-
PDF
- Split View
-
Views
-
Cite
Cite
Amen Ben Hamida, Kamil Mohamed Ali, Rennatus Mdodo, Abdinoor Mohamed, Kumlachew Mengistu, Rosemary M Nzunza, Noha H Farag, Derek T Ehrhardt, Eltayeb Elfakki, Chukwuma Mbaeyi, Using Nonpolio Enterovirus Detection to Assess the Integrity of Stool Specimens Collected From Acute Flaccid Paralysis Cases in Somalia During 2014–2017, Open Forum Infectious Diseases, Volume 7, Issue 5, May 2020, ofaa135, https://doi.org/10.1093/ofid/ofaa135
Close - Share Icon Share
Abstract
Despite insecurity challenges in Somalia, key indicators for acute flaccid paralysis (AFP) surveillance have met recommended targets. However, recent outbreaks of vaccine-derived polioviruses have raised concerns about possible gaps. We analyzed nonpolio enterovirus (NPEV) and Sabin poliovirus isolation rates to investigate whether comparing these rates can inform about the integrity of stool specimens from inaccessible areas and the likelihood of detecting circulating polioviruses.
Using logistic regression, we analyzed case-based AFP surveillance data for 1348 cases with onset during 2014−2017. We assessed the adjusted impacts of variables including age, accessibility, and Sabin-like virus isolation on NPEV detection.
NPEVs were more likely to be isolated from AFP case patients reported from inaccessible areas than accessible areas (23% vs 15%; P = .01). In a multivariable model, inaccessibility and detection of Sabin-like virus were positively associated with NPEV detection (adjusted odds ratio [AOR], 1.75; 95% confidence interval [CI], 1.14–2.65; and AOR, 1.79; 95% CI, 1.07–2.90; respectively), while being aged ≥5 years was negatively associated (AOR, 0.42; 95% CI, 0.20–0.85).
Rates of NPEV and Sabin poliovirus detection in inaccessible areas suggest that the integrity of fecal specimens tested for AFP surveillance in Somalia can generate useful AFP data, but uncertainties remain about surveillance system quality.
Poliomyelitis (commonly known as polio) is a highly contagious disease caused by poliovirus (serotypes 1, 2, or 3) infection. It is transmitted by person-to-person contact mainly through the fecal–oral route and disproportionally affects children under 5 years of age [1]. Depending on the type, about 1 in 200–2000 polio infections leads to paralysis; rarely, serious complications—including death by paralysis of the respiratory muscles—may occur in about 5% of cases [1]. Since the launch of the Global Polio Eradication Initiative (GPEI) in 1988, global polio incidence has decreased by more than 99.9%, from 350 000 cases in 1988 to only 33 reported cases in 2018 [2, 3].
Case-based surveillance for syndromic acute flaccid paralysis (AFP) among children under 15 years of age is used for both the detection of circulating polioviruses and providing evidence for the absence of circulation and is one of the key strategies used for polio eradication [3]. To standardize the implementation and evaluation of AFP surveillance, the World Health Organization (WHO) recommends using several key indicators including the rate of nonpolio AFP (NPAFP; AFP cases with adequate specimens negative for wild poliovirus [WPV] and vaccine-derived poliovirus [VDPV] on laboratory testing or those cases with inadequate specimens deemed not compatible with polio upon expert review), with a target of ≥2 cases per 100 000 children per year, and the proportion of adequate stool specimens, with a target of ≥80% for all AFP cases. Because nonpolio enterovirus (NPEV) infections are ubiquitous in children, the rate of isolation of NPEV from stool specimens has been used as a crude assessment of specimen integrity [4–7]. Although the actual NPEV rate is subject to significant variability related to seasonality, climate, elevation, NPEV type–specific epidemiology, and population sanitation and hygiene, it has been considered in assessing the process of stool specimen collection, transport (the quality of the reverse cold chain system), and how well the laboratory is able to perform in the routine virus isolation of poliovirus [5, 6, 8]. However, because of the inherent variability and wide geographic differences in the actual prevalence of enterovirus infections in a population, this measure is no longer used to assess laboratory performance (although there are other performance assessments for laboratory detection sensitivity). Independent assessments indicate that problems with the reverse cold chain are unlikely to be common sources of problems with poliovirus detection [8]. Even with these limitations, the absence of significant NPEV detection can indirectly inform the polio eradication program about possible operational deficiencies to detect polioviruses in the event of circulation in a specific area. The WHO proposes an operational NPEV identification rate of at least 10% among all specimens tested, but this value is arbitrary and not reflective of actual NPEV incidence in any specific geography [5, 6, 8]. Countries that appear to be unable to isolate NPEV or Sabin poliovirus risk the possibility of missing poliovirus circulation, as such specimens may produce false-negative laboratory results for polioviruses. This is of particular significance in countries deemed to be at high risk of poliovirus circulation because of low immunization coverage.
