-
PDF
- Split View
-
Views
-
Cite
Cite
Maddalena Giannella, Stefano Verardi, Andreas Karas, Hasania Abdel Hadi, Hervé Dupont, Alex Soriano, Anne Santerre Henriksen, Andrew Cooper, Marco Falcone, ARES Study Group , Carbapenem-Resistant Acinetobacter spp Infection in Critically Ill Patients With Limited Treatment Options: A Descriptive Study of Cefiderocol Therapy During the COVID-19 Pandemic, Open Forum Infectious Diseases, Volume 10, Issue 7, July 2023, ofad329, https://doi.org/10.1093/ofid/ofad329
Close - Share Icon Share
Abstract
Carbapenem-resistant Acinetobacter baumannii infections are difficult to treat and are a significant public health threat due to intrinsic/acquired resistance and limited treatment options.
A retrospective, observational cohort study in patients receiving cefiderocol via Shionogi's early access program for Acinetobacter spp infections (1 April 2020–30 April 2021; 27 sites; Italy, Spain, Germany, France). Primary outcome was clinical success, defined as clinical resolution of infection at day 14 or day 28 survival.
Overall, 147 patients were included. Primary infection sites were respiratory (65.3%) and bloodstream (unknown source [15.6%]; catheter-related [10.9%]); 24.5% of patients had polymicrobial infection. Of 136 patients in intensive care (92.5%), 85.3% (116/136) received mechanical ventilation. Septic shock (55.6% [70/126]) and coronavirus disease 2019 (COVID-19) (81.6%) were prevalent. Prior to cefiderocol, 85.0% of patients received gram-negative treatment, 61.2% received ≥2 antimicrobials, and most received colistin (58.5%; median duration, 11.5 days). Cefiderocol monotherapy was used in 30.6% of patients. Clinical success rate was 53.1% and was higher in patients without septic shock (62.5%), without COVID-19 (77.8%), and with lower Sequential Organ Failure Assessment (SOFA) scores (quartile 1 [median, 3; range, 0–5]: 82.9%). Day 28 survival was 44.9% and was higher in patients without septic shock (60.7%), without COVID-19 (59.3%), with lower SOFA score (quartile 1: 82.9%), and receiving first-line cefiderocol (68.2% [15/22]). Resolution of infection at day 14 occurred in 39.5% of patients.
Despite use in complex patients with limited treatment options and high septic shock/COVID-19 rates, cefiderocol treatment was associated with an overall clinical success rate of 53%.
Carbapenem-resistant (CR) Acinetobacter baumannii (CRAB) infections are difficult to treat, and treatment options are limited due to intrinsic and acquired resistance to several antimicrobial classes [1, 2].
Infections due to CRAB have been increasingly prevalent in Europe in recent years [3–5] and are an independent risk factor for mortality [6]. In patients with CRAB infection, all-cause mortality rates range between 18% and 57%, which are generally higher than other World Health Organization critical priority gram-negative pathogens—CR Enterobacterales (12%–40%) and Pseudomonas aeruginosa (20%–31%) [7, 8]. The presence of septic shock and/or coronavirus disease 2019 (COVID-19) further increases the risk of mortality [9, 10]. Therefore, the need for new treatment options is becoming more urgent.
Cefiderocol is approved in Europe for the treatment of infections due to aerobic gram-negative organisms in adults with limited treatment options. Per the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidance for treatment of CRAB infection, cefiderocol is conditionally not recommended; however, supporting evidence is recognized to be of low certainty [11, 12]. In contrast, according to Infectious Diseases Society of America (IDSA) guidance, cefiderocol is recommended for patients in whom use of other antimicrobials is precluded [13]. Current treatment guidance for infection due to CRAB from ESCMID and IDSA conditionally recommend treatment regimens with ampicillin/sulbactam when susceptibility is demonstrated, although there is limited supporting evidence [11, 13]. Colistin may be considered for CRAB infection, according to ESCMID, but not without evidence of in vitro activity and the presence of sulbactam resistance [11]. European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidance states that colistin should not be used as monotherapy [14].
In the APEKS-NP and CREDIBLE-CR phase 3 studies, survival rates of 78% (18/23; vs 83% [20/24] with meropenem at day 14) and 51% (20/39; vs 82% [14/17] with best-available therapy at end of study), respectively, were reported for patients with CR Acinetobacter spp infection [15, 16]. Data from real-world experience suggest clinical effectiveness of cefiderocol in patients with CR Acinetobacter spp infection [17–28]. Despite the limitation of an observational design, a recent study demonstrated that cefiderocol-containing regimens are associated with higher survival rates compared with colistin-containing regimens in patients with bloodstream infection (BSI) due to CR Acinetobacter spp [27]. However, these findings were not confirmed in patients with COVID-19 and when cefiderocol was used as monotherapy [28].
The aim of this study was to assess real-world evidence on the clinical characteristics, treatment effectiveness, and safety of cefiderocol (supplied via the Shionogi early access program [EAP]) in patients with CR Acinetobacter spp infection in Europe.
METHODS
Data Collection
The ARES study was a retrospective, descriptive, observational cohort study in patients who received cefiderocol via the Shionogi EAP for treatment of Acinetobacter spp infections between 1 April 2020 and 30 April 2021. The EAP was established by Shionogi B.V. in April 2020 to enable access to cefiderocol in European countries where it was not yet available on the market. A total of 27 sites across 4 countries (Italy, Spain, Germany, and France) were identified from the Shionogi EAP database for inclusion. Selected sites requested cefiderocol via the EAP for treatment of ≥1 patient(s) with a CR Acinetobacter spp infection.
Inpatient hospital medical records and laboratory reports were used for data extraction. Data recorded at the study site between hospital admission date and day 28 following cefiderocol administration, or the date of discharge from the episode of hospitalization, whichever occurred sooner, were collected.
Patients ≥18 years of age hospitalized due to gram-negative infection (excluding central nervous system infection) with suspected (based on investigator opinion) or evident carbapenem resistance were included. Cefiderocol susceptibility testing was not mandatory for EAP enrollment. Of the patients in the EAP database, the inclusion criterion for this study was ≥72 hours of cefiderocol therapy received in hospital for Acinetobacter spp infection, following no prior use of cefiderocol, either as a last-resort antibiotic or when no other alternative antibiotics were available. Patients with a gram-negative coinfection resistant to cefiderocol within 14 days of initial cefiderocol dose were excluded. The study included some patients treated for CR Acinetobacter spp with excess cefiderocol stock from previous EAP requests, which was done at the discretion of the treating clinician (Nota bene, the product was licensed in Europe and was pending national reimbursement).
This study was conducted in compliance with guidance from the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (2015), International Society for Pharmacoepidemiology (2015), Council for International Organizations of Medical Sciences (2009), and European Medicines Agency (2020). Written patient consent was obtained prior to data extraction in accordance with local regulations; assumed consent was utilized in accordance with local regulations. If the patient was unable to provide consent, this was obtained through a family member or relative in accordance with local European Committee approvals and guidance or in line with local regulations.
Outcomes, Variables, and Definitions
The primary outcome was clinical success in patients with CR Acinetobacter spp infection treated with cefiderocol. Clinical success was a composite endpoint defined as clinical resolution of investigator-assessed signs and symptoms of Acinetobacter spp infection at day 14, or survival at day 28 following first cefiderocol dose. Clinical resolution of signs and symptoms was defined as the cessation of cefiderocol treatment due to improvement in symptoms of infection.
Secondary outcomes included (1) survival at day 14 and day 28; (2) clinical outcomes of cefiderocol treatment; and (3) safety. Mortality at day 14 was imputed as treatment failure regardless of the cause of death. Clinical outcomes included (1) resolution of investigator-assessed signs and symptoms of Acinetobacter spp infection at day 7 and day 14 (excludes patients with improved symptoms); (2) treatment failure at day 7 and day 14; and (3) relapse of primary Acinetobacter spp infection (isolation of the same Acinetobacter spp ≤30 days after end of cefiderocol treatment). Safety data were adverse drug reactions (ADRs, treatment-related adverse events [AEs]); total AEs (not necessarily with a causal relationship with the treatment) were not included.
