcoming these barriers. Consistent with other studies [33, 34], the study observed a lower rate of prior testing among MSM in rural/periurban areas compared to urban areas. As SPARK was meant to provide MSM with the opportunity to conveniently access HTC services near their residences, a substantial postimplementation increase in the number of HIV tests was observed, particularly in rural areas.
MSM frequently encounter difficulties accessing venue-based HIV testing in a timely manner as they might have time constraints to get tested [35]. SPARK addresses this by offering a time-flexible testing strategy, enabling testers and volunteers to arrange appointments collaboratively. This study shows that most tests were performed during nonworking hours and holidays, accommodating individuals with jobs or commitments during regular working hours. Furthermore, by offering evening and weekend testing opportunities, the strategy caters to various schedules, including students, shift workers, and those with irregular hours, and effectively reaches high-risk groups like MSM in nightlife settings.
HIVST is recommended since many people can perform it accurately with minimal support. However, some of the concerns raised during its earlier time, like the issue of the window period, missed opportunities for counseling, linkage to care, and diagnosis of other sexually transmitted infections [36], might still persist and affect its effectiveness in fighting HIV among MSM. For those requiring assistance to do self-tests, the WHO recommends that support options tailored to local contexts and community preferences, including in-person demonstrations, should be available [37]. SPARK uses trained peer volunteers to provide testing services or directly assist MSM in completing rapid HIV self-tests, ensuring a proper testing process and accurate interpretation of the results, including the negative ones. Comprehensive counseling services, which are missing in traditional HIVST, were also given to all participants while waiting for the test results, which is crucial in improving their HIV risk perceptions. This proactive counseling will also have a constructive impact on the willingness of those with positive test results to accept linkage to care [38].
As part of the SPARK protocol, free-of-cost HIV prevention materials like condoms and lubricants were distributed to participants. This will have a significant impact on reducing some of the barriers MSM face in accessing HIV prevention services in China [39]. Ultimately, the engagement of MSM peer volunteers in SPARK eliminates the effects of stigma and discrimination, creating MSM-friendly testing environments.
For traditional HIVST, MSM may refuse testing due to a lack of immediate support or fear of a positive result [40]. In SPARK, peer volunteers promptly supported and accompanied the testers who received positive results from rapid tests through subsequent testing and treatment processes. Accordingly, 88.16% of those who tested positive were successfully linked to further confirmatory test services, of whom 90.30% started ART. This finding was significantly higher than in a Thai study, where 75.0% of online supervised HIV-reactive self-testers were linked to referred facilities and only 70.4% of them successfully initiated ART [41]. The physical availability of the volunteers to reassure and accompany HIV-reactive participants can be the reason for the higher uptake of these services in the current study.
Finally, the results of this study demonstrate that the SPARK strategy fulfills the WHO's “5 C” principles of comprehensive HIV testing service [42], indicating its potential to effectively complement existing HIV counseling and testing approaches. Moreover, the strategy can obtain test results from all testers, preventing the occasional challenge of missing HIVST results for testers who test negative, which is crucial to a full understanding of the epidemiology of the disease.
Several limitations of this study should be acknowledged. First, some participants who tested positive on the HIV rapid antibody test were either lost to follow-up or declined confirmatory testing. Second, this study was conducted only in Tianjin, and the implementation of SPARK requires the support of a mobile network and MSM CBO. Therefore, the effectiveness of SPARK needs further confirmation in other areas, and its implementation would be challenging in regions with poor mobile network infrastructure or limited MSM CBO support.
CONCLUSIONS
By leveraging peer volunteers and social apps, this new HIV strategy showed a higher capacity for mobilizing testing and provided a time-flexible and MSM-friendly environment, addressing the critical barriers of time constraints and social stigmatization. The approach improved physical access, ensured privacy, provided immediate support, and facilitated referrals, significantly increasing HIV test uptake among MSM, especially in rural areas. Despite some limitations, the study suggests that SPARK can complement traditional HIV testing methods and significantly enhance public health outcomes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. Y., Z. C., and J. Y. contributed to the concept and design of the study. H. G., P. X., X. D., Y. L., Z. W., Y. G., T. N., L. L., M. Z., and J. Y. contributed to data collection. H. H. and T. Y. contributed to the statistical analysis. All authors conducted the analyses and interpretation of the data. Z. L., H. H., and N. K. G. wrote the manuscript. C. L., J. M., M. Y., and Z. C. critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments. All authors express special thanks to the volunteers, the testers, and the staff of Tianjin Shenlan Public Health Counseling Service Center.
Disclaimer. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial support. This work was supported by the National Natural Science Foundation of China (grant number 72374153 to Z. C.); the Humanities and Social Science Fund of Ministry of Education of China (grant number 20YJAZH021 to Z. C.); Tianjin Health Science and Technology Project (grant number TJWJ2023QN092 to M. Y.); National Bureau of Disease Control and Prevention for Public Health Professionals Promotiong Project (no grant number, to M. Y.); and Tianjin Key Medical Discipline (Specialty) Construction Project (grant number TJYXZDXK-050A to M. Y.).
References
Author notes
Z. L. and H. H. contributed equally to this work as joint first authors.
Potential conflicts of interest. All authors: No reported conflicts.





Comments