Somalia, a coastal country in Eastern Africa, is a priority country for the GPEI, owing to a longstanding humanitarian crisis and its vulnerability to recurrent polio outbreaks. Several years of political instability, occasioned by civil war and protracted armed conflict and insurgency, have led to fragmented political control and to a significant increase in vulnerable populations in the country [9]. By the end of 2017, it was estimated that 2.1 million persons were internally displaced in Somalia and that 900 000 Somali refugees had fled the country due to conflict [10]. These challenges have led to a deterioration of the public health system, including the delivery of immunization services. The WHO and UNICEF estimate that childhood immunization coverage with 3 doses of oral poliovirus vaccine (OPV; Sabin) has been consistently <50% in Somalia during the last decade, so that supplementary immunization campaigns with OPV have been needed to maintain population immunity against poliovirus [11]. Further complicating the situation, insurgency groups have restricted access for the delivery of immunization services in many districts in the south and central zones of the country, leaving about 1 million children under 10 years of age unvaccinated against polio [9].
Despite these challenges, the country successfully interrupted indigenous transmission of WPV in 2002 [9]. However, it experienced 2 outbreaks following importation of WPV during 2005–2007 and 2013–2014 [9, 12, 13]. There are 2 ongoing outbreaks of cVDPVs (type 2 and type 3), which were first identified during 2017–2018 through testing of sewage samples [14, 15]. In very underimmunized populations, extended person-to-person transmission of Sabin virus following use of OPV can result in genetic reversion to neurovirulence and paralytic cVDPV disease outbreaks [16]. For these cVDPV outbreaks, genomic sequence analyses indicated that they emerged 1 to several years before the first cVDPV detection, questioning the overall AFP surveillance performance in Somalia [14].
To address its vulnerability to polio outbreaks, several novel initiatives have been undertaken by the Somalia polio program, including the use of geographic information system technology and the establishment of the Village Polio Volunteers (VPV) program, a community-based initiative established in 2013 that is aimed at strengthening AFP surveillance and improving polio vaccination coverage in the country. A comprehensive evaluation of the VPV program, comparing AFP surveillance indicators before and after its introduction, showed improvements in national and subnational NPAFP rates following program introduction in 2013 [17]. Nevertheless, given the previously cited vulnerabilities and the ongoing cVDPV outbreaks with emergence years before detection, questions remain about the overall quality of AFP surveillance in Somalia. In recent years, NPAFP rates for inaccessible areas were similar to those reported nationally (5.3 cases per 100 000 persons aged <15 years during 2016) [17]; however, a major quality concern is specimen integrity upon arrival at the polio laboratory. Against this backdrop, an evaluation of NPEV and Sabin poliovirus isolation rates over the past 4 years by access level was warranted. Like poliovirus isolation, the NPEV isolation rate is dependent on laboratory-confirmed results and, therefore, is not susceptible to data manipulation during the recording of case investigations, as may be the case with some other AFP surveillance indicators.
Using NPEV and Sabin poliovirus detection as objective data, we conducted this study to assess detection rates by access type (ie, accessible, partially accessible, and inaccessible areas), while also determining the association of other factors, such as age, OPV vaccination status, and source of reporting in Somalia.
METHODS
Dataset, Inclusion Criteria, and Sample Size
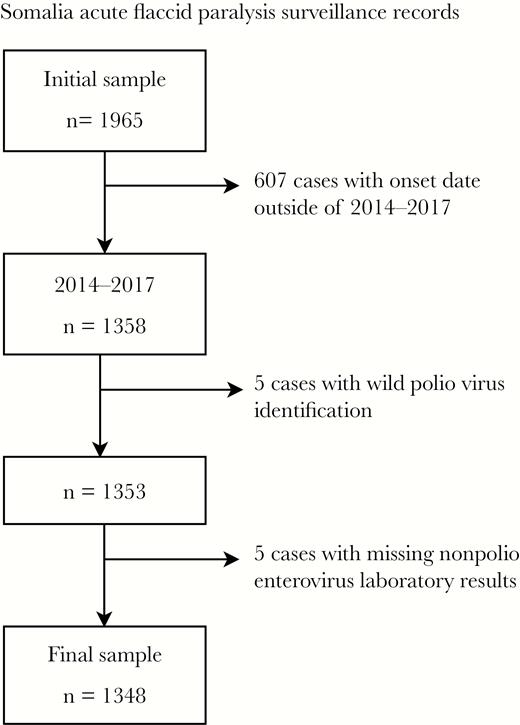
We analyzed Somalia AFP surveillance records collected from all sources of notification (including public hospitals, private clinics, traditional healers, VPVs, and immunization campaigns) obtained from the WHO Eastern Mediterranean Regional Office. For our study, we only included AFP cases that had paralysis onset during January 1, 2014–December 31, 2017, valid laboratory results for NPEV isolation (ie, yes or no), and from which WPV or VDPV was not concurrently isolated. Laboratory testing of stool specimens was conducted at the polio laboratory of the Kenya Medical Research Institute (KEMRI), located in Nairobi, Kenya. The period under review extends from the attenuation of the 2013−2014 WPV outbreak to the time of identification of the ongoing cVDPV outbreaks in 2017 [9, 14]. Figure 1 illustrates inclusion and exclusion criteria and sample sizes.
Variable Inclusion, Manipulation, and Definitions
A clinical AFP case was defined as acute onset of flaccid paralysis in any child under 15 years of age or in any person of any age with paralytic illness if polio was suspected [18]. WPV, Sabin-like virus, VDPV, and NPEV cases were defined as any AFP case patient with WPV, Sabin-like virus, VDPV, or NPEV isolated from their stool specimens, respectively. Accessibility is an operational definition used by the polio program based on its ability to conduct vaccination campaigns. There were no clearly defined criteria for accessibility categorization in Somalia; however, a district is generally considered inaccessible when the polio eradication national program is not allowed to conduct any supplementary immunization activity within its boundaries due to security concerns or bans on such activities imposed by local political actors or insurgents. A district is considered partially accessible when polio campaigns can be conducted in some (mostly urban/semi-urban) areas but not in other (mostly rural/remote) areas. We used the program’s accessibility data for 2017 as the basis for classification in this analysis.