Additional variables extracted included (1) patient characteristics, including clinical characteristics of CR Acinetobacter spp infection (mono- and polymicrobial infections), septic shock (yes/no) at time of cefiderocol initiation and/or COVID-19 status (positive/negative) during hospitalization, severity of illness at time of cefiderocol initiation (by Sequential Organ Failure Assessment [SOFA] score), and treatment for CR Acinetobacter spp infection prior to cefiderocol use; and (2) cefiderocol treatment characteristics (monotherapy/combination therapy). SOFA scores of 0 were imputed for individual SOFA domains where data were missing, providing a value was recorded for at least 1 of the 6 SOFA domains. Septic shock was defined as a score of 3 or 4 on the SOFA score cardiovascular domain, describing patients who received dopamine, epinephrine, or norepinephrine [29, 30]. Combination therapy was defined as use of an antibiotic generally used for treatment of CRAB, in conjunction with cefiderocol.
Survival probability curves were analyzed based on recorded dates of cefiderocol initiation and death.
Descriptive analyses for continuous variables were reported as median and interquartile, minimum, and maximum ranges; categorical variables were reported as frequency and percentage.
RESULTS
Study Population
A total of 227 EAP patient requests for the use of cefiderocol for Acinetobacter spp infection were received during the study period. Of 156 patients screened, 147 were included for analysis (Figure 1). The median age of patients was 62 (interquartile range [IQR], 53–71) years and 78.9% (116/147) were male; 78.9% (116/147) of hospital admissions were emergency admissions (91.2% [134/147]) (Table 1).
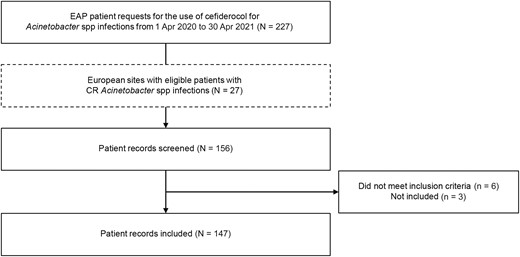
Flowchart of patient record selection from screening of the early access program database. Abbreviations: CR, carbapenem-resistant; EAP, early access program.
| Characteristic . | No. (%) . |
|---|---|
| Patient characteristics | |
| Age, y, median (range; IQR) | 62 (23–82; 53–71) |
| Sex | |
| Female | 31 (21.1) |
| Male | 116 (78.9) |
| Hospital admission | |
| Emergency | 134 (91.2) |
| Scheduled | 9 (6.1) |
| Other | 4 (2.7) |
| Infection | |
| Site of primary infection | |
| Respiratory | 96 (65.3) |
| BSI (catheter related) | 16 (10.9) |
| BSI (unknown source) | 23 (15.6) |
| Othera | 12 (8.2) |
| Secondary BSI | 47 (32.0) |
| Polymicrobial infection | 36 (24.5) |
| ICU/ventilation | |
| Any time in ICU | 136 (92.5) |
| Time in ICU, d, median (IQR) | 37 (23.5–51.0) |
| In ICU receiving mechanical ventilation | 116/136 (85.3) |
| Respiratory infection and receiving mechanical ventilation | 82/96 (85.4) |
| Ventilator-associated respiratory infection | |
| VAP | 70/82 (85.4) |
| V-HAP | 11/82 (13.4) |
| Unknownb | 1/82 (1.2) |
| ECMO during hospitalization | 22 (15.0) |
| Patient status and comorbidities | |
| Septic shockc | 70/126 (55.6) |
| COVID-19 during hospitalization | 120 (81.6) |
| Septic shockc and COVID-19 during hospitalization | 58 (39.5) |
| SOFA scored quartile (score range) in ICU, no./No. (median score) | |
| 1 (0–5) | 35/131 (3) |
| 2 (6–9) | 44/131 (8) |
| 3 (10–11) | 32/131 (11) |
| 4 (12–17) | 20/131 (13) |
| Treatment regimen | |
| No. of prior antimicrobial therapiese | |
| 0 | 22 (15.0) |
| 1 | 35 (23.8) |
| 2 | 48 (32.7) |
| 3 | 29 (19.7) |
| 4 | 10 (6.8) |
| ≥5 | 3 (2.0) |
| Prior antimicrobial therapye,f | |
| Colistin | 86 (58.5) |
| Meropenem | 62 (42.2) |
| Tigecycline | 40 (27.2) |
| Piperacillin/tazobactam | 22 (15.0) |
| Ampicillin/sulbactam | 13 (8.8) |
| Reason for discontinuation of prior antimicrobial therapyg | |
| Lack of resolution | 65 (44.2) |
| New resistance | 34 (23.1) |
| Adverse events | 5 (3.4) |
| Other | 19 (12.9) |
| Duration of cefiderocol treatment, d, median (IQR) | 10.0 (8.0–15.0) |
| Cefiderocol administered as monotherapy | 49 (33.3) |
| Septic shockc present | 21/42 (50.0) |
| SOFA scored, median (IQR) | 7.5 (3.0–9.0) |
| Cefiderocol administered as combination therapy | 98 (66.7) |
| Septic shockc present | 49/84 (58.3) |
| SOFA scored, median (IQR) | 9.0 (7.0–11.0) |
| Antibiotic therapy started with cefiderocol administration | |
| Colistin | 27 (18.4) |
| Tigecycline | 22 (15.0) |
| Fosfomycin | 19 (12.9) |
| Meropenem | 3 (2.0) |
| Ampicillin/sulbactam | 2 (1.4) |
| Trimethoprim/sulfamethoxazole | 2 (1.4) |
| Other | 12 (8.2) |
| Characteristic . | No. (%) . |
|---|---|
| Patient characteristics | |
| Age, y, median (range; IQR) | 62 (23–82; 53–71) |
| Sex | |
| Female | 31 (21.1) |
| Male | 116 (78.9) |
| Hospital admission | |
| Emergency | 134 (91.2) |
| Scheduled | 9 (6.1) |
| Other | 4 (2.7) |
| Infection | |
| Site of primary infection | |
| Respiratory | 96 (65.3) |
| BSI (catheter related) | 16 (10.9) |
| BSI (unknown source) | 23 (15.6) |
| Othera | 12 (8.2) |
| Secondary BSI | 47 (32.0) |
| Polymicrobial infection | 36 (24.5) |
| ICU/ventilation | |
| Any time in ICU | 136 (92.5) |
| Time in ICU, d, median (IQR) | 37 (23.5–51.0) |
| In ICU receiving mechanical ventilation | 116/136 (85.3) |
| Respiratory infection and receiving mechanical ventilation | 82/96 (85.4) |
| Ventilator-associated respiratory infection | |
| VAP | 70/82 (85.4) |
| V-HAP | 11/82 (13.4) |
| Unknownb | 1/82 (1.2) |
| ECMO during hospitalization | 22 (15.0) |
| Patient status and comorbidities | |
| Septic shockc | 70/126 (55.6) |
| COVID-19 during hospitalization | 120 (81.6) |
| Septic shockc and COVID-19 during hospitalization | 58 (39.5) |
| SOFA scored quartile (score range) in ICU, no./No. (median score) | |
| 1 (0–5) | 35/131 (3) |
| 2 (6–9) | 44/131 (8) |
| 3 (10–11) | 32/131 (11) |
| 4 (12–17) | 20/131 (13) |
| Treatment regimen | |
| No. of prior antimicrobial therapiese | |
| 0 | 22 (15.0) |
| 1 | 35 (23.8) |
| 2 | 48 (32.7) |
| 3 | 29 (19.7) |
| 4 | 10 (6.8) |
| ≥5 | 3 (2.0) |
| Prior antimicrobial therapye,f | |
| Colistin | 86 (58.5) |
| Meropenem | 62 (42.2) |
| Tigecycline | 40 (27.2) |
| Piperacillin/tazobactam | 22 (15.0) |
| Ampicillin/sulbactam | 13 (8.8) |
| Reason for discontinuation of prior antimicrobial therapyg | |
| Lack of resolution | 65 (44.2) |
| New resistance | 34 (23.1) |
| Adverse events | 5 (3.4) |
| Other | 19 (12.9) |
| Duration of cefiderocol treatment, d, median (IQR) | 10.0 (8.0–15.0) |
| Cefiderocol administered as monotherapy | 49 (33.3) |
| Septic shockc present | 21/42 (50.0) |
| SOFA scored, median (IQR) | 7.5 (3.0–9.0) |
| Cefiderocol administered as combination therapy | 98 (66.7) |
| Septic shockc present | 49/84 (58.3) |
| SOFA scored, median (IQR) | 9.0 (7.0–11.0) |
| Antibiotic therapy started with cefiderocol administration | |
| Colistin | 27 (18.4) |
| Tigecycline | 22 (15.0) |
| Fosfomycin | 19 (12.9) |
| Meropenem | 3 (2.0) |
| Ampicillin/sulbactam | 2 (1.4) |
| Trimethoprim/sulfamethoxazole | 2 (1.4) |
| Other | 12 (8.2) |
Data are from time of cefiderocol initiation and are presented as No. (%) or no./No. (%) unless otherwise indicated.