We included the following variables in this analysis (whenever appropriate, the first level of each variable is the reference level): accessibility (accessible, partially inaccessible, inaccessible), source of notification (all other reporting sources, VPVs), year of paralysis onset (2014–2017), age in years (0–1, 1–4, ≥5), sex (female, male), lifestyle (urban, rural, nomadic), previous OPV vaccination status (0 dose, 1–2 doses, ≥3 doses), transport time (from second stool collection to laboratory receipt in days), Sabin virus detection (no, yes), and NPEV detection (no, yes). The main outcome of interest was defined as NPEV detection, and the main predictor variable was defined as accessibility status.
Data Analysis
Descriptive analyses were performed by stratifying the variables described above by access type. For simplicity of interpretation, we assessed differences only between the “accessible” and “inaccessible” categories. For continuous variables, medians and interquartile ranges (IQRs) were calculated, and the Wilcoxon signed rank test was used to assess differences. For categorical variables, frequencies and percentages were calculated, and the chi-square test was used to assess differences. We then used logistic regression to assess univariate and multivariate associations with NPEV detection. To build the final model, we followed a mixed approach retaining the main variables of interest, variables with significant associations in univariate analyses, and confounders that were identified in multivariate analysis. An association was considered statistically significant for P values <.05. All statistical analyses were performed using R Studio, version 1.1.447 [19].
This project was reviewed in accordance with Centers for Disease Control and Prevention human research protection procedures and was determined to be nonresearch, routine disease surveillance activity.
RESULTS
Overall, 1348 AFP cases were reported in Somalia with onset during January 2014−December 2017. A majority of case patients were male (56%, n = 749), and they ranged in age from newborn to 24 years (median [IQR], 2 [1–4] years). Characteristics of AFP case patients stratified by access type are shown in Table 1. When comparing AFP case patients reported from inaccessible (n = 171) and accessible (n = 848) areas, those reported from inaccessible areas were more likely to be male (65%, n = 112, vs 55%, n = 462; P = .012), not to have received OPV—that is, “0 dose”—(62%, n = 106, vs 3%, n = 25; P < .001), to be reported by a VPV (65%, n = 112, vs 35%, n = 297; P < .001), and to originate from a rural area (61%, n = 104, vs 26%, n = 216; P < .001). Their stool specimens were also more likely to have longer transportation times (median [IQR], 6 [4–10] days vs 4 [2–5] days; P < .001) and to have NPEV detected (23%, n = 39, vs 15%, n = 123; P = .010). Figure 2 illustrates yearly NPEV detection rates during 2014–2017, which ranged from a minimum of 10% in 2014 to a maximum of 19% in 2016 within accessible areas and from 19% in 2016 to 29% in 2017 within inaccessible areas. Figure 3 illustrates the yearly Sabin-like virus detection rate, which ranged from a minimum of 7% in 2015 to a maximum of 9% in 2014 within accessible areas, and from 0% in both 2014 and 2017 to 11.8% in 2015 within inaccessible areas.
Descriptive Characteristics of Acute Flaccid Paralysis Case Patients Stratified by Access Type—Somalia, 2014–2017 (n = 1348)
| . | Access Type, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| Characteristics . | Accessible (n = 846) . | Partially Accessible (n = 331) . | Inaccessible (n = 171) . | Total (n = 1348), No. (%) . | P Valuea . |
| Age, y | |||||
| 0–1 | 120 (14) | 29 (9) | 9 (5) | 158 (12) | .002 |
| 1–5 | 618 (73) | 244 (74) | 145 (85) | 1007 (75) | |
| ≥5 | 108 (13) | 58 (18) | 17 (10) | 183 (14) | |
| Sex | |||||