Abbreviations: BSI, bloodstream infection; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; VAP, ventilator-associated pneumonia; V-HAP, ventilated hospital-acquired pneumonia.
Included other (n = 4), skin and soft tissue infections (n = 3), bone or joint infections (n = 2), intra-abdominal infections (n = 2), and urinary tract infection (n = 1).
Unknown time of mechanical ventilation initiation.
Septic shock was defined as a score of 3 or 4 on the cardiovascular domain on the SOFA score.
SOFA scores of 0 were imputed for individual SOFA domains where data were missing, providing a value was recorded for at least 1 of the 6 SOFA domains.
Therapies suitable for gram-negative infections used to treat Acinetobacter spp infections.
Five most frequently used antimicrobials are shown. Patients may have received ≥1 antimicrobial prior to cefiderocol.
Reasons per prior antimicrobial are shown.
| Characteristic . | No. (%) . |
|---|---|
| Patient characteristics | |
| Age, y, median (range; IQR) | 62 (23–82; 53–71) |
| Sex | |
| Female | 31 (21.1) |
| Male | 116 (78.9) |
| Hospital admission | |
| Emergency | 134 (91.2) |
| Scheduled | 9 (6.1) |
| Other | 4 (2.7) |
| Infection | |
| Site of primary infection | |
| Respiratory | 96 (65.3) |
| BSI (catheter related) | 16 (10.9) |
| BSI (unknown source) | 23 (15.6) |
| Othera | 12 (8.2) |
| Secondary BSI | 47 (32.0) |
| Polymicrobial infection | 36 (24.5) |
| ICU/ventilation | |
| Any time in ICU | 136 (92.5) |
| Time in ICU, d, median (IQR) | 37 (23.5–51.0) |
| In ICU receiving mechanical ventilation | 116/136 (85.3) |
| Respiratory infection and receiving mechanical ventilation | 82/96 (85.4) |
| Ventilator-associated respiratory infection | |
| VAP | 70/82 (85.4) |
| V-HAP | 11/82 (13.4) |
| Unknownb | 1/82 (1.2) |
| ECMO during hospitalization | 22 (15.0) |
| Patient status and comorbidities | |
| Septic shockc | 70/126 (55.6) |
| COVID-19 during hospitalization | 120 (81.6) |
| Septic shockc and COVID-19 during hospitalization | 58 (39.5) |
| SOFA scored quartile (score range) in ICU, no./No. (median score) | |
| 1 (0–5) | 35/131 (3) |
| 2 (6–9) | 44/131 (8) |
| 3 (10–11) | 32/131 (11) |
| 4 (12–17) | 20/131 (13) |
| Treatment regimen | |
| No. of prior antimicrobial therapiese | |
| 0 | 22 (15.0) |
| 1 | 35 (23.8) |
| 2 | 48 (32.7) |
| 3 | 29 (19.7) |
| 4 | 10 (6.8) |
| ≥5 | 3 (2.0) |
| Prior antimicrobial therapye,f | |
| Colistin | 86 (58.5) |
| Meropenem | 62 (42.2) |
| Tigecycline | 40 (27.2) |
| Piperacillin/tazobactam | 22 (15.0) |
| Ampicillin/sulbactam | 13 (8.8) |
| Reason for discontinuation of prior antimicrobial therapyg | |
| Lack of resolution | 65 (44.2) |
| New resistance | 34 (23.1) |
| Adverse events | 5 (3.4) |
| Other | 19 (12.9) |
| Duration of cefiderocol treatment, d, median (IQR) | 10.0 (8.0–15.0) |
| Cefiderocol administered as monotherapy | 49 (33.3) |
| Septic shockc present | 21/42 (50.0) |
| SOFA scored, median (IQR) | 7.5 (3.0–9.0) |
| Cefiderocol administered as combination therapy | 98 (66.7) |
| Septic shockc present | 49/84 (58.3) |
| SOFA scored, median (IQR) | 9.0 (7.0–11.0) |
| Antibiotic therapy started with cefiderocol administration | |
| Colistin | 27 (18.4) |
| Tigecycline | 22 (15.0) |
| Fosfomycin | 19 (12.9) |
| Meropenem | 3 (2.0) |
| Ampicillin/sulbactam | 2 (1.4) |
| Trimethoprim/sulfamethoxazole | 2 (1.4) |
| Other | 12 (8.2) |
| Characteristic . | No. (%) . |
|---|---|
| Patient characteristics | |
| Age, y, median (range; IQR) | 62 (23–82; 53–71) |
| Sex | |
| Female | 31 (21.1) |
| Male | 116 (78.9) |
| Hospital admission | |
| Emergency | 134 (91.2) |
| Scheduled | 9 (6.1) |
| Other | 4 (2.7) |
| Infection | |
| Site of primary infection | |
| Respiratory | 96 (65.3) |
| BSI (catheter related) | 16 (10.9) |
| BSI (unknown source) | 23 (15.6) |
| Othera | 12 (8.2) |
| Secondary BSI | 47 (32.0) |
| Polymicrobial infection | 36 (24.5) |
| ICU/ventilation | |
| Any time in ICU | 136 (92.5) |
| Time in ICU, d, median (IQR) | 37 (23.5–51.0) |
| In ICU receiving mechanical ventilation | 116/136 (85.3) |
| Respiratory infection and receiving mechanical ventilation | 82/96 (85.4) |
| Ventilator-associated respiratory infection | |
| VAP | 70/82 (85.4) |
| V-HAP | 11/82 (13.4) |
| Unknownb | 1/82 (1.2) |
| ECMO during hospitalization | 22 (15.0) |
| Patient status and comorbidities | |
| Septic shockc | 70/126 (55.6) |
| COVID-19 during hospitalization | 120 (81.6) |
| Septic shockc and COVID-19 during hospitalization | 58 (39.5) |
| SOFA scored quartile (score range) in ICU, no./No. (median score) | |
| 1 (0–5) | 35/131 (3) |
| 2 (6–9) | 44/131 (8) |
| 3 (10–11) | 32/131 (11) |
| 4 (12–17) | 20/131 (13) |
| Treatment regimen | |
| No. of prior antimicrobial therapiese | |
| 0 | 22 (15.0) |
| 1 | 35 (23.8) |
| 2 | 48 (32.7) |
| 3 | 29 (19.7) |
| 4 | 10 (6.8) |
| ≥5 | 3 (2.0) |
| Prior antimicrobial therapye,f | |
| Colistin | 86 (58.5) |
| Meropenem | 62 (42.2) |
| Tigecycline | 40 (27.2) |
| Piperacillin/tazobactam | 22 (15.0) |
| Ampicillin/sulbactam | 13 (8.8) |
| Reason for discontinuation of prior antimicrobial therapyg | |
| Lack of resolution | 65 (44.2) |
| New resistance | 34 (23.1) |
| Adverse events | 5 (3.4) |
| Other | 19 (12.9) |
| Duration of cefiderocol treatment, d, median (IQR) | 10.0 (8.0–15.0) |
| Cefiderocol administered as monotherapy | 49 (33.3) |
| Septic shockc present | 21/42 (50.0) |
| SOFA scored, median (IQR) | 7.5 (3.0–9.0) |
| Cefiderocol administered as combination therapy | 98 (66.7) |
| Septic shockc present | 49/84 (58.3) |
| SOFA scored, median (IQR) | 9.0 (7.0–11.0) |
| Antibiotic therapy started with cefiderocol administration | |
| Colistin | 27 (18.4) |
| Tigecycline | 22 (15.0) |
| Fosfomycin | 19 (12.9) |
| Meropenem | 3 (2.0) |
| Ampicillin/sulbactam | 2 (1.4) |
| Trimethoprim/sulfamethoxazole | 2 (1.4) |
| Other | 12 (8.2) |
Data are from time of cefiderocol initiation and are presented as No. (%) or no./No. (%) unless otherwise indicated.
Abbreviations: BSI, bloodstream infection; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; VAP, ventilator-associated pneumonia; V-HAP, ventilated hospital-acquired pneumonia.
Included other (n = 4), skin and soft tissue infections (n = 3), bone or joint infections (n = 2), intra-abdominal infections (n = 2), and urinary tract infection (n = 1).
Unknown time of mechanical ventilation initiation.
Septic shock was defined as a score of 3 or 4 on the cardiovascular domain on the SOFA score.
SOFA scores of 0 were imputed for individual SOFA domains where data were missing, providing a value was recorded for at least 1 of the 6 SOFA domains.
Therapies suitable for gram-negative infections used to treat Acinetobacter spp infections.
Five most frequently used antimicrobials are shown. Patients may have received ≥1 antimicrobial prior to cefiderocol.
Reasons per prior antimicrobial are shown.
Infection
Of the 147 patients included, 146 had A baumannii infection and 1 had an infection defined as “Acinetobacter–other.” Primary infection sites were respiratory (65.3% [96/147]), BSI (unknown source, 15.6% [23/147]; catheter related, 10.9% [16/147]), and other (8.2% [12/147]) (Table 1). Secondary BSIs with Acinetobacter spp occurred in 32.0% (47/147) of patients. Polymicrobial gram-negative infections were reported in 24.5% (36/147) of patients (Table 1; Supplementary Table 1), while gram-positive pathogens were isolated from 18.4% (27/147) of patients.
Most patients (92.5% [136/147]) spent some time in an intensive care unit (ICU) during hospitalization with a median length of total ICU stay of 37 (IQR, 23.5–51.0) days (Table 1). Of 136 patients in the ICU, 85.3% (116/136) received mechanical ventilation (Table 1). Of 96 patients with respiratory infection, 85.4% (82/96) received mechanical ventilation; of these 85.4% (70/82) had ventilator-associated pneumonia (VAP), 13.4% (11/82) had ventilated hospital-acquired pneumonia (V-HAP), and 1.2% (1/82) had undefined respiratory infection (Table 1).
Patient Status and Comorbidities
A high proportion of patients (55.6% [70/126]) had septic shock at time of cefiderocol initiation, and most (81.6% [120/147]) had COVID-19 during hospitalization; 39.5% (58/147) had both septic shock and COVID-19 (Table 1).
SOFA scores were reported for 96.3% (131/136) of patients who spent time in ICU (Table 1). To analyze outcomes in patients according to severity of illness, SOFA scores were divided into quartiles. At time of cefiderocol initiation, 35 patients were in SOFA score quartile 1 (median score, 3 [range, 0–5]), 44 in quartile 2 (median, 8 [range, 6–9]), 32 in quartile 3 (median, 11 [range, 10–11]), and 20 in quartile 4 (median, 13 [range, 12–17]) (Table 1). Median SOFA scores were 10 (range, 3–17; IQR, 9–11) in patients with septic shock and 5 (range, 1–14; IQR, 3–8.5) in patients without septic shock (Supplementary Table 2).
During hospitalization, 15.0% (22/147) of patients received extracorporeal membrane oxygenation (Table 1).
Treatment Regimens
Prior to cefiderocol initiation, 61.2% (90/147) of patients received ≥2 gram-negative antimicrobials (Table 1). Colistin-based therapy was administered in 58.5% (86/147) of patients with a median (IQR) treatment duration of 11.5 (6.0–18.0) days (Supplementary Tables 3 and 4). Median (IQR) treatment durations of prior ampicillin/sulbactam and tigecycline treatment were 8.0 (7.0–10.0) days and 15.0 (9.0–20.0) days, respectively (Supplementary Table 4). Reasons for discontinuation of antimicrobials prior to cefiderocol initiation included lack of resolution (44.2% [65/147]), new resistance (23.1% [34/147]), and AEs (3.4% [5/147]) (Table 1).
Median (IQR) duration of cefiderocol treatment was 10.0 (8.0–15.0) days, and it was used as first-line therapy in 15.0% (22/147) of patients. Cefiderocol was administered as monotherapy (33.3% [49/147]) and in combination with other antimicrobials, including colistin (18.4% [27/147]), tigecycline (15.0% [22/147]), and fosfomycin (12.9% [19/147]) (Table 1). In patients receiving cefiderocol monotherapy versus combination therapy, septic shock was present in 50.0% (21/42) versus 58.3% (49/84), and median (IQR) SOFA scores at time of cefiderocol initiation were 7.5 (3.0–9.0) versus 9.0 (7.0–11.0), respectively.
Primary Outcome
Clinical success rate in patients treated with cefiderocol was 53.1% (78/147) (Table 2). Rate of resolution of infection at day 14 was 39.5% (58/147) and survival at day 28 was 44.9% (66/147) (Table 2). In total, 31.3% (46/147) of patients had both resolution of infection at day 14 and were alive at day 28 (Table 2).