| Female | 383 (45) | 156 (47) | 59 (35) | 598 (44) | .012 |
| Male | 462 (55) | 175 (53) | 112 (65) | 749 (56) | |
| Notified by | |||||
| VPV | 297 (35) | 137 (42) | 112 (65) | 546 (41) | <.001 |
| Other | 544 (65) | 193 (58) | 59 (35) | 796 (59) | |
| Lifestyle | |||||
| Urban | 186 (22) | 82 (25) | 35 (21) | 303 (23) | <.001 |
| Rural | 216 (26) | 150 (45) | 104 (61) | 470 (35) | |
| Nomadic | 440 (52) | 98 (30) | 31 (18) | 569 (42) | |
| OPV doses | |||||
| 0 | 25 (3) | 82 (25) | 106 (62) | 213 (16) | <.001 |
| 1–2 | 55 (7) | 43 (13) | 27 (16) | 125 (9) | |
| ≥3 | 765 (91) | 206 (62) | 38 (22) | 1009 (75) | |
| Transport timeb | |||||
| Median (IQR) | 4 (2–5) | 5 (3–8) | 6 (4–10) | 4 (3–6) | <.001 |
| Sabin detection | |||||
| No | 776 (92) | 310 (94) | 164 (96) | 1250 (93) | .084 |
| Yes | 70 (8) | 21 (6) | 7 (4) | 98 (7) | |
| NPEV detection | |||||
| No | 723 (85) | 280 (85) | 132 (77) | 1135 (84) | .010 |
| Yes | 123 (15) | 51 (15) | 39 (23) | 213 (16) | |
| . | Access Type, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| Characteristics . | Accessible (n = 846) . | Partially Accessible (n = 331) . | Inaccessible (n = 171) . | Total (n = 1348), No. (%) . | P Valuea . |
| Age, y | |||||
| 0–1 | 120 (14) | 29 (9) | 9 (5) | 158 (12) | .002 |
| 1–5 | 618 (73) | 244 (74) | 145 (85) | 1007 (75) | |
| ≥5 | 108 (13) | 58 (18) | 17 (10) | 183 (14) | |
| Sex | |||||
| Female | 383 (45) | 156 (47) | 59 (35) | 598 (44) | .012 |
| Male | 462 (55) | 175 (53) | 112 (65) | 749 (56) | |
| Notified by | |||||
| VPV | 297 (35) | 137 (42) | 112 (65) | 546 (41) | <.001 |
| Other | 544 (65) | 193 (58) | 59 (35) | 796 (59) | |
| Lifestyle | |||||
| Urban | 186 (22) | 82 (25) | 35 (21) | 303 (23) | <.001 |
| Rural | 216 (26) | 150 (45) | 104 (61) | 470 (35) | |
| Nomadic | 440 (52) | 98 (30) | 31 (18) | 569 (42) | |
| OPV doses | |||||
| 0 | 25 (3) | 82 (25) | 106 (62) | 213 (16) | <.001 |
| 1–2 | 55 (7) | 43 (13) | 27 (16) | 125 (9) | |
| ≥3 | 765 (91) | 206 (62) | 38 (22) | 1009 (75) | |
| Transport timeb | |||||
| Median (IQR) | 4 (2–5) | 5 (3–8) | 6 (4–10) | 4 (3–6) | <.001 |
| Sabin detection | |||||
| No | 776 (92) | 310 (94) | 164 (96) | 1250 (93) | .084 |
| Yes | 70 (8) | 21 (6) | 7 (4) | 98 (7) | |
| NPEV detection | |||||
| No | 723 (85) | 280 (85) | 132 (77) | 1135 (84) | .010 |
| Yes | 123 (15) | 51 (15) | 39 (23) | 213 (16) | |
Abbreviations: IQR, interquartile range; NPEV, nonpolio enterovirus; OPV, oral poliovirus vaccine; VPV, Village Polio Volunteers.
aAccessible vs inaccessible areas (chi-square test for categorical variables and Wilcoxon signed rank test for continuous variables; P < .05 are bolded).
bFrom second stool collection to laboratory receipt (in days).
Descriptive Characteristics of Acute Flaccid Paralysis Case Patients Stratified by Access Type—Somalia, 2014–2017 (n = 1348)
| . | Access Type, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| Characteristics . | Accessible (n = 846) . | Partially Accessible (n = 331) . | Inaccessible (n = 171) . | Total (n = 1348), No. (%) . | P Valuea . |
| Age, y | |||||
| 0–1 | 120 (14) | 29 (9) | 9 (5) | 158 (12) | .002 |
| 1–5 | 618 (73) | 244 (74) | 145 (85) | 1007 (75) | |
| ≥5 | 108 (13) | 58 (18) | 17 (10) | 183 (14) | |
| Sex | |||||
| Female | 383 (45) | 156 (47) | 59 (35) | 598 (44) | .012 |
| Male | 462 (55) | 175 (53) | 112 (65) | 749 (56) | |
| Notified by | |||||
| VPV | 297 (35) | 137 (42) | 112 (65) | 546 (41) | <.001 |
| Other | 544 (65) | 193 (58) | 59 (35) | 796 (59) | |
| Lifestyle | |||||
| Urban | 186 (22) | 82 (25) | 35 (21) | 303 (23) | <.001 |
| Rural | 216 (26) | 150 (45) | 104 (61) | 470 (35) | |
| Nomadic | 440 (52) | 98 (30) | 31 (18) | 569 (42) | |
| OPV doses | |||||
| 0 | 25 (3) | 82 (25) | 106 (62) | 213 (16) | <.001 |
| 1–2 | 55 (7) | 43 (13) | 27 (16) | 125 (9) | |
| ≥3 | 765 (91) | 206 (62) | 38 (22) | 1009 (75) | |