| Outcome . | No. (%) [95% CI] . |
|---|---|
| Overall clinical success | 78 (53.1) [45.0–61.1] |
| Overall assessment of clinical benefit of cefiderocol at day 14 | |
| Resolution of infection | 58 (39.5) [31.6–47.4] |
| Improved symptoms | 18 (12.2) [7.0–17.5] |
| Unable to determine | 11 (7.5) [3.2–11.7] |
| Failurea | 56 (38.1) [30.2–46.0] |
| Missing | 4 (2.7) [.1–5.4] |
| Survival at day 28 | |
| Alive | 66 (44.9) [36.9–52.9] |
| Deceased | 75 (51.0) [42.9–59.1] |
| Unknown | 5 (3.4) [.5–6.3] |
| Missing data | 1 (0.7) [−.7 to 2.0] |
| Resolution of infection at day 14 and survival at day 28 | 46 (31.3) [23.8–38.8] |
| Clinical success according to infection | |
| Monomicrobial infection | 58/111 (52.3) [43.0–61.5] |
| Polymicrobial infection | 20/36 (55.6) [39.3–71.8] |
| Site of infection | |
| Respiratory | 44/96 (45.8) [35.9–55.8] |
| BSI (catheter related) | 12/16 (75.0) [53.8–96.2] |
| BSI (unknown source) | 12/23 (52.2) [31.8–72.6] |
| Otherb | 10/12 (83.3) |
| Ventilator-associated respiratory infection | |
| VAP | 31/70 (44.3) [32.7–55.9] |
| V-HAP | 4/11 (36.4) [7.9–64.8] |
| Unknownc | 1/1 (100.0) |
| Clinical success according to patient status and comorbidities | |
| Septic shockd present | 26/70 (37.1) [25.8–48.5] |
| Septic shockd absent | 35/56 (62.5) [49.8–75.2] |
| COVID-19 present | 57/120 (47.5) [38.6–56.4] |
| COVID-19 absent | 21/27 (77.8) [62.1–93.5] |
| Septic shockd present, COVID-19 present | 18/58 (31.0) |
| SOFA scoree quartile | |
| 1 (median, 3 [range, 0–5]) | 29/35 (82.9) [70.4–95.3] |
| 2 (median, 8 [range, 6–9]) | 19/44 (43.2) [28.6–57.8] |
| 3 (median, 11 [range, 10–11]) | 12/32 (37.5) [20.7–54.3] |
| 4 (median, 13 [range, 12–17]) | 5/20 (25.0) [6.0–44.0] |
| Clinical success according to treatment regimen | |
| Monotherapy | 30/49 (61.2) [47.6–74.9] |
| Combination therapy | 48/98 (49.0) [39.1–58.9] |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
| Survival according to key mortality risk factors | |
| At day 28 | |
| Septic shockd present | 17/70 (24.3) |
| Septic shockd absent | 34/56 (60.7) |
| COVID-19 present | 50/120 (41.7) |
| COVID-19 absent | 16/27 (59.3) |
| Survival according to treatment regimen | |
| At day 14 | 95/147 (64.6) |
| Monotherapy | 33/49 (67.3) |
| Combination therapy | 62/98 (63.3) |
| Cefiderocol as first-line therapy | 16/22 (72.7) |
| At day 28 | 66/147 (44.9) |
| Monotherapy | 26/49 (53.1) |
| Combination therapy | 40/98 (40.8) |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
| Outcome . | No. (%) [95% CI] . |
|---|---|
| Overall clinical success | 78 (53.1) [45.0–61.1] |
| Overall assessment of clinical benefit of cefiderocol at day 14 | |
| Resolution of infection | 58 (39.5) [31.6–47.4] |
| Improved symptoms | 18 (12.2) [7.0–17.5] |
| Unable to determine | 11 (7.5) [3.2–11.7] |
| Failurea | 56 (38.1) [30.2–46.0] |
| Missing | 4 (2.7) [.1–5.4] |
| Survival at day 28 | |
| Alive | 66 (44.9) [36.9–52.9] |
| Deceased | 75 (51.0) [42.9–59.1] |
| Unknown | 5 (3.4) [.5–6.3] |
| Missing data | 1 (0.7) [−.7 to 2.0] |
| Resolution of infection at day 14 and survival at day 28 | 46 (31.3) [23.8–38.8] |
| Clinical success according to infection | |
| Monomicrobial infection | 58/111 (52.3) [43.0–61.5] |
| Polymicrobial infection | 20/36 (55.6) [39.3–71.8] |
| Site of infection | |
| Respiratory | 44/96 (45.8) [35.9–55.8] |
| BSI (catheter related) | 12/16 (75.0) [53.8–96.2] |
| BSI (unknown source) | 12/23 (52.2) [31.8–72.6] |
| Otherb | 10/12 (83.3) |
| Ventilator-associated respiratory infection | |
| VAP | 31/70 (44.3) [32.7–55.9] |
| V-HAP | 4/11 (36.4) [7.9–64.8] |
| Unknownc | 1/1 (100.0) |
| Clinical success according to patient status and comorbidities | |
| Septic shockd present | 26/70 (37.1) [25.8–48.5] |
| Septic shockd absent | 35/56 (62.5) [49.8–75.2] |
| COVID-19 present | 57/120 (47.5) [38.6–56.4] |
| COVID-19 absent | 21/27 (77.8) [62.1–93.5] |
| Septic shockd present, COVID-19 present | 18/58 (31.0) |
| SOFA scoree quartile | |
| 1 (median, 3 [range, 0–5]) | 29/35 (82.9) [70.4–95.3] |
| 2 (median, 8 [range, 6–9]) | 19/44 (43.2) [28.6–57.8] |
| 3 (median, 11 [range, 10–11]) | 12/32 (37.5) [20.7–54.3] |
| 4 (median, 13 [range, 12–17]) | 5/20 (25.0) [6.0–44.0] |
| Clinical success according to treatment regimen | |
| Monotherapy | 30/49 (61.2) [47.6–74.9] |
| Combination therapy | 48/98 (49.0) [39.1–58.9] |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
| Survival according to key mortality risk factors | |
| At day 28 | |
| Septic shockd present | 17/70 (24.3) |
| Septic shockd absent | 34/56 (60.7) |
| COVID-19 present | 50/120 (41.7) |
| COVID-19 absent | 16/27 (59.3) |
| Survival according to treatment regimen | |
| At day 14 | 95/147 (64.6) |
| Monotherapy | 33/49 (67.3) |
| Combination therapy | 62/98 (63.3) |
| Cefiderocol as first-line therapy | 16/22 (72.7) |
| At day 28 | 66/147 (44.9) |
| Monotherapy | 26/49 (53.1) |
| Combination therapy | 40/98 (40.8) |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
Data are presented as No. (%) or no./No. (%), with [95% CI] where available.
Abbreviations: BSI, bloodstream infection; CI, confidence interval; COVID-19, coronavirus disease 2019; SOFA, Sequential Organ Failure Assessment; VAP, ventilator-associated pneumonia; V-HAP, ventilated hospital-acquired pneumonia.
Mortality at day 14 was imputed as treatment failure, regardless of the cause of death.
Included other infections (4/4), skin and soft tissue infection (3/3), bone and joint infection (2/2), intra-abdominal infection (1/2), and urinary tract infection (0/1).
Unknown time of mechanical ventilation initiation.
Septic shock was defined as a score of 3 or 4 on the cardiovascular domain on the SOFA score at time of cefiderocol initiation.
SOFA scores of 0 were imputed for individual SOFA domains where data were missing, providing a value was recorded for at least 1 of the 6 SOFA domains.
| Outcome . | No. (%) [95% CI] . |
|---|---|
| Overall clinical success | 78 (53.1) [45.0–61.1] |
| Overall assessment of clinical benefit of cefiderocol at day 14 | |
| Resolution of infection | 58 (39.5) [31.6–47.4] |
| Improved symptoms | 18 (12.2) [7.0–17.5] |
| Unable to determine | 11 (7.5) [3.2–11.7] |
| Failurea | 56 (38.1) [30.2–46.0] |
| Missing | 4 (2.7) [.1–5.4] |
| Survival at day 28 | |
| Alive | 66 (44.9) [36.9–52.9] |
| Deceased | 75 (51.0) [42.9–59.1] |
| Unknown | 5 (3.4) [.5–6.3] |
| Missing data | 1 (0.7) [−.7 to 2.0] |
| Resolution of infection at day 14 and survival at day 28 | 46 (31.3) [23.8–38.8] |
| Clinical success according to infection | |
| Monomicrobial infection | 58/111 (52.3) [43.0–61.5] |
| Polymicrobial infection | 20/36 (55.6) [39.3–71.8] |
| Site of infection | |
| Respiratory | 44/96 (45.8) [35.9–55.8] |
| BSI (catheter related) | 12/16 (75.0) [53.8–96.2] |
| BSI (unknown source) | 12/23 (52.2) [31.8–72.6] |
| Otherb | 10/12 (83.3) |
| Ventilator-associated respiratory infection | |
| VAP | 31/70 (44.3) [32.7–55.9] |
| V-HAP | 4/11 (36.4) [7.9–64.8] |
| Unknownc | 1/1 (100.0) |
| Clinical success according to patient status and comorbidities | |
| Septic shockd present | 26/70 (37.1) [25.8–48.5] |
| Septic shockd absent | 35/56 (62.5) [49.8–75.2] |
| COVID-19 present | 57/120 (47.5) [38.6–56.4] |
| COVID-19 absent | 21/27 (77.8) [62.1–93.5] |
| Septic shockd present, COVID-19 present | 18/58 (31.0) |
| SOFA scoree quartile | |
| 1 (median, 3 [range, 0–5]) | 29/35 (82.9) [70.4–95.3] |
| 2 (median, 8 [range, 6–9]) | 19/44 (43.2) [28.6–57.8] |
| 3 (median, 11 [range, 10–11]) | 12/32 (37.5) [20.7–54.3] |
| 4 (median, 13 [range, 12–17]) | 5/20 (25.0) [6.0–44.0] |
| Clinical success according to treatment regimen | |
| Monotherapy | 30/49 (61.2) [47.6–74.9] |
| Combination therapy | 48/98 (49.0) [39.1–58.9] |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
| Survival according to key mortality risk factors | |
| At day 28 | |
| Septic shockd present | 17/70 (24.3) |
| Septic shockd absent | 34/56 (60.7) |
| COVID-19 present | 50/120 (41.7) |
| COVID-19 absent | 16/27 (59.3) |
| Survival according to treatment regimen | |
| At day 14 | 95/147 (64.6) |
| Monotherapy | 33/49 (67.3) |
| Combination therapy | 62/98 (63.3) |
| Cefiderocol as first-line therapy | 16/22 (72.7) |
| At day 28 | 66/147 (44.9) |
| Monotherapy | 26/49 (53.1) |
| Combination therapy | 40/98 (40.8) |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
| Outcome . | No. (%) [95% CI] . |
|---|---|
| Overall clinical success | 78 (53.1) [45.0–61.1] |
| Overall assessment of clinical benefit of cefiderocol at day 14 | |
| Resolution of infection | 58 (39.5) [31.6–47.4] |
| Improved symptoms | 18 (12.2) [7.0–17.5] |
| Unable to determine | 11 (7.5) [3.2–11.7] |
| Failurea | 56 (38.1) [30.2–46.0] |
| Missing | 4 (2.7) [.1–5.4] |
| Survival at day 28 | |
| Alive | 66 (44.9) [36.9–52.9] |
| Deceased | 75 (51.0) [42.9–59.1] |
| Unknown | 5 (3.4) [.5–6.3] |
| Missing data | 1 (0.7) [−.7 to 2.0] |
| Resolution of infection at day 14 and survival at day 28 | 46 (31.3) [23.8–38.8] |
| Clinical success according to infection | |
| Monomicrobial infection | 58/111 (52.3) [43.0–61.5] |
| Polymicrobial infection | 20/36 (55.6) [39.3–71.8] |
| Site of infection | |
| Respiratory | 44/96 (45.8) [35.9–55.8] |
| BSI (catheter related) | 12/16 (75.0) [53.8–96.2] |
| BSI (unknown source) | 12/23 (52.2) [31.8–72.6] |
| Otherb | 10/12 (83.3) |
| Ventilator-associated respiratory infection | |
| VAP | 31/70 (44.3) [32.7–55.9] |
| V-HAP | 4/11 (36.4) [7.9–64.8] |
| Unknownc | 1/1 (100.0) |
| Clinical success according to patient status and comorbidities | |
| Septic shockd present | 26/70 (37.1) [25.8–48.5] |
| Septic shockd absent | 35/56 (62.5) [49.8–75.2] |
| COVID-19 present | 57/120 (47.5) [38.6–56.4] |
| COVID-19 absent | 21/27 (77.8) [62.1–93.5] |
| Septic shockd present, COVID-19 present | 18/58 (31.0) |
| SOFA scoree quartile | |
| 1 (median, 3 [range, 0–5]) | 29/35 (82.9) [70.4–95.3] |
| 2 (median, 8 [range, 6–9]) | 19/44 (43.2) [28.6–57.8] |
| 3 (median, 11 [range, 10–11]) | 12/32 (37.5) [20.7–54.3] |
| 4 (median, 13 [range, 12–17]) | 5/20 (25.0) [6.0–44.0] |
| Clinical success according to treatment regimen | |
| Monotherapy | 30/49 (61.2) [47.6–74.9] |
| Combination therapy | 48/98 (49.0) [39.1–58.9] |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
| Survival according to key mortality risk factors | |
| At day 28 | |
| Septic shockd present | 17/70 (24.3) |
| Septic shockd absent | 34/56 (60.7) |
| COVID-19 present | 50/120 (41.7) |
| COVID-19 absent | 16/27 (59.3) |
| Survival according to treatment regimen | |
| At day 14 | 95/147 (64.6) |
| Monotherapy | 33/49 (67.3) |
| Combination therapy | 62/98 (63.3) |
| Cefiderocol as first-line therapy | 16/22 (72.7) |
| At day 28 | 66/147 (44.9) |
| Monotherapy | 26/49 (53.1) |
| Combination therapy | 40/98 (40.8) |
| Cefiderocol as first-line therapy | 15/22 (68.2) |
Data are presented as No. (%) or no./No. (%), with [95% CI] where available.
Abbreviations: BSI, bloodstream infection; CI, confidence interval; COVID-19, coronavirus disease 2019; SOFA, Sequential Organ Failure Assessment; VAP, ventilator-associated pneumonia; V-HAP, ventilated hospital-acquired pneumonia.
Mortality at day 14 was imputed as treatment failure, regardless of the cause of death.
Included other infections (4/4), skin and soft tissue infection (3/3), bone and joint infection (2/2), intra-abdominal infection (1/2), and urinary tract infection (0/1).
Unknown time of mechanical ventilation initiation.
Septic shock was defined as a score of 3 or 4 on the cardiovascular domain on the SOFA score at time of cefiderocol initiation.
SOFA scores of 0 were imputed for individual SOFA domains where data were missing, providing a value was recorded for at least 1 of the 6 SOFA domains.
By Infection
Patients with monomicrobial infections (52.3% [58/111]) had similar clinical success to those with polymicrobial infections (55.6% [20/36]) (Table 2). By primary infection site, clinical success was 45.8% (44/96) in patients with respiratory infection, 52.2% (12/23) with BSI from an unknown source, and 75.0% (12/16) with catheter-related BSI (Table 2). Clinical success was 44.3% (31/70) in patients with VAP and 36.4% (4/11) in patients with V-HAP (Table 2).
By Patient Status and Comorbidities
Clinical success was 62.5% (35/56) in patients without septic shock versus 37.1% (26/70) with septic shock, and 77.8% (21/27) in patients without COVID-19 versus 47.5% (57/120) with COVID-19 (Table 2). Where both septic shock and COVID-19 were present, clinical success was 31.0% (18/58) (Table 2). A small number of patients were without either septic shock or COVID-19, and clinical success in this subset was 71.4% (5/7).
Clinical success was higher in patients with lower SOFA scores: 82.9% (29/35) in SOFA quartile 1 (range, 0–5), 43.2% (19/44) in quartile 2 (range, 6–9), 37.5% (12/32) in quartile 3 (range, 10–11), and 25.0% (5/20) in quartile 4 (range, 12–17) (Table 2).
By Treatment Regimen
Of patients treated with cefiderocol as first-line therapy, 68.2% (15/22) achieved clinical success. Additionally, clinical success was higher in patients receiving monotherapy (61.2% [30/49]) versus combination therapy (49.0% [48/98]) (Table 2).
Clinical success rates by time from Acinetobacter spp isolation to cefiderocol initiation are reported in Supplementary Table 5.
Secondary Outcomes
Survival Rate
Survival rate was 64.6% (95/147) at day 14 and 44.9% (66/147) at day 28 (Table 2; Figure 2A). Notably, survival rates at day 14 and day 28 for patients treated with cefiderocol as first-line therapy were 72.2% (16/22) and 68.2% (15/22), respectively (Table 2).
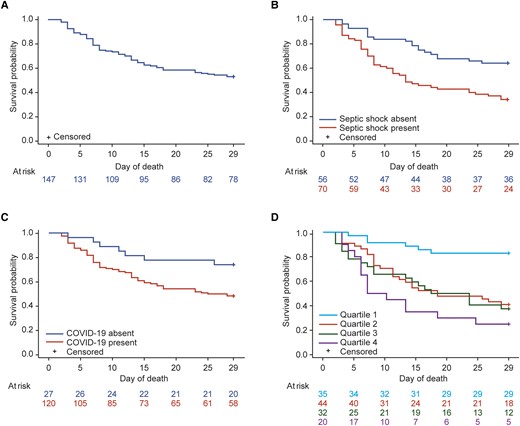
Kaplan-Meier survival rates up to day 28 (n = 147). Survival probability curves were analyzed based on recorded dates of cefiderocol initiation and death. There may be discrepancies between these recorded dates and the deaths registered at day 28 in the case report form. A, Total population. B, By presence/absence of septic shock. C, By presence/absence of coronavirus disease 2019 (COVID-19). D, By Sequential Organ Failure Assessment (SOFA) score quartiles. Septic shock was defined as a score of 3 or 4 on the cardiovascular domain on the SOFA score at time of cefiderocol initiation. SOFA scores of 0 were imputed for individual SOFA domains where data were missing, providing a value was recorded for at least 1 of the 6 SOFA domains.