| Transport timeb | |||||
| Median (IQR) | 4 (2–5) | 5 (3–8) | 6 (4–10) | 4 (3–6) | <.001 |
| Sabin detection | |||||
| No | 776 (92) | 310 (94) | 164 (96) | 1250 (93) | .084 |
| Yes | 70 (8) | 21 (6) | 7 (4) | 98 (7) | |
| NPEV detection | |||||
| No | 723 (85) | 280 (85) | 132 (77) | 1135 (84) | .010 |
| Yes | 123 (15) | 51 (15) | 39 (23) | 213 (16) | |
| . | Access Type, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| Characteristics . | Accessible (n = 846) . | Partially Accessible (n = 331) . | Inaccessible (n = 171) . | Total (n = 1348), No. (%) . | P Valuea . |
| Age, y | |||||
| 0–1 | 120 (14) | 29 (9) | 9 (5) | 158 (12) | .002 |
| 1–5 | 618 (73) | 244 (74) | 145 (85) | 1007 (75) | |
| ≥5 | 108 (13) | 58 (18) | 17 (10) | 183 (14) | |
| Sex | |||||
| Female | 383 (45) | 156 (47) | 59 (35) | 598 (44) | .012 |
| Male | 462 (55) | 175 (53) | 112 (65) | 749 (56) | |
| Notified by | |||||
| VPV | 297 (35) | 137 (42) | 112 (65) | 546 (41) | <.001 |
| Other | 544 (65) | 193 (58) | 59 (35) | 796 (59) | |
| Lifestyle | |||||
| Urban | 186 (22) | 82 (25) | 35 (21) | 303 (23) | <.001 |
| Rural | 216 (26) | 150 (45) | 104 (61) | 470 (35) | |
| Nomadic | 440 (52) | 98 (30) | 31 (18) | 569 (42) | |
| OPV doses | |||||
| 0 | 25 (3) | 82 (25) | 106 (62) | 213 (16) | <.001 |
| 1–2 | 55 (7) | 43 (13) | 27 (16) | 125 (9) | |
| ≥3 | 765 (91) | 206 (62) | 38 (22) | 1009 (75) | |
| Transport timeb | |||||
| Median (IQR) | 4 (2–5) | 5 (3–8) | 6 (4–10) | 4 (3–6) | <.001 |
| Sabin detection | |||||
| No | 776 (92) | 310 (94) | 164 (96) | 1250 (93) | .084 |
| Yes | 70 (8) | 21 (6) | 7 (4) | 98 (7) | |
| NPEV detection | |||||
| No | 723 (85) | 280 (85) | 132 (77) | 1135 (84) | .010 |
| Yes | 123 (15) | 51 (15) | 39 (23) | 213 (16) | |
Abbreviations: IQR, interquartile range; NPEV, nonpolio enterovirus; OPV, oral poliovirus vaccine; VPV, Village Polio Volunteers.
aAccessible vs inaccessible areas (chi-square test for categorical variables and Wilcoxon signed rank test for continuous variables; P < .05 are bolded).
bFrom second stool collection to laboratory receipt (in days).
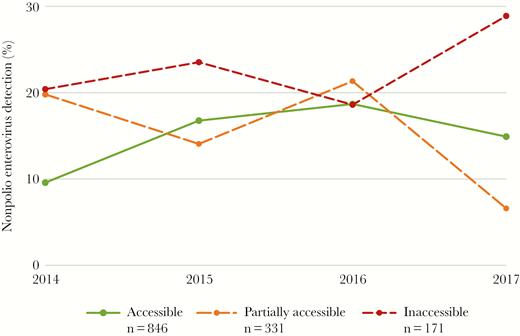
Yearly nonpolio enterovirus detection rate within stool specimens collected from acute flaccid paralysis case patients and stratified by access category—Somalia, 2014–2017 (n = 1348): n = 411 in 2014 (261 accessible, 101 partially accessible, and 49 inaccessible); n = 277 in 2015 (179 accessible, 64 partially accessible, and 34 inaccessible); n = 316 in 2016 (198 accessible, 75 partially accessible, and 43 inaccessible); and n = 344 in 2017 (208 accessible, 91 partially accessible, and 45 inaccessible).
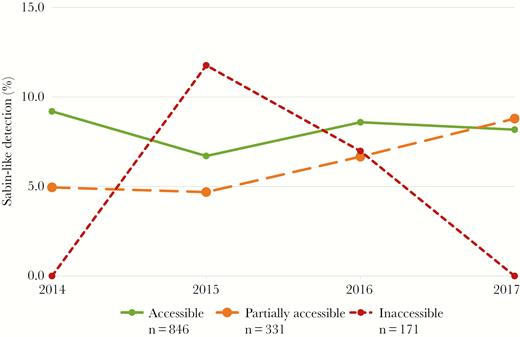
Yearly Sabin-like detection rate within stool specimens collected from acute flaccid paralysis case patients and stratified by access category—Somalia, 2014–2017 (n = 1348): n = 411 in 2014 (261 accessible, 101 partially accessible, and 49 inaccessible); n = 277 in 2015 (179 accessible, 64 partially accessible, and 34 inaccessible); n = 316 in 2016 (198 accessible, 75 partially accessible, and 43 inaccessible); and n = 344 in 2017 (208 accessible, 91 partially accessible, and 45 inaccessible).
In univariate cross-tabulations (Table 2), samples from cases reported from inaccessible areas (odds ratio [OR], 1.74; 95% confidence interval [CI], 1.15–2.59) and those in which Sabin-like virus was detected (OR, 1.82; 95% CI, 1.10–2.92) were both associated with higher odds of NPEV detection, whereas receiving at least 3 doses of OPV (OR, 0.62; 95% CI, 0.43–0.90) and being aged ≥5 years (OR, 0.41; 95% CI, 0.20–0.81) were both associated with lower NPEV detection. Stool specimens of AFP cases identified by VPVs did not differ from those of AFP cases identified by other sources of notification with respect to NPEV detection (OR, 1.15; 95% CI, 0.86–1.55).