Rate of survival at day 28 was higher both in patients without septic shock (60.7% [34/56]) versus those with septic shock (24.3% [17/70]) and in patients without COVID-19 (59.3% [16/27]) versus those with COVID-19 (41.7% [50/120]) (Table 2; Figure 2B and 2C). Additionally, rate of survival at day 28 was markedly higher in patients with lower SOFA scores (quartile 1, 82.9% [29/35]) versus those with higher scores (quartile 2, 34.1% [15/44]; quartile 3, 28.1% [9/32]; quartile 4, 10.0% [2/20]) (Figure 2D).
Clinical Benefit
At day 14, resolution of infection occurred in 39.5% (58/147) of patients, and treatment failure was recorded in 38.1% (56/147) (Table 2). At day 7, resolution of infection was reported in 19.0% (28/147) of patients and treatment failure in 15.0% (22/147) (Supplementary Table 6). Relapse of primary Acinetobacter spp infection occurred in 10.2% (15/147) of patients at a median (IQR) time of 10.0 (6.0–14.0) days following cefiderocol discontinuation.
Adverse Drug Reactions
Adverse drug reactions were reported in 4.8% (7/147) of patients, and 1.4% (2/147) experienced a total of 3 severe/serious ADRs (Table 3).
| ADR Classification . | Patients, No. (%) . | Events, No. . |
|---|---|---|
| All ADRs | 7 (4.8) | 9 |
| Mild | 3 (2.0) | 4 |
| Pancytopenia | 1 (0.7) | 1 |
| Hypernatremia | 1 (0.7) | 1 |
| Rash | 1 (0.7) | 1 |
| Hypertension | 1 (0.7) | 1 |
| Moderate | 1 (0.7) | 1 |
| Clostridium difficile colitis | 1 (0.7) | 1 |
| Severe/serious ADRs | 2 (1.4) | 3 |
| Cardiac arrest | 1 (0.7) | 1 |
| Renal tubular necrosis | 1 (0.7) | 1 |
| Tubulointerstitial nephritis | 1 (0.7) | 1 |
| Not reported | 1 (0.7) | 1 |
| Treatment withdrawala | 5 (3.4) | 7 |
| ADR Classification . | Patients, No. (%) . | Events, No. . |
|---|---|---|
| All ADRs | 7 (4.8) | 9 |
| Mild | 3 (2.0) | 4 |
| Pancytopenia | 1 (0.7) | 1 |
| Hypernatremia | 1 (0.7) | 1 |
| Rash | 1 (0.7) | 1 |
| Hypertension | 1 (0.7) | 1 |
| Moderate | 1 (0.7) | 1 |
| Clostridium difficile colitis | 1 (0.7) | 1 |
| Severe/serious ADRs | 2 (1.4) | 3 |
| Cardiac arrest | 1 (0.7) | 1 |
| Renal tubular necrosis | 1 (0.7) | 1 |
| Tubulointerstitial nephritis | 1 (0.7) | 1 |
| Not reported | 1 (0.7) | 1 |
| Treatment withdrawala | 5 (3.4) | 7 |
Abbreviation: ADR, adverse drug reaction.
Treatment withdrawals were assumed to be treatment related.
| ADR Classification . | Patients, No. (%) . | Events, No. . |
|---|---|---|
| All ADRs | 7 (4.8) | 9 |
| Mild | 3 (2.0) | 4 |
| Pancytopenia | 1 (0.7) | 1 |
| Hypernatremia | 1 (0.7) | 1 |
| Rash | 1 (0.7) | 1 |
| Hypertension | 1 (0.7) | 1 |
| Moderate | 1 (0.7) | 1 |
| Clostridium difficile colitis | 1 (0.7) | 1 |
| Severe/serious ADRs | 2 (1.4) | 3 |
| Cardiac arrest | 1 (0.7) | 1 |
| Renal tubular necrosis | 1 (0.7) | 1 |
| Tubulointerstitial nephritis | 1 (0.7) | 1 |
| Not reported | 1 (0.7) | 1 |
| Treatment withdrawala | 5 (3.4) | 7 |
| ADR Classification . | Patients, No. (%) . | Events, No. . |
|---|---|---|
| All ADRs | 7 (4.8) | 9 |
| Mild | 3 (2.0) | 4 |
| Pancytopenia | 1 (0.7) | 1 |
| Hypernatremia | 1 (0.7) | 1 |
| Rash | 1 (0.7) | 1 |
| Hypertension | 1 (0.7) | 1 |
| Moderate | 1 (0.7) | 1 |
| Clostridium difficile colitis | 1 (0.7) | 1 |
| Severe/serious ADRs | 2 (1.4) | 3 |
| Cardiac arrest | 1 (0.7) | 1 |
| Renal tubular necrosis | 1 (0.7) | 1 |
| Tubulointerstitial nephritis | 1 (0.7) | 1 |
| Not reported | 1 (0.7) | 1 |
| Treatment withdrawala | 5 (3.4) | 7 |
Abbreviation: ADR, adverse drug reaction.
Treatment withdrawals were assumed to be treatment related.
DISCUSSION
Determining the best treatment option for patients with severe illness previously treated with multiple antibiotics is challenging, as randomized controlled trials may not be feasible, so real-world experience is highly informative [31, 32]. This study provides a comprehensive collection of real-world evidence on cefiderocol as a therapeutic option for CR Acinetobacter spp infection in severely ill patients.
The primary outcome of clinical success in this study was lower with higher severity of illness, unsurprisingly. Overall clinical success was achieved in 53% of a population in which half of the patients had at least 1 key mortality risk factor, including septic shock (56%) or COVID-19 (82%), and 40% had both, with SOFA scores up to 17. The study period also encompassed the early phase of the COVID-19 pandemic, indicating that illness due to COVID-19 was likely severe. A high proportion of patients spent time in ICU (93%). Clinical success was higher in patients without septic shock (63%), without COVID-19 (78%), and with lower SOFA scores (83% in SOFA quartile 1), compared with the overall rate (53%).
The day 28 survival rate was also higher in patients without septic shock (61%) and lower in patients with septic shock (24%), compared with an overall survival rate of 45%. Previous studies have reported similar findings, with low survival rates in patients with septic shock and VAP caused by A baumannii (19% [31/159] and 23% [13/57], respectively) [9, 33, 34]. Septic shock has been shown to be significantly associated with increased risk of all-cause mortality at day 30 in patients with A baumannii and Klebsiella pneumoniae infections (odds ratio, 23.41 [95% confidence interval {CI}, 8.66–63.31]; P < .001) [35]. In addition, Russo et al reported that survival was significantly lower in patients with septic shock due to multidrug-resistant (MDR) A baumannii than with septic shock due to K pneumoniae carbapenemase–producing K pneumoniae (15% [14/92] vs 55% [71/128], respectively; P < .001) [10]. A baseline survival rate of 67% has been reported in patients with COVID-19 in European ICUs [36]; in this study, day 28 survival rates in patients with (42%) and without (59%) COVID-19 indicate that COVID-19 may have played a role in decreasing survival. These survival rates were higher than in a previous study in patients with COVID-19 and coinfection with Acinetobacter spp (14% [3/21]), albeit this was reported in a small population treated with a range of different therapies (not including cefiderocol) [9].