Unadjusted and Adjusted Associations With Nonpolio Enterovirus Detection Rate in Stool Specimens Collected From Acute Flaccid Paralysis Case Patients—Somalia, 2014–2017 (n = 1348)
| . | Univariate Analysis . | Multivariate Model 1 Excluding OPV . | Multivariate Model 2a Including OPV . |
|---|---|---|---|
| . | Unadjusted OR [95% CI] . | Adjusted OR [95% CI] . | Adjusted OR [95% CI] . |
| Sex (ref: female) | |||
| Male | 0.88 [0.66–1.19] | ||
| Lifestyle (ref: urban) | |||
| Rural | 1.19 [0.80–1.79] | ||
| Nomadic | 1.11 [0.75–1.65] | ||
| Transport timeb (continuous) | 1.01 [0.98–1.03] | ||
| OPV (ref: 0 doses) | |||
| 1–2 | 0.69 [0.38–1.22] | 0.81 [0.43–1.47] | |
| ≥3 | 0.62 [0.43–0.90] | 0.74 [0.46–1.20] | |
| Access (ref: accessible) | |||
| Partially accessible | 1.07 [0.75–1.52] | 1.13 [0.78–1.61] | 1.04 [0.70–1.53] |
| Inaccessible | 1.74 [1.15–2.59] | 1.75 [1.14–2.65] | 1.44 [0.84–2.43] |
| Age (ref: 0–1), y | |||
| 1–5 | 1.12 [0.72–1.80] | 1.07 [0.68–1.73] | 1.09 [0.69–1.79] |
| ≥5 | 0.41 [0.20–0.81] | 0.42 [0.20–0.85] | 0.44 [0.21–0.89] |
| Notified by (ref: other) | |||
| VPV | 1.15 [0.86–1.55] | 1.04 [0.76–1.42] | 1.03 [0.75–1.42] |
| Sabin detection (ref: no) | |||
| Yes | 1.82 [1.10–2.92] | 1.79 [1.07–2.90] | 1.80 [1.08–2.91] |
| Year (continuous) | 1.05 [0.93–1.19] | 1.04 [0.92–1.19] | 1.05 [0.92–1.20] |
| . | Univariate Analysis . | Multivariate Model 1 Excluding OPV . | Multivariate Model 2a Including OPV . |
|---|---|---|---|
| . | Unadjusted OR [95% CI] . | Adjusted OR [95% CI] . | Adjusted OR [95% CI] . |
| Sex (ref: female) | |||
| Male | 0.88 [0.66–1.19] | ||
| Lifestyle (ref: urban) | |||
| Rural | 1.19 [0.80–1.79] | ||
| Nomadic | 1.11 [0.75–1.65] | ||
| Transport timeb (continuous) | 1.01 [0.98–1.03] | ||
| OPV (ref: 0 doses) | |||
| 1–2 | 0.69 [0.38–1.22] | 0.81 [0.43–1.47] | |
| ≥3 | 0.62 [0.43–0.90] | 0.74 [0.46–1.20] | |
| Access (ref: accessible) | |||
| Partially accessible | 1.07 [0.75–1.52] | 1.13 [0.78–1.61] | 1.04 [0.70–1.53] |
| Inaccessible | 1.74 [1.15–2.59] | 1.75 [1.14–2.65] | 1.44 [0.84–2.43] |
| Age (ref: 0–1), y | |||
| 1–5 | 1.12 [0.72–1.80] | 1.07 [0.68–1.73] | 1.09 [0.69–1.79] |
| ≥5 | 0.41 [0.20–0.81] | 0.42 [0.20–0.85] | 0.44 [0.21–0.89] |
| Notified by (ref: other) | |||
| VPV | 1.15 [0.86–1.55] | 1.04 [0.76–1.42] | 1.03 [0.75–1.42] |
| Sabin detection (ref: no) | |||
| Yes | 1.82 [1.10–2.92] | 1.79 [1.07–2.90] | 1.80 [1.08–2.91] |
| Year (continuous) | 1.05 [0.93–1.19] | 1.04 [0.92–1.19] | 1.05 [0.92–1.20] |
P values <.05 are bolded.
Abbreviations: CI, confidence interval; OPV, oral poliovirus vaccine; OR, odds ratio; VPV, Village Polio Volunteers.
aUsing logistic regression models.
bFrom second stool collection to laboratory receipt (in days).