Day 28 survival (45% [66/147]) in the ARES population was broadly similar to previous observations in real-world case series in patients with CRAB infection by Falcone et al [27] (66% [31/47]), Pascale et al [28] (45% [19/42]), and Bavaro et al [17] (70% [7/10]). A number of patients (88% [87/99]) in these case series were treated via the Shionogi EAP and are included in the ARES patient set [17, 27, 28]. The observational comparative study by Falcone et al reported a higher survival rate with cefiderocol-containing regimens (66% [31/47]) versus colistin (44% [34/77]) (P = .018), whereas Pascale et al did not find cefiderocol to be associated with a significant lower risk of mortality versus colistin (survival, 45% [19/42] vs 42% [27/65]; hazard ratio, 0.64 [95% CI, .38–1.08]; P = .10) [27, 28]. Despite the different study designs, the survival rate of 45% that we observed in ARES is similar to that observed in the CREDIBLE-CR phase 3 study (50% [21/42]) in patients receiving cefiderocol for Acinetobacter spp infection (only 26% [11/42] had septic shock) [16].
Infection by CRAB is difficult to treat [1], and there is a lack of evidence of optimal clinical effectiveness of antimicrobial therapies, such as carbapenems, polymyxins, and tigecycline. This lack of evidence is reflected in treatment guidance, where recommendations tend to be conditional or weak [11, 13]. Additionally, colistin is not recommended as monotherapy by EUCAST or associated with a EUCAST breakpoint [14, 37]. In this study, the vast majority of patients (85%) received and failed on ≥1 prior antimicrobial commonly used against Acinetobacter spp infections, demonstrating the difficulty in treating such infections and the need for more effective therapy. Reflective of the current treatment landscape, prior therapy included >10 different agents, comprising a high proportion of colistin use (59%). Use of ampicillin/sulbactam (9%), recommended for treatment of CRAB in current ESCMID guidance, was low; however, the time period from which study data were collected predates these latest guidance [11]. Given the rate of clinical success demonstrated in this severe population, it is important that new treatments for CRAB infection such as cefiderocol are considered, not just following treatment failure, especially when offering the potential for positive patient outcomes.
When cefiderocol was used as first-line therapy (22/147), clinical success (68% [15/22]) was higher than the overall success rate (53% [78/147]) (Table 2). Severity of disease does not account for this improvement, as median SOFA score in patients treated with first-line cefiderocol was 6, aligned with SOFA score quartile 2, where clinical success was 43%. It should be noted that the survival rate in patients with first-line cefiderocol use was higher compared with overall survival at day 14 (73% [16/22] vs 65% [95/147], respectively) and day 28 (68% [15/22] vs 45% [66/147], respectively) (Table 2). In this small and heterogenous population of complex, critically unwell patients with competing risks for outcomes, we were unable to examine independent associations between time to cefiderocol therapy and clinical outcomes. Future studies in larger homogenous populations are required to fully elucidate the benefit of earlier cefiderocol treatment.
Our study has several limitations. Data indicating the reasons for why the 9 patients from the EAP were not included in the ARES population (ie, which criterium or criteria each patient did or did not meet) are not available. Due to the retrospective nature of the study, treatment data were also not always available for abstraction, so confounding of the association between cefiderocol and outcomes cannot be ruled out. This included data on the administration of steroids or immune modulators, which can affect outcomes in patients with MDR A baumannii infection and COVID-19 [38]. Some missing/partial clinical status and mortality data were imputed; however, this process was conservative, assuming that patients were less severely ill (low SOFA score) and that mortality at day 14 was due to treatment failure. This clinical outcome was assumed since additional clinical and biomarker data for subanalyses were not available, and the data would have otherwise included a high proportion of patients in whom resolution of infection was undetermined. This may have been due to the high incidence of COVID-19 (82%) in the study population, as it is challenging to confirm whether resolution of VAP is due to Acinetobacter spp infection or COVID-19 pneumonia. The definition of septic shock (use of noradrenaline or adrenaline) also overlaps with the clinical syndrome associated with COVID-19 [39]. The composite definition of clinical success differs from those previously used in clinical practice, which typically define clinical success as the achievement of ≥2 outcomes at a single timepoint [40–43]. ARES patients received cefiderocol treatment under emergency supply via the EAP; therefore, the composite outcome in this study allowed for consideration of the timeframe in which the effectiveness of cefiderocol treatment could be best assessed. Resolution of infection after 14 days of therapy could be considered, and day 28 survival enabled later deaths (likely due to underlying conditions in this severely ill population, rather than CR Acinetobacter spp infection) to be excluded. However, 7 patients with relapse of infection following day 14 were included in the subset who achieved clinical success. Long-term follow-up outcomes were not assessed as data were not collected outside each patient's hospitalization episode. Unfortunately, a lack of susceptibility testing on initial and subsequent isolates meant that development of resistance or changing resistance patterns could not be discerned.
CONCLUSIONS
This study included a patient population with limited remaining treatment options for CR Acinetobacter spp infection and high rates of septic shock and COVID-19; despite this, cefiderocol treatment was associated with an overall clinical success rate of 53%. Further studies assessing cefiderocol treatment in different clinical scenarios are warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors contributed toward data collection and analysis, drafting, and revision of the manuscript, and agree to be accountable for all aspects of the work.
Acknowledgments. The authors acknowledge Hannah James, MSc, of Ashfield MedComms, an Inizio Company, for medical writing support funded by Shionogi B.V. in accordance with Good Publication Practice (GPP3 and GPP 2022) guidelines. Thanks also go to Shionogi B.V. employees Gill Ibbotson and Maria Oppia, who provided support in the conduct of the study. The authors thank members of the ARES Study Group for their work: Pierluigi Viale, Maddalena Giannella, Renato Pascale, Marco Falcone, Giusy Tiseo, Alessandra Bandera, Toussaint Muheberimana, Valeria Pastore, Alessandra Saracino, Davide Fiore Bavaro, Lidia Dalfino, Luca Guerra, Francesco Barchiesi, Ylenia Farinaccio, Chiara Temperoni, Giustino Parruti, Simona Coladonato, Giorgia Rapacchiale, Laurenzia Ferraris, Alessandro Meloni, Andrea Bruni, Eugenio Garofalo, Carlo Torti, Paolo Grossi, Emanuele Durante Mangoni, Alessia Massa, Fabiana D’Amico, Domenico Iossa, Arta Karruli, Novella Carannante, Marco Merli, Carlo Pallotto, Elena Maria Seminari, Samantha Sottotetti, Roberto Carbone, Stefania Casolari, Elisa Vanino, Francesco Cristini, Luigi Raumer, Gennaro De Pascale, Agnese di Chiaro, Lucia Cubattoli, Alessandro Bartoloni, Nicoletta Di Lauria, Mario Venditti, Alessandra Oliva, Rosario Cultrera, Hasania Abdel Hadi, Carmen Hidalgo Tenorio, David Pérez Torres, Isabel Canas-Pérez, Luis López-Urrutia Lorente, Montserrat Rodriguez, Barbara Balandin, Raphaël Lepeule, Dominic Wichmann, Christina König, and Dominik Jarczak.
Data availability. Data are available upon reasonable request from Shionogi B.V.
Financial support. This work was sponsored by Shionogi B.V., London, UK.
References
Author notes
Potential conflicts of interest. M. G. has received honoraria for lectures, presentations, or speaker’s bureaus from MSD, Shionogi, Pfizer, and Gilead, and for participation on a data safety monitoring board or advisory board from MSD and Pfizer. S. V., A. K., and A. C. are employees of Shionogi B.V. H. D. has received fees from Shionogi for conferences and expert advisory boards. A. S. has received honoraria for lectures and advisory boards from Pfizer, MSD, Shionogi, Menarini, and Gilead. A. S. H. is an employee of Maxel Consulting ApS (Jyllinge, Denmark) and is a contractor for Shionogi B.V. M. F. has received grants and/or speaker honoraria from MSD, Angelini, Shionogi, Pfizer, Gilead, Menarini, and Nordic Pharma. H. A. H. reports no potential conflicts of interest.





Comments