Unadjusted and Adjusted Associations With Nonpolio Enterovirus Detection Rate in Stool Specimens Collected From Acute Flaccid Paralysis Case Patients—Somalia, 2014–2017 (n = 1348)
| . | Univariate Analysis . | Multivariate Model 1 Excluding OPV . | Multivariate Model 2a Including OPV . |
|---|---|---|---|
| . | Unadjusted OR [95% CI] . | Adjusted OR [95% CI] . | Adjusted OR [95% CI] . |
| Sex (ref: female) | |||
| Male | 0.88 [0.66–1.19] | ||
| Lifestyle (ref: urban) | |||
| Rural | 1.19 [0.80–1.79] | ||
| Nomadic | 1.11 [0.75–1.65] | ||
| Transport timeb (continuous) | 1.01 [0.98–1.03] | ||
| OPV (ref: 0 doses) | |||
| 1–2 | 0.69 [0.38–1.22] | 0.81 [0.43–1.47] | |
| ≥3 | 0.62 [0.43–0.90] | 0.74 [0.46–1.20] | |
| Access (ref: accessible) | |||
| Partially accessible | 1.07 [0.75–1.52] | 1.13 [0.78–1.61] | 1.04 [0.70–1.53] |
| Inaccessible | 1.74 [1.15–2.59] | 1.75 [1.14–2.65] | 1.44 [0.84–2.43] |
| Age (ref: 0–1), y | |||
| 1–5 | 1.12 [0.72–1.80] | 1.07 [0.68–1.73] | 1.09 [0.69–1.79] |
| ≥5 | 0.41 [0.20–0.81] | 0.42 [0.20–0.85] | 0.44 [0.21–0.89] |
| Notified by (ref: other) | |||
| VPV | 1.15 [0.86–1.55] | 1.04 [0.76–1.42] | 1.03 [0.75–1.42] |
| Sabin detection (ref: no) | |||
| Yes | 1.82 [1.10–2.92] | 1.79 [1.07–2.90] | 1.80 [1.08–2.91] |
| Year (continuous) | 1.05 [0.93–1.19] | 1.04 [0.92–1.19] | 1.05 [0.92–1.20] |
| . | Univariate Analysis . | Multivariate Model 1 Excluding OPV . | Multivariate Model 2a Including OPV . |
|---|---|---|---|
| . | Unadjusted OR [95% CI] . | Adjusted OR [95% CI] . | Adjusted OR [95% CI] . |
| Sex (ref: female) | |||
| Male | 0.88 [0.66–1.19] | ||
| Lifestyle (ref: urban) | |||
| Rural | 1.19 [0.80–1.79] | ||
| Nomadic | 1.11 [0.75–1.65] | ||
| Transport timeb (continuous) | 1.01 [0.98–1.03] | ||
| OPV (ref: 0 doses) | |||
| 1–2 | 0.69 [0.38–1.22] | 0.81 [0.43–1.47] | |
| ≥3 | 0.62 [0.43–0.90] | 0.74 [0.46–1.20] | |
| Access (ref: accessible) | |||
| Partially accessible | 1.07 [0.75–1.52] | 1.13 [0.78–1.61] | 1.04 [0.70–1.53] |
| Inaccessible | 1.74 [1.15–2.59] | 1.75 [1.14–2.65] | 1.44 [0.84–2.43] |
| Age (ref: 0–1), y | |||
| 1–5 | 1.12 [0.72–1.80] | 1.07 [0.68–1.73] | 1.09 [0.69–1.79] |
| ≥5 | 0.41 [0.20–0.81] | 0.42 [0.20–0.85] | 0.44 [0.21–0.89] |
| Notified by (ref: other) | |||
| VPV | 1.15 [0.86–1.55] | 1.04 [0.76–1.42] | 1.03 [0.75–1.42] |
| Sabin detection (ref: no) | |||
| Yes | 1.82 [1.10–2.92] | 1.79 [1.07–2.90] | 1.80 [1.08–2.91] |
| Year (continuous) | 1.05 [0.93–1.19] | 1.04 [0.92–1.19] | 1.05 [0.92–1.20] |
P values <.05 are bolded.
Abbreviations: CI, confidence interval; OPV, oral poliovirus vaccine; OR, odds ratio; VPV, Village Polio Volunteers.
aUsing logistic regression models.
bFrom second stool collection to laboratory receipt (in days).
In multivariable regression models (Table 2), the inclusion of OPV status in the model confounded the effect of inaccessibility on NPEV detection (when OPV was excluded from the model: adjusted odds ratio [AOR], 1.75; 95% CI, 1.14–2.65; vs when OPV was included: AOR, 1.44; 95% CI, 0.84–2.43). In the multivariate model without OPV, inaccessible areas (AOR, 1.75; 95% CI, 1.14–2.65) and detection of Sabin-like virus (AOR, 1.79; 95% CI, 1.07–2.90) both had a positive association with NPEV detection, whereas being ≥5 years of age (AOR, 0.42; 95% CI, 0.20–0.85) had a negative association with NPEV detection. In the multivariate model including OPV, only the detection of Sabin-like virus was positively associated with NPEV detection (AOR, 1.80; 95% CI, 1.08–2.91), while being aged ≥5 years remained negatively associated with NPEV detection (AOR, 0.44; 95% CI, 0.21–0.89).
Discussion
In our study, we used case-based AFP surveillance data for Somalia to assess the independent association between accessibility and NPEV detection in stool specimens collected from AFP case patients with onset during 2014–2017. Except for partially accessible areas in 2017, all areas—regardless of access type—exceeded an NPEV detection rate of 10% during each year of the study. In multivariate analyses, specimens from inaccessible areas were consistently associated with higher odds of NPEV detection when excluding OPV history from the model. Stool specimens collected from AFP case patients who were ≥5 years of age were consistently associated with a >55% decrease in the odds of detecting NPEVs, whereas Sabin-like virus detection in a stool specimen was associated with an overall 80% increase in the odds of detecting NPEV. Of note, specimens collected from AFP case patients who were reported by VPVs did not differ significantly in the rates of NPEV detection from those of cases reported through other means.
The confounding effect of OPV vaccination status on the association between inaccessibility and NPEV detection is unsurprising, given the strong correlation between the 2 predictor variables. Inaccessibility, as earlier defined, indicates a lack of access for polio vaccination activities [17], which would in turn affect the OPV vaccination status of children living in such areas. In most inaccessible areas, a large proportion of the population has no access to health services [20] and no access to good hygiene and sanitation systems; as such, it is possible that children within these areas who have received fewer doses of OPV may also have a poorer health status or more substantial crowding, and are thus more likely to become infected with and shed NPEVs. However, from an operational standpoint, the levels of NPEV detection in inaccessible areas serve as an objective measure reflecting positively on some specimen quality upon arrival in the laboratory within these areas. These results are of even greater importance given the ongoing outbreaks of circulating vaccine-derived polioviruses in Somalia and the substantial delays noted for stool specimen transportation from inaccessible areas.
Additionally, our analysis suggests that although the program is heavily reliant on VPVs within inaccessible areas (65% of all reported AFP cases), this did not have any apparent impact on NPEV detection and, by inference, the integrity of specimens collected from case patients reported from inaccessible areas upon testing. After controlling for other variables of interest, NPEV detection in specimens taken from AFP case patients reported by VPVs did not differ from the detection rate among cases identified by other sources of notification. A previous assessment of the VPV program noted significant improvements over time after introduction of the program in NPAFP rates, stool adequacy, and timeliness of case detection [17]. Our study builds on and validates these findings by providing an additional assessment of the VPV program using a different surveillance measure. Given constraints on the ground, our results inform the program about resource allocation, strategic planning, and confidence in the data on cases reported by teams in the field.
Among other predictors of interest, increased age was associated with lower odds of detecting NPEV, reaching statistical significance for AFP cases that were ≥5 years of age or older, who had 56% decreased odds of NPEV isolation compared with AFP case patients younger than 1 year of age. This outcome is biologically plausible, as older children are more likely to have developed acquired immunity against enteroviruses and may have decreased exposure as well, thus decreasing their odds of being infected with or shedding these viruses following exposure [21].
Conversely, although Sabin-like virus detection rates were lower in inaccessible areas, reflecting fewer opportunities for exposure to OPV in these areas, the detection of Sabin-like viruses in stool specimens was associated with an overall 80% increase in the odds of detecting NPEVs. This may reflect the unexpectedly high isolation rate of Sabin-like virus from inaccessible areas during 2015 and 2016.
To our knowledge, this is the first study of its kind using logistic regressions to examine NPEV detection as a binary outcome to inform a national program on the impact of inaccessibility on the quality of poliovirus surveillance activities in the field, particularly regarding specimen integrity. Our study has several limitations. First, there is no clear definition for access categories. In this analysis, we relied on the program classification for 2017, which relied on the United Nations Department of Safety and Security classification augmented with “soft” intelligence from program field staff. It is possible that for some years, some districts were misclassified; however, we do not expect this to have made a substantial impact on our results. Second, the number of previous doses of OPV was ascertained through the recall of the caregivers of AFP cases, which could be subject to both recall and social desirability biases. Third, our sample may have been subject to selection bias, which could pose threats to the generalizability of our findings. For example, there were significantly more males (65%) among AFP cases reported from inaccessible areas, which could be related to gender-based differences in cultural norms of disease surveillance and health-seeking behavior. Also, there was a high rate of Sabin isolation for cases from inaccessible areas in 2015 and 2016, which is not consistent with other years and is difficult to explain. Fourth, the use of NPEV detection in assessing stool specimen integrity upon arrival in the laboratory needs to be interpreted with caution, as its increase could serve as a proxy for both good-quality specimens and increased NPEV prevalence. Additionally, the use of NPEV detection as a binary outcome does not take into consideration the wide diversity of NPEV types, the complex patterns of intestinal infection, and the variability in NPEV detection depending on factors such as climate, geography, seasonality, and urbanization [22, 23]. Lastly, the variability in surveillance indictors and the heterogenicity within each access type were not assessed in this investigation. Nevertheless, this analysis, while recognizing its limitations, provides critical information to guide the national polio program in the interpretation of AFP data and in light of the ongoing cVDPV outbreaks.
In conclusion, NPEV isolation was variable by year and by category of access but found in all areas in Somalia—regardless of access—with inaccessible areas having higher odds of NPEV detection. No differences were recorded in terms of NPEV detection between AFP cases who were reported by the VPVs and those reported by other means of notification. The polio eradication program in Somalia still faces significant challenges in the field, including critical access limitations, fragmented political control, and significant gaps in immunization activities; nevertheless, our findings suggest that the program is capable of generating surveillance data that are useful for interpretation. This analysis does not preclude the existence of surveillance gaps—as evidenced by the ongoing outbreaks of circulating vaccine-derived polioviruses in the country for which genomic sequence analysis indicated undetected circulation for 1–3 years (for cVDPV types 3 and 2, respectively) [14]—but it does provide a measure of confidence in the ability of field staff to detect poliovirus circulation. The network of VPVs, which was developed to overcome significant access limitations in many parts of South and Central Somalia, should continue to be supported, as they complement the traditional health facility–based AFP surveillance system in the country. The findings of this study provide guidance to the polio program on the presence of a level of AFP surveillance in inaccessible areas, which has implications for other countries with similar accessibility challenges.
Acknowledgments
The authors would like to acknowledge the World Health Organization Eastern Mediterranean Regional Offices and African Regional Offices for providing the Somalia AFP surveillance data and approving this work.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was conducted as part of the normal duties of employees of the World Health Organization and the US Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments